Abstract
Sexual behavior involves motivational processes. Findings from both animal models and neuroimaging in humans suggest that the recruitment of neural motor networks is an integral part of the sexual response. However, no study so far has directly linked sexual motivation to physiologically measurable changes in cerebral motor systems in humans. Using transcranial magnetic stimulation in hetero- and homosexual men, we here show that sexual motivation modulates cortical excitability. More specifically, our results demonstrate that visual sexual stimuli corresponding with one’s sexual orientation, compared with non-corresponding visual sexual stimuli, increase the excitability of the motor cortex. The reflection of sexual motivation in motor cortex excitability provides evidence for motor preparation processes in sexual behavior in humans. Moreover, such interrelationship links theoretical models and previous neuroimaging findings of sexual behavior.
Keywords: sexual motivation, cortical excitability, transcranial magnetic stimulation, motor evoked potentials
INTRODUCTION
Sexual behavior involves a state of sexual excitement induced by preferred sexual stimuli. Based on neuroimaging studies investigating neural networks for sexual stimulus-driven processing, a neurobehavioral model of sexual arousal proposed autonomic, cognitive, emotional and motivational components (Stoléru et al., 2012). This neurobehavioral model is commensurate with an information processing model that distinguishes between automatic, pre-attentive stages of stimulus appraisal and subsequent emotional and motivational states (Janssen et al., 2000). A comprehensive theoretical framework for sexual motivation, arousal and behavior integrates biological and cognitive-emotional aspects by focusing on the pathway between incentive and response (Toates, 2009). It follows incentive motivation theories and highlights that sexual motivation emerges from an interaction of external incentives and internal states (Singer and Toates, 1987). In this context, motivation directs thoughts and behavior to a sexual goal, including the perceived urge to express sexual behavior (Stoléru, 2014), and is mediated by cortico-basal ganglia loops (Tanaka et al., 2004; Kühn and Gallinat, 2011; Stoléru, 2014). The notion of motivation representing an integral part of sexual behavior is endorsed by meta-analytic evidence for the recruitment of basal ganglia and cerebral motor regions during sexual stimulation (Stoléru et al., 2012; Poeppl et al., 2014). In this regard, it has also been noted that the functional neuroanatomy of sex is remarkably similar to that of other incentives such as food (Georgiadis and Kringelbach, 2012). Moreover, animal research suggests that generalized brain arousal characterized by increased motor activity and responsivity to sensory stimuli impacts sexual behavior (Weil et al., 2010).
Cortical excitability depends on cortical arousal level (Fischer et al., 2008) and is modulated by motivational states particularly within the motor system (Kapogiannis et al., 2008; Gupta and Aron, 2011). Importantly, no study has as yet investigated the link between sexual motivation and cortical excitability in the motor system. Therefore, this study sought to test the hypothesis that sexual motivation induced by appropriate sexual stimuli is reflected by changes in excitability of the motor cortex. Motor-evoked potentials (MEPs) are electromyographic markers for motor cortex excitability and can be non-invasively elicited by single pulses of transcranial magnetic stimulation (TMS) over the corresponding area of the motor cortex. More specifically, a single TMS pulse over the motor cortex induces electric currents in the brain that can depolarize neurons. Transsynaptically, the excitation propagates via pyramidal motor neurons to the periphery and finally results in a contraction of corresponding muscles contralateral to the stimulated motor cortex, which can be recorded as MEP by surface electrodes (Hallett, 2007; Rossini and Rossi, 2007). The amplitude of the MEP hence represents an aggregate measure of the excitation state of efferent cells in the motor cortex (Rothwell et al., 1991; Wassermann and Zimmermann, 2012). Alterations in cortical excitability have been demonstrated in clinical populations [for a review see Bunse et al. (2014)] and also following therapeutic interventions (Frank et al., 2014; Schecklmann et al., 2014). In addition, cortical excitability has been shown to depend on cognitive and emotional mental states including motivation (Gupta and Aron, 2011; Klein et al., 2012; Borgomaneri et al., 2014). We used TMS-induced MEP measurements during visual sexual stimulation in healthy hetero- and homosexual men to assess the relationship between cortical excitability and stimulus-induced sexual motivation. Subjects were presented with non-explicit but sexually arousing pictures and neutral control pictures, while motor cortex excitability was measured (Figure 1). After the experimental procedures, pictures were rated with respect to emotional valence, sexual arousal and sexual urge.
Fig. 1.
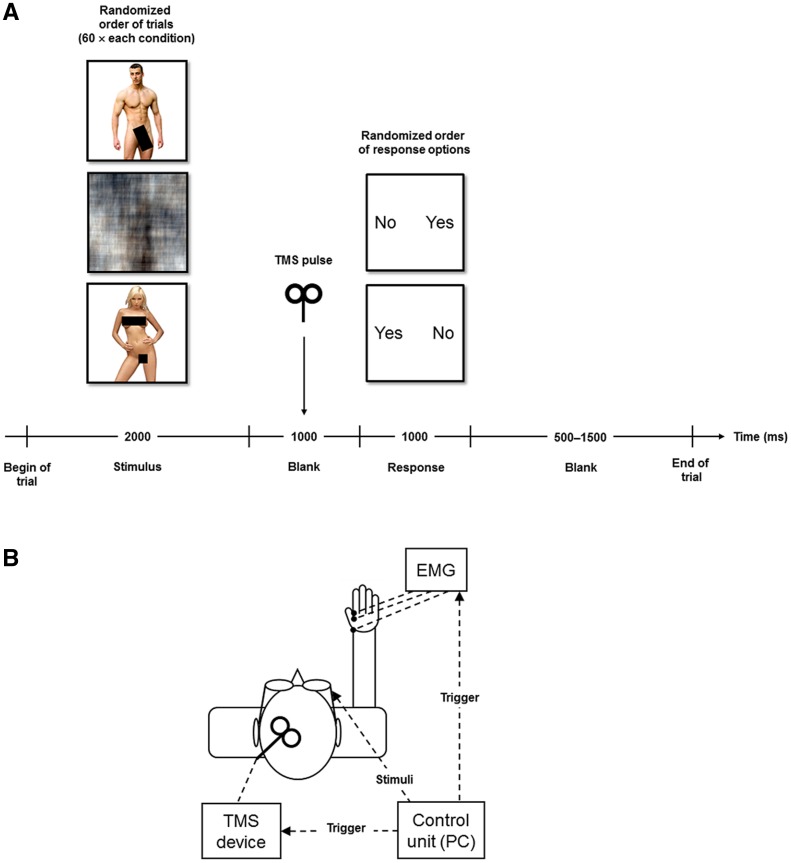
Experimental setup. (A) Paradigm. Subjects were presented with non-explicit but sexually arousing and neutral pictures in a two-alternative forced-choice task. Motor cortex excitability was measured before actual active response was required. (B) Schematic illustration of the technical interface. MEPs were assessed using TMS over the left motor cortex and concomitant EMG of the right index finger. EMG = electromyography, MEP = motor evoked potential, TMS = transcranial magnetic stimulation.
MATERIALS AND METHODS
Subjects
For this study, we recruited 23 heterosexual and 17 homosexual healthy men without any record of psychiatric or neurological disorders, who fulfilled the safety criteria for TMS. All participants were examined by a trained psychiatrist and completed a screening standard questionnaire assessing eligibility for TMS (Rossi et al., 2009). Subjects with past or current psychiatric or neurologic disorders did not enter the study. Similarly, candidates taking any medication or with contraindications for TMS were not eligible. Participants’ sexual orientation was self-reported using the Sell Assessment of Sexual Orientation (Sell, 1996), which revealed a clear distinction between groups with mean scores of 7.6 ± 1.2 SD for the heterosexual and −4.8 ± 2.8 for the homosexual participants (positive values indicate heterosexual, while negative values indicate homosexual orientation; P < 0.001). All subjects were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Both groups were comparable [mean (M) ± SD] with respect to age (years; 23.7 ± 3.1 vs 24.7 ± 4.2), intelligence (IQ; 109.0 ± 17.3 vs 115.4 ± 14.0) (Oswald and Roth, 1987), and days since last sexual intercourse (30.4 ± 48.0 vs 26.5 ± 39.8) and masturbation (11.4 ± 37.8 vs 2.2 ± 2.5) (all P-values > 0.232). There were also no differences with respect to days since last sexual outlet (4.2 ± 8.3 vs 1.3 ± 1.2; P = 0.166).
All participants gave written informed consent after comprehensive explanation of the procedures. The study was approved by the ethics committee at the University of Regensburg.
Paradigm
Subjects were presented with 120 non-explicit but sexually arousing pictures and 60 neutral control pictures during the experimental session. Sexual stimuli comprised 60 photographs of adult females and 60 photographs of adult males. The images had an identical background color and black bars covering the genitalia (Santtila et al., 2009). Neutral pictures had been generated from a random selection of half of these (30 female and 30 male pictures) with a Fourier transformation, scrambling the figural shapes but keeping the amplitude spectrum and the overall color appearance of the images (Näsänen, 1999; Poeppl et al., 2011). After the experimental procedures, pictures were also rated with respect to emotional valence (unpleasant–pleasant), sexual arousal (not at all–extremely arousing) and sexual urge (none–highly motivated to see the person completely naked) on five-point Likert scales.
Before the experiment, subjects were instructed that they would have to answer the question ‘Do you want to see this person naked?’ after each stimulus. Stimuli were presented in random order (same for all subjects). Each experimental session consisted of 180 trials of the following sequence: After presentation of the stimulus for 2 s, a blank was shown for 1 s, during which a TMS pulse was applied (500 ms after blank onset) and the MEP assessed. Since actual active response to an incentive may boost its effect on changes in cortical excitability (Gupta and Aron, 2011), subjects then had to respond within 1 s to the question whether they wanted to see the depicted person naked by pressing the left or right arrow button of a common computer keyboard with the index finger. The answers ‘yes’ or ‘no’ were randomly presented on the left or right side of the visual field and participants had been instructed to choose the button corresponding to their response. Each trial ended with a jittered blank screen (0.5–1.5 s) (Figure 1A). Stimuli and instructions were presented via video goggles (EVG920V; Prober Industrial Ltd., China). Prior to the actual experiment, subjects were familiarized with the paradigm in a short practice session using 12 novel sexual and neutral stimuli.
MEP measurement
Participants were seated in a reclining chair with electromyography electrodes placed on the right hand. TMS was delivered by two Magstim 200 stimulators (Magstim Co., UK) connected via a Bistim module to a figure-of-eight coil (double-circular 70-mm coil). The coil was held tangential to the skull, with the handle pointing backwards, and 45° away from the midline. The optimal coil position for stimulation was defined as the position above the left motor cortex for eliciting MEP of maximal amplitude in the right first dorsal interosseus (FDI) muscle (index finger). Once this position was found, it was marked on a head cap and the coil was held in this position by the investigator. The resting motor threshold (RMT) was determined as the lowest stimulation intensity that evoked in at least four out of eight consecutive trials a MEP of at least 50 µV in the resting FDI (Rossini et al., 1994). MEPs were measured with a stimulation intensity of 110% RMT and were recorded with surface cup electrodes placed over the belly and tendon of the index finger with the ground placed on the head of the radius. Data were registered with a V-Amp 16-channel amplifier (Brain Products, Germany) and recorded with a band-pass filter of 30 Hz–1 kHz with a sampling rate of 2 kHz. The signals of the belly and tendon electrode were subtracted offline. MEP amplitudes were measured peak-to-peak (Devanne et al., 1997). MEPs with bimodal negative or positive peaks were excluded from analysis. The experimental setup is illustrated in Figure 1B.
Statistical analysis
The medians of the MEP peak-to-peak amplitudes for the female, male and control pictures, respectively, were used as dependent variables. We normalized the female and male pictures condition (i.e. their MEP medians) by dividing them by the neutral pictures condition (i.e. its MEP median). Sexual motivation is regulated by the individual’s sexual orientation insofar as sexual desire should only be observed in response to sexual stimuli being in accordance with individual sexual preference. To investigate the influence of sexual motivation on motor cortex excitability (i.e. MEPs), we calculated a 2 × 2 analysis of variance with the within-subjects factor stimulus condition (female vs male pictures) and the between-subjects factor sexual orientation (hetero- vs homosexual). For post-hoc analyses we used Student’s t tests. The significance threshold for all statistical tests was set to P < 0.05, two-sided.
RESULTS
As expected, subjects rated the sexual stimuli according to their sexual orientation: heterosexuals reported higher emotional valence (P < 0.001), sexual arousal (P < 0.001) and sexual urge (P < 0.001) for female stimuli compared with male stimuli, while homosexuals’ ratings showed the opposite pattern [all P-values < 0.001 except for emotional valence (P = 0.071)]. That is, in line with the self-reported sexual orientation female sexual stimuli increased sexual motivation in heterosexual men, while male sexual stimuli increased sexual motivation in homosexual men. Therefore, the MEPs may be interpreted as reflecting the subjects’ response to the particular stimulus category (i.e. sexually arousing pictures of males or females).
Several subjects responded with ‘no’ in the forced-choice task also to stimuli corresponding with their sexual orientation (i.e. preferred gender stimuli). The mean ratio between ‘yes’ and ‘no’ answers for preferred gender stimuli was 68%. However, analysis of mean Likert scores and coefficients of variation (M; CV) for stimuli corresponding with subjects’ sexual orientation demonstrated that these stimuli were rated equally as indicated by relatively low CV with respect to emotional valence (3.17; 0.20), sexual arousal (3.31; 0.17) and sexual urge (3.40; 0.18).
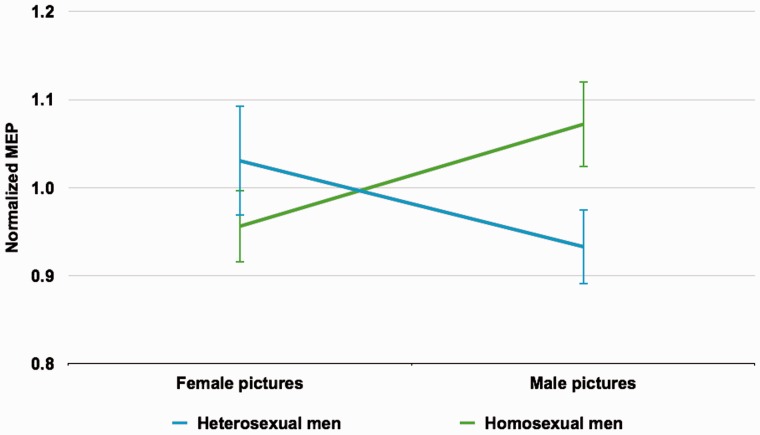
We found a significant crossed interaction effect between stimulus condition and sexual orientation (F = 7.966; df = 1,38; P = 0.008). Post-hoc tests indicated that this interaction was driven by stimulus-specific MEP increases corresponding with the subjects’ sexual orientation. Heterosexual subjects showed higher MEP amplitudes for female in contrast to male stimuli (T = 1.788; df = 22; P = 0.088). Homosexual subjects showed the opposite response pattern with increased MEP amplitudes for male stimuli (T = 2.434; df = 16; P = 0.027) (Figure 2).
Fig. 2.
Interaction effect. Crossed interaction between stimulus condition and sexual orientation (P = 0.008) driven by stimulus-specific MEP increases corresponding with the subjects’ sexual orientation. Heterosexual subjects showed higher MEP amplitudes for female in contrast to male stimuli (P = 0.088), while homosexual subjects showed the opposite response pattern with increased MEP amplitudes for male stimuli (P = 0.027). MEP = motor evoked potential.
DISCUSSION
By using TMS for the assessment of stimulus-dependent MEP changes in hetero- and homosexual men, we could demonstrate that cortical excitability is modulated by sexual motivation. More specifically, the results show that visual sexual stimuli corresponding with one’s sexual orientation, compared with non-corresponding visual sexual stimuli, increase the excitability of the motor cortex. These findings corroborate both psychological and neurobehavioral models of the human sexual response.
Theoretical models of sexual motivation and behavior have been based on the incentive theory of motivation (Toates, 2009). In brief, it has been proposed that a sexual incentive triggers sexual motivation, which in turn links to genital reactions through the autonomic nervous system and to behavior via the somatic nervous system (i.e. voluntary motor responses). Information on the consequences of the genital and behavioral responses feed back to affect motivation (Toates, 2009). Notably, according to this model, the translation of motivation into behavior is mediated by the basal ganglia (Toates, 2009), which hold strong structural and functional connections with motor areas (Postuma and Dagher, 2006; Draganski et al., 2008) and together with these areas play a pivotal role in motor preparation (Elsinger et al., 2006; Monchi et al., 2006; Purzner et al., 2007). Our results further place the basic incentive motivation model of sex on a neurobiological foundation to the extent that they provide a link between sexual motivation and quantitatively measurable states of neural motor systems, specifically motor cortex excitability. In this regard, they also complement earlier findings showing that amplitudes of spinal reflexes, as a measure for motor preparation, are modulated by sexual motivation (Both et al., 2005).
A previous investigation into the relationship between motor cortex excitability and non-sexual motivation revealed that an increase in motor cortex excitability exclusively occurs in the phase of response preparation. However, it was demonstrated that such increase is not a function of response preparation per se but likely indexes the degree of motivation (Gupta and Aron, 2011). Accordingly, the observed stimulus-dependent changes of cortical excitability in this study should be linked to motor preparation processes relating to sexual motivation, although the present paradigm is not capable of isolating sexual motivation (i.e. urge) from sexual arousal. Visual sexual stimuli reliably activate basal ganglia and motor cortices (Kühn and Gallinat, 2011; Stoléru et al., 2012; Poeppl et al., 2014). Such activity has been interpreted as a correlate of motor preparation in the context of motivational processes in a neurophenomenological model of sexual arousal (Kühn and Gallinat, 2011; Stoléru, 2014). Since cortical excitability correlates with neuronal activity (Siebner et al., 2001; Takano et al., 2004), our finding of motor cortex excitability depending on sexual motivation corroborates the attribution of neuronal activity in corticobasal ganglia loops to the motivational component of sexual arousal (Kühn and Gallinat, 2011; Stoléru, 2014). Moreover, the interdependence between motivation-dependent changes in cortical excitability and an urge to perform action (Gupta and Aron, 2011) suggests that neuronal activity in the motor system indeed relates to motor preparation within the motivational component of the neurophenomenological model of sexual processing (Stoléru et al., 2012; Stoléru, 2014).
It has to be noted that the effect of non-preferred stimuli on MEPs considerably contributed to the observed interaction. This holds particularly true for heterosexual men watching male stimuli. The corresponding MEP amplitudes being lower as compared with those in the neutral condition might reflect sexual aversion leading to less sexual readiness. Since preferred stimuli in contrast were associated with an increase in MEP amplitudes as compared with the neutral condition, the paradigm seems to capture both sexual motivation and sexual aversion. However, we cannot exclude that other processes account for the decreases in MEPs associated with stimuli non-corresponding with subjects’ sexual orientation. It seems conceivable that these decreases reflect suppression of a response (e.g. because heterosexual men tend to be afraid to show the wrong behavior, or because they secretly want to see the male stimuli naked). Such possibility certainly represents a limitation that future research needs to address. Future studies on the topic might for instance employ preferred gender stimuli that show high variability with respect to induced sexual urge and allow for the assessment of a linear relationship between cortical excitability and sexual motivation, as done in previous research on non-sexual motivation (Gupta and Aron, 2011). Moreover, it should be tested if the paradigm is robust against manipulation and deception.
In summary, this study provides strong evidence that stimulus-dependent cortical excitability reflects sexual motivation and preference. By demonstrating that sexual motivation induces physiological brain changes in the motor cortex, our findings fill the gap between theoretical models of sexual motivation and corresponding neuroimaging results that involve neural motor systems.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Pekka Santtila (University of Turku, Finland) for providing the stimulus material used in this study. T.B.P. was supported by the Foundation for Mental Health [German Association for Psychiatry, Psychotherapy and Psychosomatics (DGPPN)].
REFERENCES
- Borgomaneri S, Gazzola V, Avenanti A. Temporal dynamics of motor cortex excitability during perception of natural emotional scenes. Social Cognitive and Affective Neuroscience. 2014;9:1451–7. doi: 10.1093/scan/nst139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both S, Boxtel G, Stekelenburg J, Everaerd W, Laan E. Modulation of spinal reflexes by sexual films of increasing intensity. Psychophysiology. 2005;42:726–31. doi: 10.1111/j.1469-8986.2005.00364.x. [DOI] [PubMed] [Google Scholar]
- Bunse T, Wobrock T, Strube W, et al. Motor cortical excitability assessed by transcranial magnetic stimulation in psychiatric disorders: a systematic review. Brain Stimulation. 2014;7:158–69. doi: 10.1016/j.brs.2013.08.009. [DOI] [PubMed] [Google Scholar]
- Devanne H, Lavoie BA, Capaday C. Input–output properties and gain changes in the human corticospinal pathway. Experimental Brain Research. 1997;114:329–38. doi: 10.1007/pl00005641. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. Journal of Neuroscience. 2008;28:7143–52. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsinger CL, Harrington DL, Rao SM. From preparation to online control: reappraisal of neural circuitry mediating internally generated and externally guided actions. Neuroimage. 2006;31:1177–87. doi: 10.1016/j.neuroimage.2006.01.041. [DOI] [PubMed] [Google Scholar]
- Fischer T, Langner R, Birbaumer N, Brocke B. Arousal and attention: self-chosen stimulation optimizes cortical excitability and minimizes compensatory effort. Journal of Cognitive Neuroscience. 2008;20:1443–53. doi: 10.1162/jocn.2008.20101. [DOI] [PubMed] [Google Scholar]
- Frank E, Landgrebe M, Poeppl TB, et al. Antipsychotic treatment with quetiapine increases the cortical silent period. Schizophrenia Research. 2014;156:128–32. doi: 10.1016/j.schres.2014.03.028. [DOI] [PubMed] [Google Scholar]
- Georgiadis JR, Kringelbach ML. The human sexual response cycle: brain imaging evidence linking sex to other pleasures. Progress in Neurobiology. 2012;98:49–81. doi: 10.1016/j.pneurobio.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Gupta N, Aron AR. Urges for food and money spill over into motor system excitability before action is taken. European Journal of Neuroscience. 2011;33:183–8. doi: 10.1111/j.1460-9568.2010.07510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M. Transcranial magnetic stimulation: a primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Janssen E, Everaerd W, Spiering M, Janssen J. Automatic processes and the appraisal of sexual stimuli: toward an information processing model of sexual arousal. Journal of Sex Research. 2000;37:8–23. [Google Scholar]
- Kapogiannis D, Campion P, Grafman J, Wassermann EM. Reward-related activity in the human motor cortex. European Journal of Neuroscience. 2008;27:1836–42. doi: 10.1111/j.1460-9568.2008.06147.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P-A, Olivier E, Duque J. Influence of reward on corticospinal excitability during movement preparation. Journal of Neuroscience. 2012;32:18124–36. doi: 10.1523/JNEUROSCI.1701-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. A quantitative meta-analysis on cue-induced male sexual arousal. Journal of Sexual Medicine. 2011;8:2269–75. doi: 10.1111/j.1743-6109.2011.02322.x. [DOI] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Annals of Neurology. 2006;59:257–64. doi: 10.1002/ana.20742. [DOI] [PubMed] [Google Scholar]
- Näsänen R. Spatial frequency bandwidth used in the recognition of facial images. Vision Research. 1999;39:3824–33. doi: 10.1016/s0042-6989(99)00096-6. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oswald WD, Roth E. Der Zahlen-Verbindungs-Test (ZVT). Ein sprachfreier Intelligenz-Test zur Messung der “kognitiven Leistungsgeschwindigkeit”. Handanweisung. 2nd edn. Göttingen: Hogrefe; 1987. [Google Scholar]
- Poeppl TB, Langguth B, Laird AR, Eickhoff SB. The functional neuroanatomy of male psychosexual and physiosexual arousal: a quantitative meta-analysis. Human Brain Mapping. 2014;35:1404–21. doi: 10.1002/hbm.22262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poeppl TB, Nitschke J, Dombert B, et al. Functional cortical and subcortical abnormalities in pedophilia: a combined study using a choice reaction time task and fMRI. Journal of Sexual Medicine. 2011;8:1660–74. doi: 10.1111/j.1743-6109.2011.02248.x. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cerebral Cortex. 2006;16:1508–21. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Purzner J, Paradiso GO, Cunic D, et al. Involvement of the basal ganglia and cerebellar motor pathways in the preparation of self-initiated and externally triggered movements in humans. Journal of Neuroscience. 2007;27:6029–36. doi: 10.1523/JNEUROSCI.5441-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalography And Clinical Neurophysiology. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic, and research potential. Neurology. 2007;68:484–8. doi: 10.1212/01.wnl.0000250268.13789.b2. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Thompson PD, Day BL, Boyd S, Marsden CD. Stimulation of the human motor cortex through the scalp. Experimental Physiology. 1991;76:159–200. doi: 10.1113/expphysiol.1991.sp003485. [DOI] [PubMed] [Google Scholar]
- Santtila P, Mokros A, Viljanen K, et al. Assessment of sexual interest using a choice reaction time task and priming: a feasibility study. Legal and Criminological Psychology. 2009;14:65–82. [Google Scholar]
- Schecklmann M, Landgrebe M, Kleinjung T, et al. State- and trait-related alterations of motor cortex excitability in tinnitus patients. PLoS One. 2014;9:e85015. doi: 10.1371/journal.pone.0085015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell RL. The Sell assessment of sexual orientation: background and scoring. Journal of Gay, Lesbian, and Bisexual Identity. 1996;1:295–310. [Google Scholar]
- Siebner HR, Takano B, Peinemann A, Schwaiger M, Conrad B, Drzezga A. Continuous transcranial magnetic stimulation during positron emission tomography: a suitable tool for imaging regional excitability of the human cortex. Neuroimage. 2001;14:883–90. doi: 10.1006/nimg.2001.0889. [DOI] [PubMed] [Google Scholar]
- Singer B, Toates FM. Sexual motivation. Journal of Sex Research. 1987;23:481–501. [Google Scholar]
- Stoléru S. Reading the Freudian theory of sexual drives from a functional neuroimaging perspective. Frontiers in Human Neuroscience. 2014;8:157. doi: 10.3389/fnhum.2014.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoléru S, Fonteille V, Cornélis C, Joyal C, Moulier V. Functional neuroimaging studies of sexual arousal and orgasm in healthy men and women: a review and meta-analysis. Neuroscience and Biobehavioral Reviews. 2012;36:1481–509. doi: 10.1016/j.neubiorev.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Takano B, Drzezga A, Peller M, et al. Short-term modulation of regional excitability and blood flow in human motor cortex following rapid-rate transcranial magnetic stimulation. Neuroimage. 2004;23:849–59. doi: 10.1016/j.neuroimage.2004.06.032. [DOI] [PubMed] [Google Scholar]
- Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nature Neuroscience. 2004;7:887–93. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- Toates F. An integrative theoretical framework for understanding sexual motivation, arousal, and behavior. Journal of Sex Research. 2009;46:168–93. doi: 10.1080/00224490902747768. [DOI] [PubMed] [Google Scholar]
- Wassermann EM, Zimmermann T. Transcranial magnetic brain stimulation: therapeutic promises and scientific gaps. Pharmacology and Therapeutics. 2012;133:98–107. doi: 10.1016/j.pharmthera.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil ZM, Zhang Q, Hornung A, Blizard D, Pfaff DW. Impact of generalized brain arousal on sexual behavior. Proceedings of the National Acadamy of Sciences of the United States of America. 2010;107:2265–70. doi: 10.1073/pnas.0914014107. [DOI] [PMC free article] [PubMed] [Google Scholar]