Abstract
An extensive body of literature has indicated that there is increased activity in the frontoparietal control network (FPC) and decreased activity in the default mode network (DMN) during working memory (WM) tasks. The FPC and DMN operate in a competitive relationship during tasks requiring externally directed attention. However, the association between this FPC-DMN competition and performance in social WM tasks has rarely been reported in previous studies. To investigate this question, we measured FPC-DMN connectivity during resting state and two emotional face recognition WM tasks using the 2-back paradigm. Thirty-four individuals were instructed to perform the tasks based on either the expression [emotion (EMO)] or the identity (ID) of the same set of face stimuli. Consistent with previous studies, an increased anti-correlation between the FPC and DMN was observed during both tasks relative to the resting state. Specifically, this anti-correlation during the EMO task was stronger than during the ID task, as the former has a higher social load. Intriguingly, individual differences in self-reported empathy were significantly correlated with the FPC-DMN anti-correlation in the EMO task. These results indicate that the top-down signals from the FPC suppress the DMN to support social WM and empathy.
Keywords: social working memory, default mode network, frontoparietal control network, empathy
INTRODUCTION
Humans possess unique social cognitive abilities that set us apart from other species. These abilities may be partially supported by another crucial capacity for maintaining and manipulating social information, such as people’s concerns, traits and mental states. Therefore, efficient social working memory (WM) is essential to successful social interactions (Meyer et al., 2012). One of the commonly utilized experimental paradigms for functional brain imaging studies of WM is the n-back task, in which participants are asked to monitor a series of stimuli and to respond when the currently presented stimulus is the same as the one presented n trials previously (a meta-analysis can be seen in Owen et al., 2005). Previous WM studies using the n-back paradigm consistently reported activation in the frontoparietal control network (FPC), consisting primarily of the dorsolateral prefrontal cortex (dlPFC), posterior parietal cortex, as well as the dorsal anterior cingulate cortex (Wager and Smith, 2003; Owen et al., 2005). Recently, increasing evidence has highlighted the role of the FPC in cognitive control (Cole and Schneider, 2007; Dosenbach et al., 2008; Cole et al., 2013). The top-down control function of the FPC allows for focusing attention on goal-relevant information and suppressing goal-irrelevant information (Smallwood et al., 2012; Wen et al., 2013). Declines in frontoparietal control mechanisms may result in greater distractibility (Campbell et al., 2012).
In contrast to the FPC, the activity of the default mode network (DMN) has been documented as typically diminishing during performing tasks demanding externally directed attention, which certainly encompasses WM. The DMN, including the medial prefrontal cortex (mPFC), the precuneus/posterior cingulate cortex, angular gyrus and medial temporal cortex, is thought to support internally oriented and self-referential mental processes, such as mind wandering, autobiographical memory retrieval, imagining the future and theory of mind (Buckner et al., 2008; Spreng et al., 2009; Andrews-Hanna, 2012). Therefore, DMN activity may produce internal interference which interrupts externally oriented attention tasks (Buckner et al., 2008; Wen et al., 2013). The deactivation of the DMN serves to suppress irrelevant thoughts and focus more attention on the task. Insufficient suppression of the DMN leads to attention lapses (Weissman et al., 2006), poor cognitive task performance and even mental illness (Anticevic et al., 2012).
The FPC and DMN operate in a competitive or antagonistic relationship during tasks requiring externally directed attention (Newton et al., 2011; Gao and Lin, 2012), although the exact relationship between them during task performance depends on the paradigm employed and task demands (Spreng et al., 2010; Smallwood et al., 2012; Gerlach et al., 2014). A recent study indicated that the top-down signals from the FPC suppressed the internal noise from the DMN to improve externally oriented task performance (Wen et al., 2013). Converging evidence concludes that the FPC-DMN anti-correlation is affected in schizophrenia patients (Anticevic et al., 2013; Palaniyappan et al., 2013). The WM dysfunction in schizophrenia may not only be associated with the failure to recruit the frontoparietal system but also due to a conjoint failure to suppress the task-irrelevant DMN system. However, this proposed anti-correlation between the FPC and DMN needs to be further ascertained in social WM, because some recent findings suggest that social WM and classical WM may rely on different neural mechanisms (Meyer and Lieberman, 2012; Thornton and Conway, 2013).
Based on large-sample data sets, the FPC was found anatomically situated between components of the DMN and the dorsal attention network (DAN), providing a spatial convenience to reallocate cognitive resources from different systems (Vincent et al., 2008). Furthermore, with nodes of three different types in mediating internetwork communication, the FPC plays a pivotal gate-keeping role in goal-directed cognition, modulating the dynamic balance between the DMN and DAN (Spreng et al., 2010; Gao and Lin, 2012; Smallwood et al., 2012; Spreng et al., 2013). However, the modulatory role played by the FPC has rarely been investigated in previous studies related to social WM. In this study, we aimed to fill this gap by exploring the functional connectivity of the FPC-DMN and FPC-DAN during social WM task performance.
Another interesting topic is the association between the inter-subjective variability of the FPC-DMN anti-correlation and cognitive ability. By connectivity–cognition relationship analysis, we planned to investigate the psychological significance of the FPC-DMN anti-correlation. Since social WM involves social cognition, we supposed that individuals with higher social cognition ability (i.e. empathy) would exhibit better performance in social WM tasks. Empathy is an ability to understand and share the mental states of others, and it is crucial to human emotional experiences and social interactions (Davis, 1994; Baron-Cohen and Wheelwright, 2004). Although to date there has been a great deal of research regarding the neural bases of empathy, they mostly focused on some specific brain regions and rarely explored from the level of functional connectivity between large-scale networks (Bernhardt and Singer, 2012; Zaki and Ochsner, 2012). A large body of evidence has suggested that the DMN plays an important role in empathy (Spreng et al., 2009; Schnell et al., 2011; Bernhardt and Singer, 2012). Some core regions in the FPC have also been reported to be involved in empathy, including the dorsal anterior cingulate cortex, right temporoparietal junction, anterior insula and supplementary motor area (Corbetta et al., 2008; Fan et al., 2011; Bernhardt and Singer, 2012). Intriguingly, the core nodes of the FPC and DMN, i.e. the dlPFC and mPFC (Buckner et al., 2008; Vincent et al., 2008), were indicated to be associated with trait emotional intelligence in a recent resting-state study (Takeuchi et al., 2013). On the basis of these results, we speculated that empathy might be associated with FPC-DMN connectivity, and individuals with higher empathic ability would perform better in social WM tasks.
To investigate the aforementioned questions, 34 participants were recruited to perform resting state and load-variable social WM tasks (emotional face recognition 2-back tasks). During the social WM tasks, the participants were instructed to focus on either the emotion (EMO) or the identity (ID) of the same set of face stimuli. By analyzing FPC-DMN connectivity during resting state and task performance, we expected to find the functional connectivity related to social WM, as well as its association with empathy.
MATERIALS AND METHODS
Participants
Thirty-four, healthy, right-handed university students (17 female; mean age = 22 ± 1.6 years, range: 18–26 years) participated in this study. All participants were recruited from the local community through advertisements. They were screened for a history of psychiatric or neurological illness as confirmed by a psychiatric clinical assessment. Written informed consent was obtained after a detailed explanation of the study protocol. The study was approved by the Ethics Committee of Southwest University, China, and all procedures involved were in accordance with the sixth revision of the Declaration of Helsinki. One participant was excluded from analyses due to making a large number of inappropriate selections, so the final analyses contained data from 33 participants.
Psychometric testing
Before the functional magnetic resonance imaging (fMRI) scanning, each participant completed a self-report measure of empathy, the empathy quotient (EQ) questionnaire (Baron-Cohen and Wheelwright, 2004). This questionnaire consists of 40 empathy items and 20 filler/control items. For each empathy item a person can score 0, 1 or 2, corresponding to ‘disagree’, ‘slightly agree’ and ‘strongly agree’, so the EQ has a minimum score of zero and a maximum score of 80. A sample item from the EQ is ‘I am good at predicting what someone will do’. Baron-Cohen and Wheelwright (2004) reported that the EQ is a valid and reliable measure of empathic ability for adults of normal intelligence, with excellent test–retest reliability and high internal consistency.
Experimental design and procedure
Three stages of fMRI scans were performed on each participant: first, a 5 min resting-state scan (Rest), and then two social WM task runs. After a training session outside the scanner, a 5 min resting-state fMRI scan was conducted. In this period, the participants were instructed to fixate on a black screen and keep as motionless as possible. During each WM task run, participants performed an emotional face recognition task based on the 2-back paradigm (Figure 1), but they were instructed to match faces according to one aspect of the face stimuli and ignore another (Neta and Whalen, 2011). The participants were asked to focus on the EMO of the faces in the first task run and were asked to focus on the ID of the faces in the second run. For example, in the EMO task, they were asked to press the left button each time the current EMO of the face was the same as the one presented two trials earlier. No response was required during the first two trials, as there were no faces 2-back to match with them. The order of task runs was counterbalanced across participants such that half of the participants saw the EMO run first and the other half saw the ID run first.
Fig. 1.
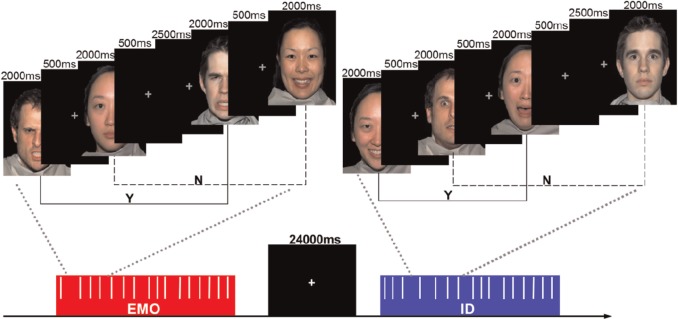
Emotional face recognition 2-back task. 4 identities (2 females, 2 males) with 4 expressions (angry, happy, fearful and neutral) were presented. Each face was displayed for 2000 ms followed by a 500 ms gray crosshair fixation. Five 2500 ms jittered gray crosshair fixations were randomly inserted. Participants were instructed to respond with their right index finger if the current stimulus matched the stimulus presented two trials prior (Y), and with their right middle finger if the current stimulus was different (N).
For the task stimuli, we chose a total of 16 face images from the NimStim standardized facial expression stimulus set (Tottenham et al., 2009). There were 4 identities (2 females, 2 males), each posing 4 expressions (angry, happy, fearful and neutral). The word ‘emotion’ or ‘identity’ appeared to instruct the participants, which task they would perform at the start of each task run. Each run contained four of the same blocks (EMO or ID). Within each block, 16 faces were presented in a pseudo-random sequence. Each face stimulus was displayed for 2000 ms followed by a 500 ms cross fixation. Before the next face appeared, five jittered crosshairs were inserted randomly, and each was displayed for 2500 ms. Participants were given a 24 s rest period after each block.
Image acquisition
High-resolution T1-weighted structural images were acquired using a 3T Siemens Trio scanner. The 3D spoiled gradient recalled (SPGR) sequence used the following parameters: repetition time (TR) = 8.5 ms, echo time (TE) = 3.4 ms, field of view (FOV) = 240 × 240 mm2, flip angle = 12°, acquisition matrix = 512 ×512, thickness = 1 mm with no gap. The high-resolution T1-weighted structural images provided an anatomical reference for the functional scans. Subsequently, 204 fMRI volumes were acquired using an echo-planar imaging (EPI) sequence with the following parameters: TR/TE = 1500/29 ms, flip angle = 90°, acquisition matrix = 64 × 64, in-plane resolution = 3.0 ×3.0 mm2, FOV = 192 × 192 mm2, axial slices = 25, thickness/gap =5/0.5 mm. The first four volumes were discarded to ensure steady-state longitudinal magnetization. Head movements were minimized by using a cushioned head fixation device.
fMRI data analysis
Functional network definition
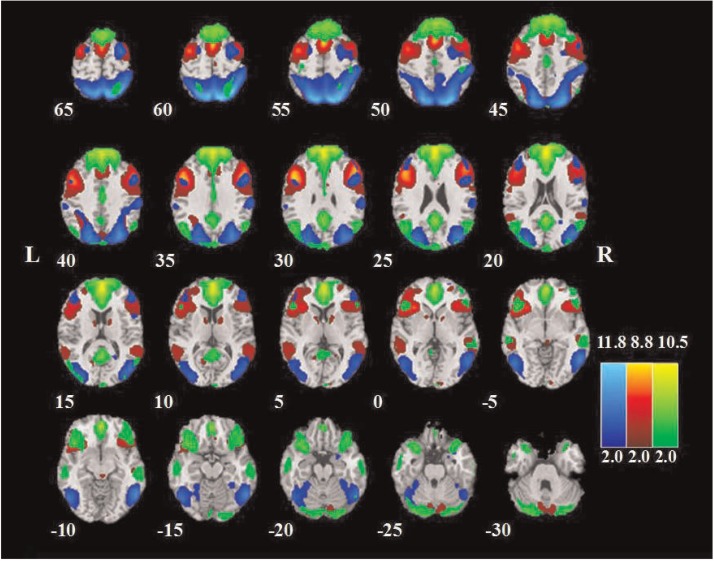
All the data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/, Welcome Department of Cognitive Neurology, UK). The preprocessing steps included slice timing, head motion correction, spatial normalization and smoothing (6 mm full-width at half maximum Gaussian kernel). After fMRI data preprocessing, we performed group independent component analysis (ICA) using the GIFT (http://icatb.sourceforge.net/) (Calhoun et al., 2001) to retrieve resting-state networks and subsequently identify our networks of interest. The preprocessed data from all three runs (Rest, EMO and ID) were submitted to GIFT. The optimal number of components was set to 30, which was estimated using the minimum description length criterion (Li et al., 2007). After data reduction by principal component analysis, ICA decomposition was performed on concatenated datasets using the Extended Infomax algorithm. Independent components and time courses for each subject were back-reconstructed, and the mean spatial maps for each group were transformed to z-scores for display purposes. We employed the FPC, DMN and DAN maps from one of our previous resting-state fMRI studies (Lei et al., 2013) as spatial templates for component classification. The selected networks corresponded to those components with the largest spatial correlations with the templates (Lei et al., 2013) and with correlation values at least double that of all other networks. The spatial distribution of the three networks is presented in Figure 2, and the anatomical locations, abbreviations and the corresponding Montreal Neurological Institute (MNI) template space (Tzourio-Mazoyer et al., 2002) coordinates of the brain regions within each network are summarized in Table 1.
Fig. 2.
The spatial distribution of the FPC (red), DMN (green) and DAN (blue). Brain areas with intensities of two s. d. greater than the mean are shown.
Table 1.
Peak foci for the group-level DMN, DAN and FPC defined by ICA in resting state and both task runs combined
| Regions | Abbreviation | MNI coordinates | No. of voxels | ||
|---|---|---|---|---|---|
| DMN | |||||
| Anterior medial prefrontal cortex | amPFC | −6 | 45 | 9 | 1713 |
| Dorsal medial prefrontal cortex | dmPFC | −6 | 54 | 24 | 790 |
| Posterior cingulate | PCC | −4 | −56 | 23 | 442 |
| L Precuneus | lPCu | −9 | −69 | 51 | 32 |
| R Precuneus | rPCu | 9 | −66 | 51 | 68 |
| L Anterior temporal lobe | laTL | −60 | −21 | −15 | 145 |
| R Anterior temporal lobe | raTL | 63 | −21 | −18 | 134 |
| L Inferior frontal gyrus | lIFG | −38 | 22 | −18 | 170 |
| R Inferior frontal gyrus | rIFG | 39 | 21 | −20 | 155 |
| R Hippocampus formation | rHF | 21 | −24 | −36 | 37 |
| L Angular gyrus | lAG | −48 | −66 | 33 | 83 |
| R Angular gyrus | rAG | 36 | −60 | 42 | 12 |
| L Posterior inferior parietal lobule | lpIPL | −36 | −48 | 39 | 51 |
| DAN | |||||
| L Inferior precentral sulcus | lIPCS | −48 | 9 | 30 | 72 |
| R Inferior precentral sulcus | rIPCS | 48 | 9 | 33 | 41 |
| L Frontal eye fields | lFEF | −27 | 9 | 63 | 20 |
| R Frontal eye fields | rFEF | 36 | 3 | 63 | 42 |
| L Middle temporal motion complex | lMT+ | −51 | −66 | −5 | 257 |
| R Middle temporal motion complex | rMT+ | 51 | −66 | 3 | 384 |
| L Intraparietal sulcus | lIPS | −24 | −69 | 42 | 146 |
| R Intraparietal sulcus | rIPS | 27 | −75 | 45 | 274 |
| R Cuneus | rCS | 9 | −105 | 15 | 23 |
| FPC | |||||
| L Dorsolateral prefrontal cortex | ldlPFC | −45 | 15 | 27 | 627 |
| R Dorsolateral prefrontal cortex | rdlPFC | 48 | 24 | 24 | 882 |
| L Medial superior prefrontal cortex | lmsPFC | 3 | 24 | 57 | 176 |
| Dorsal anterior cingulate cortex | dACC | −1 | 38 | 47 | 334 |
| L Anterior inferior parietal lobule | laIPL | −51 | −63 | 39 | 33 |
| R Anterior inferior parietal lobule | raIPL | 51 | −57 | 57 | 58 |
| L Precuneus | lPCu | −12 | −60 | 66 | 276 |
| L Anterior insula | laINS | −36 | 24 | 0 | 153 |
| R Anterior insula | raINS | 36 | 30 | 0 | 45 |
| L Caudate | lCD | −12 | 6 | 9 | 13 |
| R Caudate | rCD | 12 | 3 | 12 | 31 |
Note. Abbreviations used: L, left hemisphere; R, right hemisphere. The significance threshold was set to P < 0.001, FDR–corrected, with a minimum cluster size equal to 10 adjacent voxels.
Activation and functional connectivity analysis
In the task runs, analysis was restricted to task performance by removing the time-series corresponding to the 24 s between-block rest periods. In activation analyses, we assessed the mean task-related hemodynamic response within each of the three networks. For each participant, the mean blood oxygen level-dependent (BOLD) signal within each network was calculated for both the EMO and ID task conditions, relative to the Rest condition. We removed the effects of spurious signal variations, including head-movement parameters and average signals arising from the ventricles, white matter and whole brain (the so-called global signal), by means of linear regression (Van Dijk et al., 2010). A complementary activation analysis based on the region-of-interest (ROI) approach was also conducted. The ROIs were spheres (radius = 5 mm) centered on the peak coordinates within each network.
In functional connectivity analyses, the time courses of the selected components were used as inputs for correlation calculations. Given that nuisance signals may exert a potential influence on functional connectivity analyses, we repeated our analyses by regressing out the effects of spurious signal variations before computing correlations between time courses. These nuisance variables included head-movement parameters and average signals arising from the ventricles, white matter and whole brain (the so-called global signal). We also repeated our analyses using the functional network connectivity toolbox (Jafri et al., 2008) and the ROI approach to test if the results remained. The correlation coefficients were normalized to z-scores with Fisher’s r-to-z transformation to increase the normality of the distribution, allowing further statistical analysis of correlation strengths.
Statistical analysis
Paired-samples t-tests were conducted on accuracy (ACC) and reaction times to compare the statistical differences between the EMO and ID tasks. Then, Pearson correlations were computed to assess the correspondence between the ACC during each task run and empathy scores across subjects. For each network in the EMO and ID tasks, one-sample t-tests were performed to determine significant differences in percent BOLD signal changes from the Rest baseline condition. Three additional paired-samples t-tests comparing percent BOLD signal changes between the EMO and the ID task conditions were also conducted. Pearson correlations between the time courses of FPC, DMN and DAN components were also calculated for between-network (FPC-DMN, FPC-DAN) functional connectivity. Correlation coefficients for each condition of each subject were introduced into a two-factor analysis of variance (ANOVA). The two within-subjects factors were task (Rest, ID and EMO), and network pair (FPC-DMN and FPC-DAN). We then used post hoc two-tailed paired-samples t-tests to identify the differences between the Rest, EMO and ID conditions. Finally, we used the Pearson correlations to assess the correspondence between the FPC-DMN anti-correlation during each task run and the empathy scores across subjects. The Bonferroni correction was employed when performing multiple statistical tests simultaneously in all the functional networks. The differences were considered significant if the associated probability was below 0.05.
RESULTS
Behavior results
Social WM task performance
The behavioral results indicated that participants were significantly more accurate and reacted more quickly in the ID task (mean ± standard error: response time (RT) = 926 ± 22 ms; ACC = 90.8% ± 1.2%) compared with the EMO task (mean ± standard error: RT = 1130 ±25 ms; mean ACC = 79.6% ± 1.9%), which supported the notion that the EMO task was more challenging than the ID task (ACC and RT paired-samples t-tests, P < 0.001) (Figure 3). Further analysis of RT data considering only correct trials revealed the same effects. These results were consistent with previous research findings (Neta and Whalen, 2011), demonstrating the increased cognitive load involved in EMO recognition compared with identity recognition.
Fig. 3.
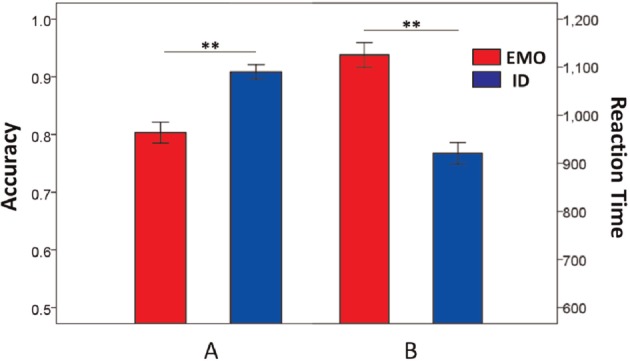
Behavioral performance (mean ± standard error) on the EMO (red) and ID (blue) 2-back tasks. Participants were significantly (paired-samples t-test, P < 0.001) less accurate (A) and slower (B) on the EMO task than the ID task. ** indicates significant differences at P < 0.01.
Association between task performance and empathic ability
Participants in this study had a minimum EQ score of 25 and a maximum of 49, whilst the average score was 35.79. We examined whether empathic ability was associated with WM task performance. As expected, there was a significant positive correlation between ACC in the EMO task and empathy scores [r(31) = 0.39, P = 0.024 < 0.05; Figure 4], but the corresponding correlation was not significant in the ID task [r(31) = 0.24, P = 0.18]. Moreover, the correlation between RTs and empathy scores was not significant within either task, P = 0.53 for EMO task, and P = 0.65 for ID task, respectively. These results indicated that the participants with higher empathic ability performed better in terms of ACC (but not processing speed) in the EMO task.
Fig. 4.
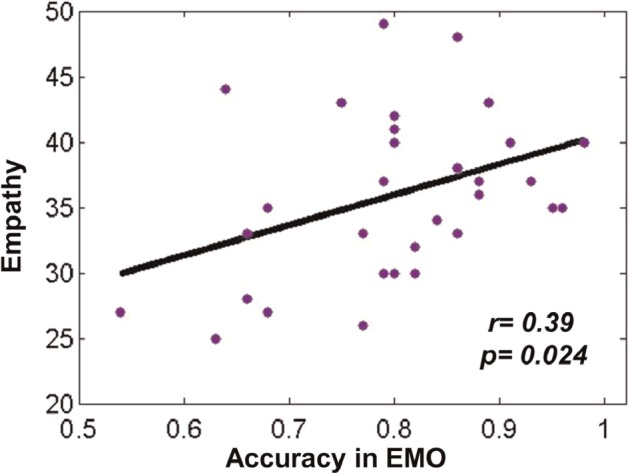
A significant positive correlation between ACC in the EMO task and empathy scores (r = 0.39, P < 0.05).
Task-related functional network activation
We examined the mean magnitude of the task-related hemodynamic response within each of the three networks (Figure 5). In the FPC and DMN, there were significant task-related differences in percent signal changes relative to the resting state. Within the FPC, there were significant increases in BOLD signals for both EMO (P < 0.001) and ID (P < 0.001) tasks. Within the DMN, there were significant decreases in BOLD signals for both EMO (P < 0.001) and ID (P < 0.001) tasks. Differences were also observed in a direct comparison between the EMO and ID tasks in percent BOLD signal changes in the FPC (P < 0.01) and DAN (P < 0.05) networks. No significant differences were observed in percent BOLD signal changes in the DMN between the two tasks (P = 0.0559). The complementary activation analysis based on the ROI approach showed similar results.
Fig. 5.
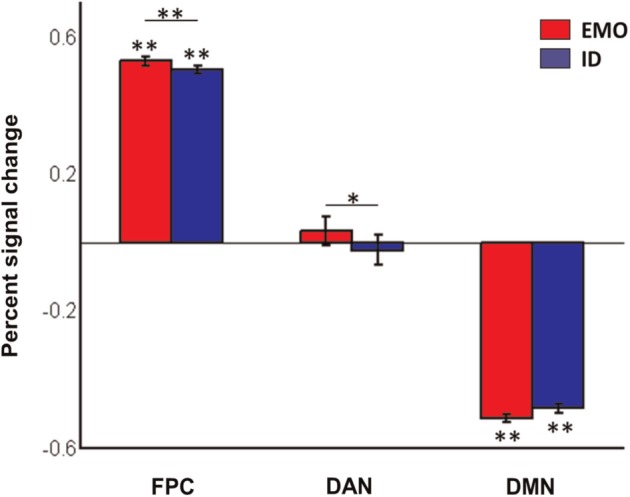
Mean and standard error of task-related percent BOLD signal changes within each network. * and ** indicate significant differences at P < 0.05 and P < 0.01, respectively.
Altered functional connectivity among the FPC and DMN, DAN
We conducted a 3 × 2 repeated measures ANOVA on these correlations, testing for the main effects and interaction of tasks (Rest, EMO and ID) and network pairs (FPC-DMN and FPC-DAN). A main effect of network pairs [F(1,32) = 64.02, P < 0.001] and an interaction between network pairs and tasks [F(2,64) = 19.98, P < 0.001] were observed. However, no main effect of tasks [F(2,64) = 1.48, P = 0.24] was found. The between-network functional connectivity altered significantly between low and high social load (Figure 6). Relative to the Rest condition, the FPC-DMN connectivity became more negative in the EMO task (EMO-Rest: mean r = −0.16, P < 0.01), whereas FPC-DAN connectivity became more positive (EMO-Rest: mean r = 0.24, P < 0.001; ID-Rest: mean r = 0.10, P < 0.01). Moreover, compared with the ID task, the EMO task with higher social load produced more modulation of FPC-DMN connectivity (EMO-ID: mean r = −0.11, P < 0.01) and FPC-DAN connectivity (EMO-ID: mean r = 0.14, P < 0.001). A complementary analysis with nuisance signals regression revealed similar effects (see Supporting Information Figure S2). In fact, the ICA technique is capable of extracting noise (e.g. scanner, physiological and motion artifacts) from the desired dataset. After ICA, the low-frequency nuisance signals, such as heart rate, respiration, head movement and the ventricles were detected and separated independently. For these reasons, we chose to report the functional connectivity results without removing nuisance signals independently. Finally, the complementary study based on the FNC toolbox and ROI analysis (see Supporting Information Figure S1) also revealed similar effects.
Fig. 6.
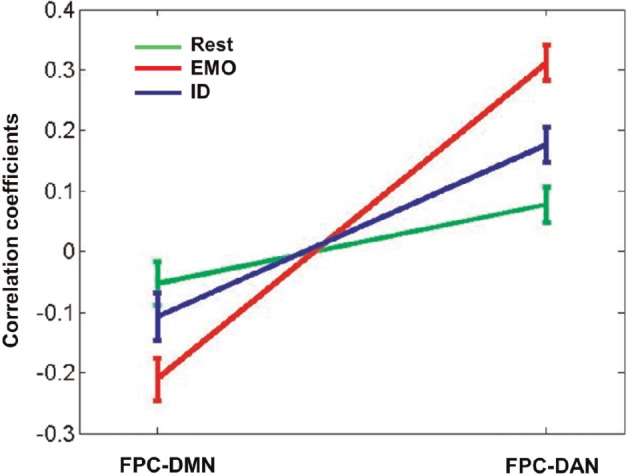
Functional network connectivity among three large-scale brain networks altered by social WM task. The line graph shows the correlation coefficients (mean ± standard error) between two pairs of networks (FPC-DMN and FPC-DAN) in three states [Rest (green), EMO (red) and ID (blue)]. Relative to the Rest, the FPC-DMN connectivity became more negative, whereas the FPC-DAN connectivity became more positive during tasks. The EMO task produced more modulation of the FPC-DMN connectivity than the ID task. Significant alterations between conditions were assessed by means of paired-samples t-tests.
Association between FPC-DMN connectivity and empathic ability
We also examined whether FPC-DMN connectivity was associated with empathic ability. Intriguingly, there was a significant negative correlation between the FPC-DMN connectivity in the EMO task and participants’ empathy scores [r(31) = −0.38, P < 0.05; Figure 7]. We found an increased FPC-DMN anti-correlation corresponding to higher empathic ability.
Fig. 7.
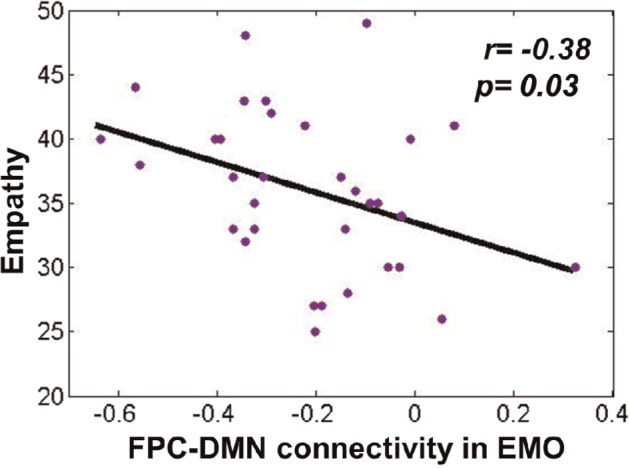
A significant negative correlation between FPC-DMN connectivity and empathy scores in the EMO task (r = −0.38, P < 0.05).
DISCUSSION
To explore the relationship between brain functional connectivity and task performance in social WM, we investigated the anti-correlation between the FPC and DMN under three conditions: a resting state, an facial EMO recognition WM task and an facial identity recognition WM task. There were three main findings. First, relative to the resting state, the anti-correlation between the FPC and DMN strengthened during the WM tasks. Second, compared with the ID task, the EMO task with greater social load conduced increased FPC-DMN anti-correlations. Third, the participants with higher empathic ability demonstrated better task performance and stronger FPC-DMN anti-correlations in the EMO task. These results indicate that competition between the FPC and DMN supports social WM and empathy. A potential neural mechanism may be that the top-down signals from the FPC inhibit goal-irrelevant activity in the DMN to enhance behavioral performance in social WM functions.
Social WM induced increased FPC-DMN anti-correlation
The altering of functional connectivity between the FPC and DMN as a function of task condition and increasing load has been investigated in a number of classical WM studies (Newton et al., 2011; Gordon et al., 2012; Repovs and Barch, 2012; Gordon et al., 2014). However, this study is the first to have explored social WM related large-scale network connectivity.
Similar to some classical WM findings (Newton et al., 2011; Gordon et al., 2012; Repovs and Barch, 2012), we observed increased competition between the FPC and DMN during social WM tasks. Yet, in opposition to the FPC-DMN anti-correlation, the FPC showed more cooperation with the DAN as the task performance and task load increased. As previous literature has reported, the DMN is commonly associated with internally oriented and stimulus-independent mental processes, whereas the DAN is primarily involved in externally oriented visual attention (Corbetta and Shulman, 2002; Fornito et al., 2012). The FPC may be uniquely positioned to integrate information coming from the DMN and DAN by mediating competition between internally and externally directed processes (Vincent et al., 2008). By selectively cooperating with the DMN or DAN according to the attentional demands of task, the FPC may serve as a cortical mediator linking the two networks to support adaptive cognitive processes (Spreng et al., 2010; Gao and Lin, 2012; Smallwood et al., 2012). During externally oriented attention processes, the FPC suppresses the DMN to reallocate cognitive resources from internal irrelevant thoughts and focus on goal-directed tasks. However, the FPC may cooperate with the DMN to help maintain the internal information stream against disruption by the external world for producing coherent internal trains of thought (Smallwood et al., 2012). Here, the FPC coupled with the DAN but decoupled from the DMN to focus attention on externally oriented social WM tasks and suppress irrelevant spontaneous thoughts. In a recent spatial attention study, top-down signals from the task control network to the DMN were found to regulate activity in the DMN to enhance behavioral performance, whereas the signals from the DMN to the task control network were thought to have acted as internal noise to interfere with goal-oriented behaviors (Wen et al., 2013). The same mechanism may have manifested itself in our study.
Although the FPC-DMN anti-correlation has been well documented during WM tasks, some recent studies suggested a contrary phenomenon that the FPC and DMN may co-activate and cooperate during WM for social cognitive information (Meyer and Lieberman, 2012). Meyer et al. (2012) conducted a study involving a delayed-response social WM task, and found that activation in both the FPC and DMN linearly increased with rising social load. However, the DMN showed decreased activity with rising social load in our study, although our task condition also involved social WM. To account for the inconsistency between Meyer’s results and those of this study, one possible interpretation is that the social WM task paradigms differed across the two studies. According to the review by Lieberman (2007), social cognition can be divided into internally focused cognition and externally focused cognition. Internally focused cognition refers to mental processes that focus on one’s own or another’s inner thoughs and feelings, whereas externally focused cognition refers to mental processes that focus on one’s own or another’s physical and visible features and actions. Since social WM definitely belongs to the category of social cognition processes, we can infer that there is also a division between internally focused and externally focused social WM. Regarding the social WM task employed by Meyer et al. (2012), participants were almost certainly required to focus their attention on their friends’ internal characteristics to mentally rank them along a trait dimension. However, in our social WM task, participants only needed to focus their attention on the external and visual features of faces. Relative to the ID task, perhaps it was necessary for participants to process and integrate more subtle facial information during the EMO task. Abundant evidence has provided support for the competitive relationship between the FPC and DMN during externally focused processing (Fransson, 2006; Newton et al., 2011; Gao and Lin, 2012).
Taken together, our task employed an externally focused social WM paradigm, thus participants needed to allocate resources away from internally task-irrelated thoughts and towards external stimuli to adaptively perform goal-directed processes. These results provide evidence that the FPC competes with the DMN to support externally focused social WM. To further confirm our inferences, future research should explore internally focused and externally focused social WM using the same task paradigm, and then analyze the FPC-DMN connectivity during these two types of task performance. In addition, simultaneous EEG-fMRI should be utilized to reveal more dynamic details of the FPC-DMN anti-correlation during social WM (Lei et al., 2010; Lei et al., 2014).
Association between social WM performance and empathy
This study found that the participants with higher empathic ability performed better in the EMO task, during which their higher empathic ability helped them to correctly identify and remember the facial expressions. Empathy not only consists of conjecture regarding the other person’s mental state (cognitive empathy, similar to ‘theory of mind’) but also involves simple facial EMO detection and understanding (affective empathy). Previous studies have reported that higher empathic ability corresponded to better EMO perception performance (Chakrabarti et al., 2006; Sucksmith et al., 2013). In our study, though the EMO task was a combination of facial expression perception and WM, we still observed this association. One possible explanation is that empathic ability influences social WM performance. Participants with higher empathic ability likely focused more attention on facial EMO information and ignored irrelevant information during their EMO task performance. Thus, they could encode face affect information more precisely and preprocess it into chunks more efficiently, thus facilitating WM processing. Another explanation is that social WM ability influences empathy. WM plays an important role in social interactions, and impaired WM can interfere with the ability to hold and manipulate social and emotional information. Thus, excellent WM may improve empathic processes. Overall, there may be an interaction between social WM and empathy. Indeed, there is some recent evidence supporting this association (Smith et al., 2014). This is perhaps one of the reasons why schizophrenics show impairment both in WM and empathy (Goldman-Rakic, 1994; Shamay-Tsoory et al., 2007; Anticevic et al., 2013). However, this hypothesis remains to be further investigated.
No relationship between task performance and empathy was observed during the ID task. This is possibly because there was no sufficient empathy involved in completing the ID task. Unlike the EMO recognition task, the identity recognition task only required participants to process invariant facial structure features, and EMO was just an implicit element. In addition, we found significant gender differences in empathy, with females scoring higher than males, which was similar to the results of previous research (Hoffman, 1977; Eisenberg and Lennon, 1983). Moreover, females performed better in the EMO task than males in this study.
This finding may possess some important value in clinical applications. Many mental disorders including schizophrenia and autism spectrum disorder are characterized by deficits in empathy (Shamay-Tsoory et al., 2007; Fan et al., 2014). What’s more, schizophrenia patients are also impaired in WM ability (Goldman-Rakic, 1994; Palaniyappan et al., 2013). Our finding may provide help for the diagnosis and treatment of these diseases. We expect that social WM training (Meyer and Lieberman, 2012; Meyer et al., 2012) may simultaneously improve WM and empathic ability in both healthy individuals and psychiatric patients. This may be an interesting area for future research.
Association between FPC-DMN connectivity and empathy
To the best of our knowledge, this study is the first to have explored the association between FPC-DMN connectivity and empathy. We found that an increased FPC-DMN anti-correlation was associated with higher empathic ability during the EMO task. Takeuchi et al. (2013) were the first to investigate the association between trait emotional intelligence and resting-state functional connectivity. They demonstrated that increased anti-correlation between the key nodes of the DMN and FPC, the mPFC and the anterior part of the right dlPFC, was associated with a higher intrapersonal factor score in trait emotional intelligence. In this study, we found a similar phenomenon simply in the EMO task, which required more empathy recruitment.
It has been mentioned earlier that the subjects with higher empathic ability were able to perform facial EMO perception better. A possible explanation may be that the individuals with higher empathy could fully focus their attention on facial EMO perception and exclude irrelevant thoughts. Our neuroimaging results provide further support for this explanation. To balance internal and external mental processes, the FPC acts as a modulator by suppressing the DMN. This suppression allows a reallocation of cognitive resources from task-irrelevant processes to task-relevant processes. Due to the limited empathic involvement required by the ID task, no association between FPC-DMN connectivity and empathy was observed in the ID task.
Limitations
This study has several limitations, especially with respect to data analyses and experimental design. The main disadvantage in data analyses is the ICA employed in network extraction. It has been established that component selection is the primary problem for the application of ICA in fMRI analysis (Lei et al., 2010). In particular, available prior information (for example, a priori regions of interest) is not well utilized in ICA. In addition, the correlation between the behavioral performance (ACC) and the FPC-DMN anti-correlation was non-significant in the EMO task [r(31) = −0.10, P = 0.58] in this study. This was unexpected given that we were able to discern the correlations between empathy and behavior, and between empathy and the FPC-DMN anti-correlation.
The design of this study was not ideal. First, many face images were repeated across trials, resulting in priming effects and potentially boosting ID memory performance to some extent. Although we have demonstrated that the priming effects did not produce a significant influence on our results, this design needs to be improved. Second, the two conditions of EMO and ID being separated in two independent runs may bring the differences in the BOLD response between runs. To solve this issue, each run should contain all the conditions. However, the order of task runs has been counterbalanced across participants in this study. Furthermore, an additional analysis of two task-irrelevant regions revealed no run-wise differences in both BOLD signal and functional connectivity. Thus, the possibility that this design limitation could account for our findings was ruled out. Third, all of the subjects were young Chinese college students. This limited sample brings two problems. On the one hand, it has been proved that cultural differences have an important influence on empathy. Eastern cultures and western cultures are distinct in empathic processing patterns (Adams Jr et al., 2010; Cassels et al., 2010; Birkett, 2014). On the other hand, participants in this study were well educated, so these results may limit their generalizability to other populations. Fourth, it is not possible to determine the extent to which the relationship between FPC-DMN connectivity and empathy is mediated by WM based on our current data. As mentioned before, there is some evidence supporting the association between WM and empathy (Smith et al., 2014). The empathy we measured here may also reflect WM to some extent. The relative contribution of empathy and WM to the FPC-DMN connectivity is worthy of further investigation. Last but not least, we only employed a single (albeit well-validated) empathy metric here. Inclusion of more measures would strengthen the conclusion that the FPC-DMN connectivity specifically reflects empathy.
CONCLUSIONS
In this study, we explored the anti-correlation between the FPC and DMN in social WM task performance. Consistent with previous classical WM studies, an increased anti-correlation was observed between the FPC and DMN as participants engaged in the WM tasks. Specifically, this anti-correlation during the EMO task was stronger than during the ID task, as the former had a higher social load. Furthermore, we observed a connectivity-cognition relationship. In the EMO task, individuals with higher empathic ability demonstrated better task performance. More interestingly, higher empathy corresponded to stronger FPC-DMN anti-correlations. These results suggest that individual differences in social WM task performance may be related to individual differences in the functional connectivity between the FPC and DMN, and that the FPC suppresses the DMN to support efficient social WM performance and empathy.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Nature Science Foundation of China (31200857) and the Major Project of National Social Science of China (14ZDB016).
REFERENCES
- Adams RB, Jr, Rule NO, Franklin RG, Jr, et al. Cross-cultural reading the mind in the eyes: an fMRI investigation. Journal of Cognitive Neuroscience. 2010;22(1):97–108. doi: 10.1162/jocn.2009.21187. [DOI] [PubMed] [Google Scholar]
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18(3):251–70. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends in Cognitive Sciences. 2012;16(12):584–92. doi: 10.1016/j.tics.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophrenia Bulletin. 2013;39(1):168–78. doi: 10.1093/schbul/sbr107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of autism and developmental disorders. 2004;34(2):163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Singer T. The neural basis of empathy. Annual Review of Neuroscience. 2012;35:1–23. doi: 10.1146/annurev-neuro-062111-150536. [DOI] [PubMed] [Google Scholar]
- Birkett MA. Self-compassion and empathy across cultures: comparison of young adults in China and the United States. International Journal of Research Studies in Psychology. 2014;3(1):25–34. [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Pekar JJ. A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping. 2001;14(3):140–51. doi: 10.1002/hbm.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, Hasher L. Age differences in the frontoparietal cognitive control network: implications for distractibility. Neuropsychologia. 2012;50(9):2212–23. doi: 10.1016/j.neuropsychologia.2012.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassels TG, Chan S, Chung W. The role of culture in affective empathy: cultural and bicultural differences. Journal of Cognition and Culture. 2010;10(3):309–26. [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Society of Neuroscience. 2006;1(3–4):364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nature Neuroscience. 2013;16(9):1348–55. doi: 10.1038/nn.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole MW, Schneider W. The cognitive control network: integrated cortical regions with dissociable functions. Neuroimage. 2007;37(1):343–60. doi: 10.1016/j.neuroimage.2007.03.071. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58(3):306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews Neuroscience. 2002;3(3):201–15. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Davis MH. Empathy: A Social Psychological Approach. Boulder, CO, US: Westview Press; 1994. [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Lennon R. Sex differences in empathy and related capacities. Psychological Bulletin. 1983;94(1):100. [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2011;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Fan YT, Chen C, Chen SC, Decety J, Cheng Y. Empathic arousal and social understanding in individuals with autism: evidence from fMRI and ERP measurements. Social Cognitive and Affective Neuroscience. 2014;9(8):1203–13. doi: 10.1093/scan/nst101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Harrison BJ, Zalesky A, Simons JS. Competitive and cooperative dynamics of large-scale brain functional networks supporting recollection. Proceedings of the National Academy of Sciences of the USA. 2012;109(31):12788–93. doi: 10.1073/pnas.1204185109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–45. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Gao W, Lin W. Frontal parietal control network regulates the anti-correlated default and dorsal attention networks. Human Brain Mapping. 2012;33(1):192–202. doi: 10.1002/hbm.21204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach KD, Spreng RN, Madore KP, Schacter DL. Future planning: default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Social Cognitive and Affective Neuroscience. 2014;9(12):1942–51. doi: 10.1093/scan/nsu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. The Journal of Neuropsychiatry and Clinical Neurosciences. 1994;6(4):348–57. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gordon EM, Breeden AL, Bean SE, Vaidya CJ. Working memory-related changes in functional connectivity persist beyond task disengagement. Human Brain Mapping. 2014;35(3):1004–17. doi: 10.1002/hbm.22230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Stollstorff M, Devaney JM, Bean S, Vaidya CJ. Effect of dopamine transporter genotype on intrinsic functional connectivity depends on cognitive state. Cereb Cortex. 2012;22(9):2182–96. doi: 10.1093/cercor/bhr305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman ML. Sex differences in empathy and related behaviors. Psychological bulletin. 1977;84(4):712. [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39(4):1666–81. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Qiu C, Xu P, Yao D. A parallel framework for simultaneous EEG/fMRI analysis: methodology and simulation. Neuroimage. 2010;52(3):1123–34. doi: 10.1016/j.neuroimage.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Lei X, Wang Y, Yuan H, Mantini D. Neuronal oscillations and functional interactions between resting state networks. Human Brain Mapping. 2014;35(7):3517–28. doi: 10.1002/hbm.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhao Z, Chen H. Extraversion is encoded by scale-free dynamics of default mode network. Neuroimage. 2013;74:52–7. doi: 10.1016/j.neuroimage.2013.02.020. [DOI] [PubMed] [Google Scholar]
- Li YO, Adali T, Calhoun VD. Estimating the number of independent components for functional magnetic resonance imaging data. Human Brain Mapping. 2007;28(11):1251–66. doi: 10.1002/hbm.20359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Clinical Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Meyer ML, Lieberman MD. Social working memory: neurocognitive networks and directions for future research. Fronteirs in Psychology. 2012;3:571. doi: 10.3389/fpsyg.2012.00571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer ML, Spunt RP, Berkman ET, Taylor SE, Lieberman MD. Evidence for social working memory from a parametric functional MRI study. Proceedings of the National Academy of Sciences of the USA. 2012;109(6):1883–8. doi: 10.1073/pnas.1121077109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta M, Whalen PJ. Individual differences in neural activity during a facial expression vs. identity working memory task. Neuroimage. 2011;56(3):1685–92. doi: 10.1016/j.neuroimage.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton AT, Morgan VL, Rogers BP, Gore JC. Modulation of steady state functional connectivity in the default mode and working memory networks by cognitive load. Human Brain Mapping. 2011;32(10):1649–59. doi: 10.1002/hbm.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: a meta-analysis of normative functional neuroimaging studies. Human Brain Mapping. 2005;25(1):46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79(4):814–28. doi: 10.1016/j.neuron.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repovs G, Barch DM. Working memory related brain network connectivity in individuals with schizophrenia and their siblings. Frontiers in Human Neuroscience. 2012;6:137. doi: 10.3389/fnhum.2012.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54(2):1743–54. doi: 10.1016/j.neuroimage.2010.08.024. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology. 2007;21(4):431. doi: 10.1037/0894-4105.21.4.431. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Brown K, Baird B, Schooler JW. Cooperation between the default mode network and the frontal-parietal network in the production of an internal train of thought. Brain Research. 2012;1428:60–70. doi: 10.1016/j.brainres.2011.03.072. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Horan WP, Cobia DJ, Karpouzian TM, Fox JM, Reilly JL, Breiter HC. Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophrenia Bulletin. 2014;40(4):824–34. doi: 10.1093/schbul/sbt084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21(3):489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Sepulcre J, Turner GR, Stevens WD, Schacter DL. Intrinsic architecture underlying the relations among the default, dorsal attention, and frontoparietal control networks of the human brain. Journal of Cognitive Neuroscience. 2013;25(1):74–86. doi: 10.1162/jocn_a_00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. Neuroimage. 2010;53(1):303–17. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucksmith E, Allison C, Baron-Cohen S, Chakrabarti B, Hoekstra RA. Empathy and emotion recognition in people with autism, first-degree relatives, and controls. Neuropsychologia. 2013;51(1):98–105. doi: 10.1016/j.neuropsychologia.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. Resting state functional connectivity associated with trait emotional intelligence. Neuroimage. 2013;83C:318–28. doi: 10.1016/j.neuroimage.2013.06.044. [DOI] [PubMed] [Google Scholar]
- Thornton MA, Conway AR. Working memory for social information: chunking or domain-specific buffer? Neuroimage. 2013;70:233–9. doi: 10.1016/j.neuroimage.2012.12.063. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168(3):242–9. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. Journal of Neurophysiology. 2010;103(1):297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology. 2008;100(6):3328–42. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Smith EE. Neuroimaging studies of working memory. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(4):255–74. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- Weissman D, Roberts K, Visscher K, Woldorff M. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9(7):971–8. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- Wen X, Liu Y, Yao L, Ding M. Top-down regulation of default mode activity in spatial visual attention. Journal of Neuroscience. 2013;33(15):6444–53. doi: 10.1523/JNEUROSCI.4939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Ochsner K. The neuroscience of empathy: progress, pitfalls and promise. Nature Neuroscience. 2012;15(5):675–80. doi: 10.1038/nn.3085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.