Abstract
Food is an innate reward stimulus related to energy homeostasis and survival, whereas money is considered a more general reward stimulus that gains a rewarding value through learning experiences. Although the underlying neural processing for both modalities of reward has been investigated independently from one another, a more detailed investigation of neural similarities and/or differences between food and monetary reward is still missing. Here, we investigated the neural processing of food compared with monetary-related rewards in 27 healthy, normal-weight women using functional magnetic resonance imaging. We developed a task distinguishing between the anticipation and the receipt of either abstract food or monetary reward. Both tasks activated the ventral striatum during the expectation of a reward. Compared with money, greater food-related activations were observed in prefrontal, parietal and central midline structures during the anticipation and lateral orbitofrontal cortex (lOFC) during the receipt of food reward. Furthermore, during the receipt of food reward, brain activation in the secondary taste cortex was positively related to the body mass index. These results indicate that food-dependent activations encompass to a greater extent brain regions involved in self-control and self-reflection during the anticipation and phylogenetically older parts of the lOFC during the receipt of reward.
Keywords: neural processing of food rewards, eating disorders, BMI, ventral striatum, orbitofrontal cortex
INTRODUCTION
Motivation for food intake in humans is not only dependent on energy homeostasis but also determined by hedonic aspects of food intake. From an evolutionary account, the latter constitute important factors formerly ensuring survival in times when food supply was rare. However, in our present obesogenic environment, a heightened food reward sensitivity facilitates the overconsumption of food. As a result, the obesity epidemic has become a serious public health and socioeconomic problem (Swinburn et al., 2011). In recent years, brain imaging studies have shown that alterations in brain reward pathways influence food intake (Stice et al., 2009). These findings offer promising routes to identify neural markers that may underlie the overconsumption of food and other pathological eating behaviors (Morton et al., 2006).
Anticipated intake of high-calorie food leads to an activation of the mesocorticolimibic reward system (O’Doherty et al., 2002; Pelchat et al., 2004). In contrast, consummatory food reward mainly involves the primary (insula/frontal operculum), as well as the secondary gustatory cortex [lateral orbitofrontal cortex (lOFC)] (O’Doherty et al., 2002; Rolls, 2007). Nevertheless, activations in these regions are not only restricted to the consumption of palatable food but can also be found during the exposure to appetizing visual food stimuli (Beaver et al., 2006; Porubská et al., 2006), suggesting that abstract visual stimuli can inherit similar motivational and hedonic qualities. Furthermore, previous studies have shown that direct and indirect measures of food intake, such as the body mass index (BMI) or frequency of overeating, are directly related to activations in reward-related brain regions such as the ventral striatum (VS) and OFC during the processing of food stimuli (Stoeckel et al., 2008; Lawrence et al., 2012; Murdaugh et al., 2012). However, the direction of this association remains unclear as some studies report a positive relation between weight and activity in reward-related regions, whereas other studies report the opposite (Burger and Stice, 2011; Ziauddeen et al., 2012).
Given the evolutionary relevance of food reward, one would expect that the processing of food as an innate reward stimulus differs to some extent from more general monetary reward processing. However, there is a paucity of studies directly comparing food and monetary reward (Metereau and Dreher, 2013; Sescousse et al., 2013). Previous studies have assessed food and secondary reward processing independently from one another. These studies found both common as well as distinct activation patterns in the brain reward network for the two reward modalities (Diekhof et al., 2012; Sescousse et al., 2013). Specifically, learned reward associations (i.e. money) are additionally represented in evolutionary more recent brain regions, whereas innate food rewards also recruit the somatosensory cortex (Sescousse et al., 2013). In this study, we were interested in assessing both the anticipation as well as the receipt of food and monetary rewards intra-individually. To our knowledge, this is the first study directly comparing food and monetary reward using the same experimental task for both modalities of reward.
Because the VS and medial OFC (mOFC) have been strongly related to subjective experiences of pleasantness (Kühn and Gallinat, 2012), we hypothesized that both the VS and mOFC will show significant activations independently from the reward modality (Knutson et al., 2001b; Morton et al., 2006). Additionally, we expected that food rewards will be associated with higher general activation during the anticipation phase and greater activation in gustatory brain regions during the receipt phase (frontal operculum and lOFC, respectively). Furthermore, we expected that the latter observation will be modulated by the individual participant's BMI.
SUBJECTS AND METHODS
Subjects
Thirty healthy right-handed female adults participated in the study. Mean age was 26.5 years ( ± 6.3), mean BMI was 21.8 kg/m2 ( ± 1.8). Data from two subjects were excluded from the analysis due to excessive head movement (greater than 3 mm in any one direction); data from one subject was excluded due to a technical error, leaving 27 subjects for the final analysis. All had normal or corrected to normal vision and were screened for medical and psychic diseases by taking the medical history, measuring body weight and height and by interviewing them with the Structured Clinical Interview for DSM-IV (SCID, Wittchen, 1997). Chronically ill, overweight and participants with a lifetime psychic diagnoses were excluded. Furthermore, participants were excluded if they were regular smokers. This study complies with the Code of Ethics of the World Medical Association (Declaration of Helsinki, version 2008) and was approved by the Ethics Committee of the Medical School of the University of Heidelberg. Written informed consent was obtained from all participants after the procedures had been fully explained.
Study procedure
All participants were asked to come to the clinic without having breakfast and to refrain from consuming alcoholic drinks for 24 h before the experiment. The study began with a light standardized breakfast at 9:00 a.m., followed by the SCID. After the interview, all subjects were asked to conduct a battery of neuropsychological tasks and to complete a package of questionnaires. The findings of the latter are not reported in this study. The magnetic resonance imaging (MRI) scanning was performed for all subjects at 12:00 p.m., corresponding to the lunchtime of most of the participants.
Stimuli and task
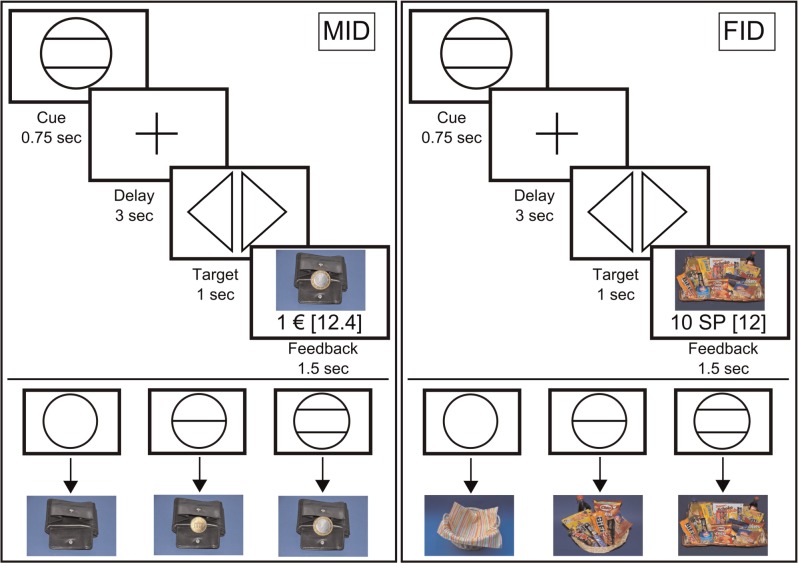
We used a modified version of the ‘monetary incentive delay’ (MID) task as proposed by Abler et al. (2001a) and Knutson et al. (2001a)). In a previous study, we showed that this paradigm allows an efficient probing of both anticipation and consumption of reward (Simon et al., 2010). Additionally, we employed a ‘food incentive delay’ (FID) task: instead of money, subjects were able to win ‘snack points’ (SP), which they then could exchange for sweet and salty snacks immediately after the MRI measurement (Simon et al., 2014). There were four blocks of reward tasks, consisting of 55 trials each. The block sequence was either SMSM (S = Snacks, M = Money) or MSMS and was counterbalanced over the participants. Before entering the scanner, subjects performed a practice version of the task lasting 3 min for each condition for which they received neither payment nor snacks. The degree of potential rewards varied on three levels as indicated via graphical cues (see Figure 1). In both tasks, each trial started with the presentation of a symbol (‘cue’, 750 ms), indicating the amount of money/number of SPs they could win with a correct response (i.e. 1 EUR, 20 cents, 0 EUR or 10 SP, 2 SP, 0 SP, respectively). After an anticipation period (‘delay’, 3000 ms) subjects had to correctly react to one of two symbols (‘targets’; i.e. triangle inclined to the right or a triangle inclined to the left) with a left or right button press corresponding to the direction of the triangle (index or middle finger of dominant hand) within a fixed interval of 1000 ms. This leads to a low task difficulty with a very high success rate, independent of the reaction speed of the participant. In order to guarantee a steady rate of reward vs non-reward throughout all subjects, we used a probabilistic reward pattern, i.e. reward was not paid out in 30 predefined trials (out of the 80 reward trials). Immediately after target presentation, feedback appeared (‘feedback’, 1500 ms), notifying participants about the amount of money/SPs they had won and about their cumulative total. In order to increase statistical efficiency, trials were separated by jittered intertrial intervals (ITIs) ranging from 1 to 8 s, with a mean of 3.5 s. The MID task used graphical depictions showing a wallet filled with the corresponding amount of money won during each trial. The FID task used pictures of either a large basket filled with snacks, a small basket filled with snacks or an empty basket, depending on the amount of SPs won. An incorrect button press resulted in a zero payout. To ensure that subjects paid attention and responded to every experimental condition, a penalty of −1 EUR/−10 SP was applied if they missed to press one of the two buttons. In the MID task, subjects were able to win a maximum of 30 EUR. In the FID task, the maximum amount to be won was 300 SP, with any snack of the basket being 50 SP worth.
Fig. 1.
Graphical depiction of the money (MID task) and food incentive delay task (FID task). Cues representing possible reward outcomes (1 EUR, 20 cents and 0 EUR/10 SPs, 2 SP and 0 SP, respectively) and task structure of the MID/FID. Participants were presented with a cue stipulating the amount of money/SP they could win if they reacted correctly during the following discrimination task. Immediately after target presentation, participants were informed about the amount of money/SP they had won during the trial and their cumulative total win so far.
Functional magnetic resonance imaging acquisition
Images were collected using a 3-T Siemens Trio MRI scanner (Siemens Medical Solutions, Erlangen, Germany) equipped with a standard 32-channel head coil. The subjects performed four functional runs lasting 9.3 min each with 280 volumes per run. In order to minimize susceptibility artifacts in the OFC 30 oblique slices (interleaved acquisition) with a 10° angle relative to the AC-PC axis were acquired with 1-mm interslice gap, using a T2*-sensitive single-shot EPI sequence with following parameters: repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, resulting in an in-plane resolution of 3 × 3 × 4 mm3, flip angle = 80°, field of view =192 × 192 mm. Participants viewed visual stimuli on a projection screen via a mirror fixed to the head coil and responded with the right hand using a button box. High-resolution T1 MPRAGE anatomical images were acquired (192 slices, voxel size 1 × 1 × 1 mm3, TR 1570 ms, TE 2.63 ms, 9° flip angle) for anatomical reference.
Functional magnetic resonance imaging data analysis
Functional MRI (fMRI) data were preprocessed and analyzed with SPM8 (Statistical Parametric Mapping, Wellcome Department of Cognitive Neurology, London, UK). To account for magnetic field equilibration, four volumes from the start of each functional run were excluded from analysis. Preprocessing of functional scans included slice time correction (reference to the first slice, using SPM8's Fourier phase shift interpolation), within-subject registration and unwarping of time-series (to correct for motion artifacts), coregistration of the T1 image with the mean T2*-image, spatial normalization of both the functional and structural images to a standard T1 template brain [ICBM152, Montreal Neurological Institute (MNI)], resulting in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for high-resolution anatomic images and smoothing with an 8-mm full-width half-maximum isotropic Gaussian kernel. A 128-s high-pass filter was used to remove low-frequency noise and signal drift.
At the first level of analysis, preprocessed fMRI data for both tasks were analyzed separately in the context of the general linear model (GLM) approach (Friston et al., 1994). Regressors were modeled separately for the three different anticipation phases (anticipation of 1 EUR, 20 cents and 0 EUR for the MID task, anticipation of 10 SP, 2 SP and 0 SP for the FID task) and the five different outcome phases (receipt of 1 EUR, omission of 1 EUR, receipt of 20 cents, omission of 20 cents and receipt of 0 EUR/neutral outcome for the MID task, receipt of 10 SP, omission of 10 SP, receipt of 2 SP, omission of 2 SP and receipt of 0 SP/neutral outcome for the FID task) as explanatory variables convolved with the gamma-variate function described by Cohen (1997). Targets and error trials were included as additional regressors of no interest. Linear combinations of the estimated GLM parameters allow the assessment of changes in the blood oxygenation level dependent (BOLD) responses of individual participants, contingent on the experimental condition. Individual contrast images corresponding to the effects of interest were subsequently constructed. For the analysis of reward anticipation, we contrasted the anticipation of a high reward (1 EUR or 10 SP) with the anticipation of no reward (0 EUR or 0 SP, anticipation_high). For the analysis of the impact of a rewarding outcome, we contrasted the receipt of a high reward (1 EUR or 10 SP) with the receipt of no reward (0 EUR or 0 SP, receipt_high), controlling for the anticipation phase that preceded both outcome types. All contrasts were modeled separately for each task.
At the second level of analysis, the individual contrast images of all participants were included in a random-effects analysis, allowing population inference (Holmes and Friston, 1998). A whole-brain analysis using the specific contrasts of interests was carried out in order to identify reward-sensitive brain areas. Within-group activation was compared using a one-sample t-test, and between-tasks activation using a two-sample t-test. We report results significant at an uncorrected voxel level threshold of P < 0.001 (Spreckelmeyer et al., 2009). The location of the peak activity associated with each cluster of activation is reported in MNI coordinates.
Region of interest analysis
In order to assess brain activation in reward-related brain regions, we constructed functional region of interests (ROIs) based on the results of the whole-brain analysis during the FID task, using a threshold of P < 0.001 uncorrected. Functional ROIs were constructed based on activation clusters found in reward-related regions during both the anticipation and the receipt of food rewards. Spheres of 8-mm diameter were created around the respective peak voxels of activation. We constructed four ROIs: left VS, right VS, left lOFC and right lOFC. The left and right VS ROIs were based on peak activation in the left (MNI coordinates: x = −6, y = 5, z = 2) and right (MNI: 12, 2, 2) VS, respectively, passing a threshold of P < 0.001 during the contrast anticipation_high. The left (MNI: −39, 47, −6) and right (MNI: 42, 41, −14) lOFC ROIs were constructed using peak activation in lOFC regions during the contrast receipt_high. Mean percent signal change was extracted for each ROI using MarsBaR (Brett et al., 2002). Simple correlation (Pearson's r) analyses were performed for psychometric scales and mean percent signal change in the ROIs using SPSS version 20.
RESULTS
Behavioral results
Reaction times and error rates for each task are given in supplementary Table S1 in the supplementary materials. We did not observe a significant difference in reaction time or error rate between tasks (Ps > 0.29).
Neuroimaging results
Anticipation of reward
During the contrast anticipation_high, the FID task displayed activations in the bilateral VS, bilateral thalamus, subgenual anterior cingulate cortex (ACC), right anterior insula and bilateral occipital cortex (Table 1, Figure 2). The cluster in the right VS extends to the anterior thalamus. For the MID task, we observed activations in the left VS, right anterior insula, bilateral thalamus and a portion of the midbrain (Table 1). The extracted signal change at the VS ROI for the FID task during different reward conditions (anticipation of 10 SP, 2 SP, 0 SP) showed a noticeable linear decrease from 10 SP via 2 SP to 0 SP for both the lVS and rVS (see Figure 2).
Table 1.
Whole-brain results for the anticipation/receipt of primary and secondary rewards
| Area | Food | Money | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t-value at peak voxel | MNI coordinates |
BA | t-value at peak voxel | MNI coordinates |
BA | |||||
| x | y | z | x | y | z | |||||
| Ventral striatum | 4.25 | 12 | 2 | 2** | ||||||
| 4.49 | −6 | 5 | 2 | 4.43 | −9 | 2 | −2 | |||
| Thalamus | 4.24 | 6 | −13 | 2** | 3.87 | 3 | −13 | −6*** | ||
| 4.24 | −9 | −22 | −2*** | |||||||
| Midbrain | 6.23 | −3 | −31 | −6*** | ||||||
| Subgenual ACC | 4.17 | −6 | 32 | −2*** | 24 | |||||
| Anterior insula | 3.6 | 36 | 17 | −6 | 13 | 3.8 | 30 | 20 | −2 | 13 |
| 3.74 | −36 | 17 | −6 | 13 | ||||||
| Medial occipital cortex | 6.07 | 24 | −85 | −2*** | 17 | 4 | 27 | −91 | −2 | 18 |
| 5.19 | −15 | −91 | 2*** | 17 | 4.2 | −30 | −79 | −6 | 18 | |
| Lateral OFC | 4.38 | 42 | 41 | −14 | 47 | |||||
| 5.09 | −39 | 47 | −6*** | 47 | ||||||
| ACC/mOFC | 4.7 | 6 | 35 | −6*** | 32 | |||||
| Inferior prefrontal cortex | 5.21 | 51 | 41 | 18*** | 46 | 7.08 | 45 | 38 | 18*** | 46 |
| 5.64 | −51 | 35 | 18*** | 46 | 4.36 | −45 | 20 | 26*** | ||
| Frontal inferior operculum | 4.47 | −45 | 11 | 30*** | 9 | 31/23 | ||||
| Anterior insula | 5.54 | 33 | 17 | −10*** | ||||||
| Posterior cingulum | 7.25 | 3 | −37 | 34*** | ||||||
| Medial occipital cortex | 5.44 | 24 | −91 | −2*** | 18 | |||||
| 6.31 | −21 | −91 | 2*** | 18 | ||||||
| Temporal medial cortex | 6.84 | 60 | −43 | −2*** | 21 | |||||
| Temporal inferior cortex | 5.47 | −51 | −58 | −10*** | 37 | 5.62 | −54 | −58 | −10*** | 37 |
| Parietal cortex, angular gyrus | 5.06 | 30 | −61 | 46*** | 7 | 7.33 | 36 | −64 | 42*** | 7 |
| 4.96 | −27 | −61 | 42*** | 7 | 5.66 | −30 | −64 | 42*** | 7 | |
All results P < 0.001 voxelwise uncorrected, except: *voxelwise FWE corrected P < 0.05, **Cluster level P < 0.001 uncorrected, ***Cluster level FWE corrected P < 0.05. BA, Brodamnn area; L, left; R, right; MNI, Montreal Neurological Institute; ACC, anterior cingulate cortex; OFC, orbitofrontal cortex.
Fig. 2.
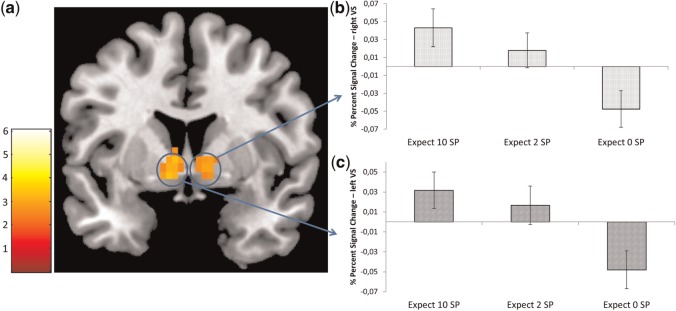
(a) Significant brain activation during the contrast anticipation_high for the FID task. The threshold was set at P < 0.001 uncorrected, with a cluster-defining threshold of five voxels for illustrative purposes. The blue circles denote the localization of the VS ROIs. SPM t-map was rendered on a T1-weighted template image (coronal slice, y coordinate = 4 mm) supplied with mricron (Colin brain). (b) Bar chart depicting percent signal change from the right VS during different anticipation conditions for the FID task. (c) Bar chart depicting percent signal change from the left VS during different anticipation conditions for the FID task. Error bars depict SEM.
Receipt of reward
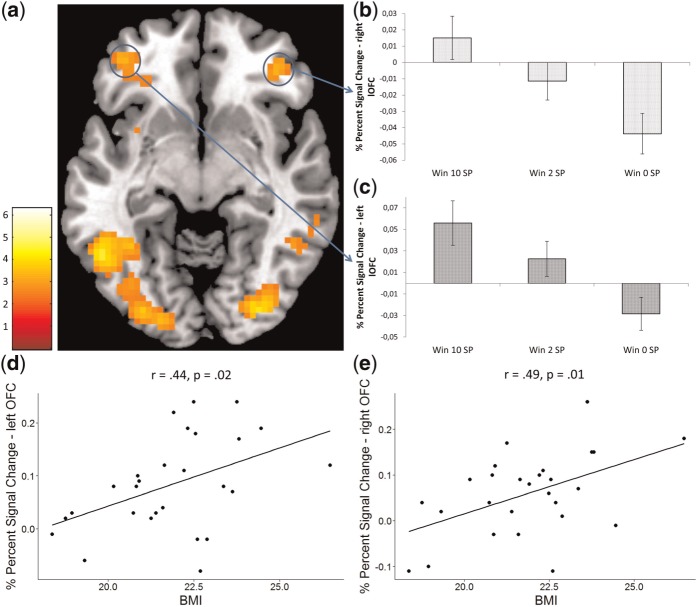
During the contrast receipt_high, the FID task showed activations in the bilateral OFC, bilateral inferior lPFC, left inferior operculum, bilateral parietal cortex and bilateral occipitotemporal cortex (Table 1, Figure 3). For the MID task, we observed a large cluster with punctum maximum in the subgenual ACC, extending into the mOFC. Additionally, we observed activity in the bilateral inferior lPFC, left anterior insula, posterior cingulum, bilateral parietal and occipitotemporal cortex (Table 1). The extracted signal change showed a linear decrease from the receipt of 10 SP via 2 SP to 0 SP for both the left and right lOFC (see Figure 3) in the FID task.
Fig. 3.
(a) Significant brain activation during the contrast receipt_high for the FID task. The threshold was set at P < 0.001 uncorrected, with a cluster-defining threshold of five voxels for illustrative purposes. The blue circles denote the localization of the OFC ROIs. SPM t-map was rendered on a T1-weighted template image (transversal slice, z-coordinate = −8 mm) supplied with mricron (Colin brain). (b) Bar chart depicting percent signal change from the right lOFC during different SP receipt conditions. (c) Bar chart depicting percent signal change from the left lOFC during different SP receipt conditions. Error bars depict SEM. (d) Correlation between percent signal change extracted at the right lOFC during the contrast receipt_high and BMI. (e) Correlation between percent signal change extracted at the left lOFC during the contrast receipt_high and BMI.
Comparison between monetary and food reward
Compared with the MID task, the FID task showed significant differences in brain activation using whole-brain analysis. During anticipation_high, the FID task showed stronger activations in the inferior lPFC, OFC, medial frontal cortex, posterior cingulum, angular gyrus and middle temporal gyrus. During the receipt_high, the FID task showed stronger activations in the posterior lOFC and occipital cortex (Table 2, Figure 4). There were no brain regions that showed greater brain activations in the MID compared with the FID task.
Table 2.
Group maximum t-values and MNI coordinates of all activation foci found during the comparison of the MID and FID task
| Area | Anticipation of high vs no reward |
Receipt of high vs no reward |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| t-value at peak voxel | MNI coordinates |
BA | t-value at peak voxel | MNI coordinates |
BA | |||||
| x | y | z | x | y | z | |||||
| FID > MID | ||||||||||
| Inferior frontal cortex | 4.03 | −54 | 20 | 10*** | 45 | |||||
| Medial frontal cortex | 3.82 | −3 | 56 | 6 | 10 | |||||
| Posterior cingulum cortex | 4.56 | 0 | −49 | 26*** | 23/31 | |||||
| Parietal cortex, angular gyrus | 4.16 | −45 | −58 | 30*** | 39 | |||||
| Middle temporal gyrus | 4.13 | 66 | −10 | −14*** | 21 | |||||
| Posterior lateral OFC | 3.84 | −27 | 32 | −14 | 47/11 | |||||
| Occipital medial cortex | 3.86 | 21 | −91 | 6 | 18 | |||||
| 3.85 | −15 | −91 | −2 | 17 | ||||||
All results P < 0.001 voxelwise uncorrected, except: *voxelwise FWE corrected P < 0.05, **Cluster level P < 0.001 uncorrected, ***Cluster level FWE corrected P < 0.05. BA, Brodamnn area; L, left; R, right; MNI, Montreal Neurological Institute.
Fig. 4.
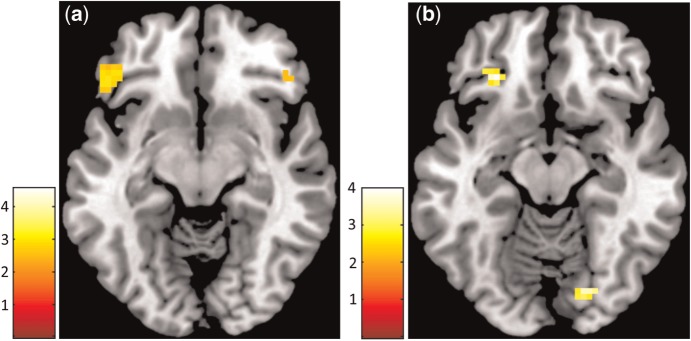
(a) Whole-brain analysis depicting areas with stronger activation during the FID then MID task during the contrast anticipation_high. (b) Whole-brain analysis depicting areas with stronger activation during the FID then MID task during the contrast receipt_high. The threshold was set at P < 0.001 uncorrected, SPM t-maps were overlaid on a T1-weighted template image (transversal slice, z-coordinate = −12 mm) supplied with mricron (Colin brain).
Correlational analysis
We found a positive correlation for the FID task between percent signal change extracted from the right and left lOFC ROI during the receipt_high contrast and the BMI of the individual participants (Figure 3). There were no significant correlations for the FID task between signal change extracted from the left and right VS and the BMI. For the MID task, no correlations between the signal change of the VS nor the lOFC or the individual BMI were observed.
DISCUSSION
The aim of this study was to investigate common and distinct patterns in neural processing of food- and more general monetary-related rewards in healthy individuals. In line with our hypotheses, we observed an increased activation in the VS during the anticipation of reward, irrespective of the reward modality. Contrary to our assumptions, we only observed mOFC activity during the receipt of monetary rewards, whereas the receipt of food reward was associated with heightened activation of the bilateral lOFC. Furthermore, we observed increased activation in the lateral ventral prefrontal cortex, central midline structures (ventromedial PFC and posterior cingulum) and parietal cortex during food anticipation. In addition, greater activation in the secondary gustatory cortex (posterior lOFC) but not in the primary gustatory cortex (insula/frontal operculum) could be observed during the receipt of food reward. Finally, the BMI was positively correlated with bilateral lOFC activation during the receipt of food reward.
The VS is considered a central region of the reward system, responding to the anticipation of different types of reward (Knutson et al., 2001a; Rademacher et al., 2010; Sescousse et al., 2013). Particularly, the overall saliency of a stimulus is related to the activity in the VS (Zink et al., 2004). Correspondingly, we observed a linear increase in VS activation to increasing food reward. Furthermore, we observed bilateral anterior insula activation during the anticipation of food. The anterior insula is known to be related to interoceptive awareness including craving elicited by different drug cues (Craig, 2009). Since the gustatory cortex is located in the insula and overlying frontal operculum (Craig, 2009; Veldhuizen et al., 2011), this observation likely represents conscious urges for food rather than primary gustatory processing. Additionally, we found dorsal thalamic activation during the anticipation, but not the receipt, of food reward which is in line with previous studies (Komura et al., 2001; Knutson and Greer, 2008), indicating that dorsal thalamus activation reflects increased arousal and attention primarily to reward predicting cues. In sum, the observed activations indicate a recruitment of the general incentive salience network during the anticipation of food-related stimuli (Berridge and Robinson, 1998).
Receipt of food rewards recruited the bilateral lOFC and frontal operculum, indicating that the receipt of abstract food-related stimuli recruits similar regions than those commonly observed during the processing of gustatory properties of food (Rolls, 2008; Veldhuizen et al., 2011; Small, 2012). Furthermore, receipt of food reward was associated with bilateral activations of the lPFC. This is in accord with a previous meta-analysis that found right lPFC activations during the processing of taste reward (Sescousse et al., 2013). The lPFC plays a central role in processes of attention, working memory and inhibitory control (Owen et al., 1999; Tanji and Hoshi, 2008). In healthy subjects, suppression of cue-induced food craving was associated with increased activation in the lPFC (Siep et al., 2012). Furthermore, repetitive transcranial magnetic stimulation of the left lPFC was found to reduce the desire for food consumption (Uher et al., 2005). Thus, bilateral lPFC activations during the receipt of food reward may reflect the recruitment of inhibitory control processes. We did not observe any significant activity in the mOFC during the receipt of food rewards. The mOFC is considered relevant for the evaluation of food reward (Gearhardt et al., 2011). Although some previous studies found mOFC activation during the processing of different types of reward including food rewards (Peters and Büchel, 2010), we only observed activation in the lateral part of the OFC. Our findings are in line with previous studies, showing that the intensity of lOFC activation during food ingestion is closely related to subjective pleasantness ratings (Kringelbach et al., 2003). Hence, our results indicate that the neural coding of the receipt of abstract food rewards specifically involves the primary and secondary gustatory cortex rather than common reward-related brain regions (i.e. mOFC).
As expected, both tasks activated the VS during the anticipation of rewards. Heightened VS activity in response to different kinds of reward such as food, money, erotic stimuli or even pleasant music (Menon and Levitin, 2005; Sescousse et al., 2010; Simon et al., 2010, 2014) confirm the role of the VS as a site of modality-independent computation of reward-related salience (Metereau and Dreher, 2013). Differences in VS activation found in previous studies could be due to the fact that the delivery of monetary rewards often depends on the participants' motor reactions, whereas food rewards are often delivered in a passive manner (Sescousse et al., 2013). Here, we compared for the first time food with monetary rewards in the same context. Our results are in line with a study by Sescousse et al. (2010), who compared erotic with monetary rewards using the same task and also failed to observe significant differences in VS activity between tasks.
Furthermore, a cluster of activation located in the posterior cingulate cortex (PCC) was stronger activated during the anticipation of food rewards compared with monetary rewards. Activation of the PCC was found to be increased in response to high caloric visual food and taste stimuli (Siep et al., 2009) and has been linked to craving for different types of reward. Together with the medial PFC, the PCC forms a system of cortical midline structures involved in self-related cognitions and attentional tracking of self-relevant stimuli (Small et al., 2003; Northoff et al., 2006). This is further corroborated by higher activation in the angular gyrus of the parietal cortex, which is an additional region involved in self-awareness and attentional processing (Seghier, 2013). Therefore, compared with monetary reward, food reward seems to be associated with increased self-referential processing (i.e. food is to a greater extent analyzed in relation with one’s self (Brewer et al., 2013)). Additionally, the observation of a stronger activation in the posterior lOFC during the receipt of food rewards is in line with previous research stating that innate rewards recruit phylogenetically older regions in the posterior OFC, whereas learned reward associations recruit phylogenetically newer regions in the anterior OFC (Kringelbach and Rolls, 2004). Lateral portions of the OFC are strongly interconnected with the primary gustatory cortex, and both brain regions are involved in the conscious experience of pleasure from palatable foods (Friederich et al., 2013).
The positive relation between activations in the bilateral lOFC and BMI may demonstrate the impact of neural responsiveness to food stimuli on eating habits. This observation is in line with some, but not all, previous studies: Hyperactivation in the lOFC while viewing pictures of high-calorie food has been found to be associated with higher BMI levels (Rothemund et al., 2007; Stoeckel et al., 2008; Yokum et al., 2011). However, another study found an inverse relation between BMI and activation in the right lOFC during the observation of food pictures (Killgore and Yurgelun-Todd, 2005). Because activity in lateral orbitofrontal regions has been linked to the current reward value of tastes (Rolls et al., 2003; Simmons et al., 2005) and seems to be increased by hunger (Porubská et al., 2006), one may argue that a stronger activation in those regions contributes to increased food intake. Indeed, there is preliminary evidence that future increases in BMI are related to increased activity in the lOFC, but to a reduced sensitivity in the common reward circuitry, specifically the VS (Stice et al., 2010; Yokum et al., 2011).
A possible limitation of our study may arise from the use of a task employing abstract food stimuli that may differ from tasks that include the receipt of actual taste stimuli during an fMRI scan. However, since the primary aim of this study was to compare food- with monetary-related reward stimuli, we used the same task design for both conditions. Furthermore, as all participants were women, results should be generalized to men with caution. Finally, all our participants had a BMI within the normal weight range. Therefore, it remains unclear whether our findings are also valid for overweight and obese people.
Taken together, the present findings support the notion that food and monetary reward processing engage both common and distinct brain networks. The direct comparison of both types of reward, assessed with the same task design in the same sample, revealed stronger activation in a brain network subserving self-referential processing. During the receipt of food reward, higher activation was observed in phylogenetically older portions of the lOFC. This underlines the posterior–anterior distinction of innate and learned reward processing in the OFC. Finally, the present task proved valid that both the anticipation and receipt of food reward can be reliably measured using abstract stimuli. Therefore, the present fMRI task offers a valuable method to further investigate brain circuits underlying reward processing in obesity and eating disorders.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by a DFG grant (FR 2626/3-1).
REFERENCES
- Abler B, Walter H, Erk S. Neural correlates of frustration. Neuroreport. 2005;16:669–72. doi: 10.1097/00001756-200505120-00003. [DOI] [PubMed] [Google Scholar]
- Beaver JD, Lawrence AD, Ditzhuijzen J, van Davis MH, Woods A, Calder AJ. Individual differences in reward drive predict neural responses to images of food. The Journal of Neuroscience. 2006;26:5160–6. doi: 10.1523/JNEUROSCI.0350-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using the MarsBar toolbox for SPM 99. Neuroimage. 2002;16:S497. [Google Scholar]
- Brewer JA, Garrison KA, Whitfield-Gabrieli S. What about the “self” is processed in the posterior cingulate cortex? Frontiers in Human Neuroscience. 2013;7:647. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. Variability in reward responsivity and obesity: evidence from brain imaging studies. Current drug abuse reviews. 2011;4:182–9. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Diekhof EK, Kaps L, Falkai P, Gruber O. The role of the human ventral striatum and the medial orbitofrontal cortex in the representation of reward magnitude - an activation likelihood estimation meta-analysis of neuroimaging studies of passive reward expectancy and outcome processing. Neuropsychologia. 2012;50:1252–66. doi: 10.1016/j.neuropsychologia.2012.02.007. [DOI] [PubMed] [Google Scholar]
- Friederich H-C, Wu M, Simon JJ, Herzog W. Neurocircuit function in eating disorders. International Journal of Eating Disorders. 2013;46:425–32. doi: 10.1002/eat.22099. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Human brain mapping. 1994;2:189–210. [Google Scholar]
- Gearhardt AN, Yokum S, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of general psychiatry. 2011;68:808–16. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AP, Friston KJ. Generalisability, Random Effects & Population Inference. Neuroimage. 1998;7:S754. [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. Neuroreport. 2005;16:859–63. doi: 10.1097/00001756-200505310-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001a;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong G, Adams C, Varner J, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001b;12:3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3771–86. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komura Y, Tamura R, Uwano T, Nishijo H, Kaga K, Ono T. Retrospective and prospective coding for predicted reward in the sensory thalamus. Nature. 2001;412:546–9. doi: 10.1038/35087595. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cerebral cortex. 2003;13:1064–71. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kühn S, Gallinat J. The neural correlates of subjective pleasantness. NeuroImage. 2012;61:289–94. doi: 10.1016/j.neuroimage.2012.02.065. [DOI] [PubMed] [Google Scholar]
- Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. NeuroImage. 2012;63:415–22. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- Menon V, Levitin DJ. The rewards of music listening: response and physiological connectivity of the mesolimbic system. NeuroImage. 2005;28:175–84. doi: 10.1016/j.neuroimage.2005.05.053. [DOI] [PubMed] [Google Scholar]
- Metereau E, Dreher J-C. Cerebral correlates of salient prediction error for different rewards and punishments. Cerebral Cortex. 2013;23:477–87. doi: 10.1093/cercor/bhs037. [DOI] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–95. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook EW, III, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59:2709–21. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP, Deichmann R, Critchley HD, Dolan RJ. Neural responses during anticipation of a primary taste reward. Neuron. 2002;33:815–26. doi: 10.1016/s0896-6273(02)00603-7. [DOI] [PubMed] [Google Scholar]
- Owen AM, Herrod NJ, Menon DK, et al. Redefining the functional organization of working memory processes within human lateral prefrontal cortex. European Journal of Neuroscience. 1999;11:567–74. doi: 10.1046/j.1460-9568.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- Pelchat ML, Johnson A, Chan R, Valdez J, Ragland JD. Images of desire: food-craving activation during fMRI. NeuroImage. 2004;23:1486–93. doi: 10.1016/j.neuroimage.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. Neural representations of subjective reward value. Behavioural Brain Research. 2010;213:135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Porubská K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. NeuroImage. 2006;32:1273–80. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- Rademacher L, Krach S, Kohls G, Irmak A, Gründer G, Spreckelmeyer KN. Dissociation of neural networks for anticipation and consumption of monetary and social rewards. NeuroImage. 2010;49:3276–85. doi: 10.1016/j.neuroimage.2009.10.089. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Sensory processing in the brain related to the control of food intake. The Proceedings of the Nutrition Society. 2007;66:96–112. doi: 10.1017/S0029665107005332. [DOI] [PubMed] [Google Scholar]
- Rolls ET. Functions of the orbitofrontal and pregenual cingulate cortex in taste, olfaction, appetite and emotion. Acta Physiologica Hungarica. 2008;95:131–64. doi: 10.1556/APhysiol.95.2008.2.1. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Scott TR, Doty RL. Central taste anatomy and neurophysiology. In: Doty RL, editor. Handbook of Olfaction and Gustation. New York: Marcel Dekker, Inc.; 2003. pp. 679–705. [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37:410–21. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Seghier ML. The angular gyrus multiple functions and multiple subdivisions. The Neuroscientist. 2013;19:43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sescousse G, Caldú X, Segura B, Dreher J-C. Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and biobehavioral reviews. 2013;37:681–96. doi: 10.1016/j.neubiorev.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Sescousse G, Redouté J, Dreher J-C. The architecture of reward value coding in the human orbitofrontal cortex. The Journal of Neuroscience. 2010;30:13095–104. doi: 10.1523/JNEUROSCI.3501-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research. 2009;198:149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. NeuroImage. 2012;60:213–20. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Martin A, Barsalou LW. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cerebral Cortex. 2005;15:1602–8. doi: 10.1093/cercor/bhi038. [DOI] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Hamze Sinno M, et al. Impaired cross-talk between mesolimbic food reward processing and metabolic signaling predicts body mass index. Frontiers in Behavioral Neuroscience. 2014;8:359. doi: 10.3389/fnbeh.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Walther S, Fiebach CJ, et al. Neural reward processing is modulated by approach- and avoidance-related personality traits. NeuroImage. 2010;49:1868–74. doi: 10.1016/j.neuroimage.2009.09.016. [DOI] [PubMed] [Google Scholar]
- Small DM. Flavor is in the brain. Physiology & behavior. 2012;107:540–52. doi: 10.1016/j.physbeh.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam M-M. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. NeuroImage. 2003;18:633–41. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Spreckelmeyer KN, Krach S, Kohls G, et al. Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience. 2009;4:158–65. doi: 10.1093/scan/nsn051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Spoor S, Ng J, Zald DH. Relation of obesity to consummatory and anticipatory food reward. Physiology & behavior. 2009;97:551–60. doi: 10.1016/j.physbeh.2009.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. The Journal of Neuroscience. 2010;30:13105–9. doi: 10.1523/JNEUROSCI.2105-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook EW, III, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41:636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Sacks G, Hall KD, et al. The global obesity pandemic: shaped by global drivers and local environments. The Lancet. 2011;378:804–14. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiological Reviews. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Uher R, Yoganathan D, Mogg A, et al. Effect of left prefrontal repetitive transcranial magnetic stimulation on food craving. Biological psychiatry. 2005;58:840–2. doi: 10.1016/j.biopsych.2005.05.043. [DOI] [PubMed] [Google Scholar]
- Veldhuizen MG, Albrecht J, Zelano C, Boesveldt S, Breslin P, Lundström JN. Identification of human gustatory cortex by activation likelihood estimation. Human brain mapping. 2011;32:2256–66. doi: 10.1002/hbm.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H-U. Strukturiertes Klinisches Interview Für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an fMRI study. Obesity. 2011;19:1775–83. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nature Reviews Neuroscience. 2012;13:279–86. doi: 10.1038/nrn3212. [DOI] [PubMed] [Google Scholar]
- Zink CF, Pagnoni G, Martin-Skurski ME, Chappelow JC, Berns GS. Human striatal responses to monetary reward depend on saliency. Neuron. 2004;42:509–17. doi: 10.1016/s0896-6273(04)00183-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.