Abstract
Few studies have investigated how attentional control is affected by transient affective states while taking individual differences in affective traits into consideration. In this study, participants completed a color-word Stroop task immediately after undergoing a positive, neutral or negative affective context manipulation (ACM). Behavioral performance was unaffected by any ACM considered in isolation. For individuals high in trait negative affect (NA), performance was impaired by the negative but not the positive or neutral ACM. Neuroimaging results indicate that activity in primarily top-down control regions of the brain (inferior frontal gyrus and dorsal anterior cingulate cortex) was suppressed in the presence of emotional arousal (both negative and positive ACMs). This effect appears to have been exacerbated or offset by co-occurring activity in other top-down control regions (parietal) and emotion processing regions (orbitofrontal cortex, amygdala and nucleus accumbens) as a function of the valence of state affect (positive or negative) and trait affect (trait NA or trait PA). Neuroimaging results are consistent with behavioral findings. In combination, they indicate both additive and interactive influences of trait and state affect on top-down control of attention.
Keywords: trait affect, state affect, attention, cognitive control, interference
INTRODUCTION
Emotional experiences frequently occur during performance of cognitive tasks in daily life. Depending on the type and extent of emotion, it can either improve or impair various aspects of cognition (Ohman et al., 2001; Pessoa, 2009; Dolcos et al., 2011). There has been particular interest in the impact of emotion on executive function, including the top-down control of attention. An ability to protect attention from interfering emotional stimuli or situations is especially important in maintaining on-going task performance and pursuing goal-directed behaviors. In clinical disorders, such as depression and anxiety, that are characterized by pervasive and prolonged negative emotional states, various aspects of executive functions, such as attention control and problem-solving, are impaired (Derakshan and Eysenck, 2009; Snyder, 2013), in turn exacerbating overall impairment in daily life. Despite abundant evidence of a close relationship between emotion and cognitive control, the circumstances under which and the mechanisms by which emotion impairs or enhances cognitive control have yet to be fully identified.
Some findings indicate an impairing effect of negative affect (NA) on task performance resulting from interference by distracting emotional information. For example, self-reported state NA was associated with increased reaction times (RTs) for distracting emotional stimuli compared to neutral stimuli in an emotional Stroop task (Crocker et al., 2012), suggesting that in a negative mood it becomes harder to ignore task-irrelevant emotional meaning of stimuli. In contrast, some studies have failed to find an impairing effect of negative emotion on cognitive control. Although sad mood elicited a bias in recognition memory for negative words and interfered with facial emotion recognition, it failed to affect performance of cognitive tasks without emotional content (e.g. Go/No-Go task and Color-Word Stroop task), suggesting that state NA effects are restricted to certain emotion-related cognitive processes (Chepenik et al., 2007).
In none of these investigations has trait or dispositional affect been taken into account. However, trait NA, defined as a tendency to experience negative emotions such as anxiety, anger, guilt and depressed mood, has also been shown to have an impairing effect on cognitive control, separate from the influence of state NA (e.g. Crocker et al., 2012). Trait NA is associated with bias toward enhanced processing of negative or diminished processing of positive emotional information (Canli, 2008). Studies manipulating mood (state affect) as a function of trait affect have also shown an interaction between the two; McCabe et al. (2000) found that, in a neutral mood, both never-depressed and previously depressed individuals exhibited bias to avoid negative stimuli. In a sad mood, however, never-depressed individuals maintained this bias, but previously depressed individuals did not.
The presence or absence of trait NA may account for mixed findings of state affect on cognitive task performance. However, most studies have only examined one of these factors (state or trait) or have not distinguished their influences, making it difficult to draw conclusions about their additive or interactive effects. Furthermore, studies that suggested an interactive influence of state and trait affect on cognition used tasks with emotional distractors, while using mood manipulation procedures for eliciting state affect, making it difficult to distinguish the specific and interactive impact of state and trait affect on cognitive processing. In addition, to our knowledge, there is no research systematically investigating the interactive effects of state and trait affect in processing non-emotional information.
Presence or absence of trait positive affect (PA), defined as a tendency to experience positive emotions (Watson and Clark, 1997), may also play a role. Although few studies have examined the role of trait PA in cognitive task performance, a substantial literature supports a role for state PA in cognitive control (for review, see Ashby et al., 1999). State PA can promote cognitive flexibility and creative problem solving (e.g. Isen, 2009). It also broadens attentional scope while reducing selectivity (Dreisbach and Goschke, 2004; Rowe et al., 2007). Since most research has focused on state PA, questions remain about how trait PA might affect cognitive processes or interact with other affective states.
Studies have examined neural correlates of state and trait affect as a means to elucidate mechanisms of impact on cognitive control. Interactions between dorsal executive and ventral affective systems (Pessoa, 2009; Dolcos et al., 2011) have been proposed to implement emotion–cognition interactions. A dorsal system implicated in non-emotional executive processing involves frontoparietal regions, including the dorsolateral prefrontal cortex (DLPFC), inferior frontal gyrus (IFG), lateral/inferior parietal cortex and dorsal anterior cingulate cortex (dACC) (Banich et al., 2009; Pessoa, 2009; Silton et al., 2010; Dolcos et al., 2011). In contrast, a ventral system has been broadly implicated in affective processing, including amygdala, orbitofrontal cortex (OFC) and ventral striatum (Davidson and Irwin, 1999; Pessoa, 2009; Dolcos et al., 2011). Studies have shown that, while performing cognitive tasks in the presence of emotional distractors, dorsal regions are deactivated and ventral regions activated, suggesting a dynamic interplay between them in processing emotional distractors (Drevets and Raichle, 1998; Dolcos et al., 2011). Clinical research has also supported an interaction between dorsal and ventral systems. Studies have shown that depression is associated with decreased activity in the executive system (e.g. DLPFC and dACC) and increased activity in the ventral affective system (e.g. amygdala and rostral ACC) (Davidson et al., 2002; Fales et al., 2008; Herrington et al., 2010).
Recent findings examining brain activity during an emotional Stroop task indicated that trait NA was associated with reduced activity in left posterior DLPFC, which is involved in top-down, goal-directed control of attention, while self-reported state NA was associated with increased activity in a region involved in more stimulus-driven attentional control, mid-DLPFC (Crocker et al., 2012), a functional and anatomical distinction emphasized in the cascade-of-control model (Banich et al., 2009). Thus, relevant brain circuits associated with state and trait NA appear to be dissociable. Related constructs (trait anxiety, neuroticism) have been investigated, with results indicating reduced activity in lateral prefrontal cortical regions and increased activity in ventral regions for processing task-irrelevant threat-related stimuli (e.g. fearful faces) (Bishop et al., 2004; Etkin et al., 2004).
Another line of research has examined the impact of state PA on cognitive control. Exposure to task-irrelevant positive stimuli was associated with increased activity in DLPFC, accompanied by improved performance (Herrington et al., 2005), and state PA was associated with decreased amygdala activity (Damasio et al., 2000; Blood and Zatorre, 2001).
To examine the possibility that trait and state affect have distinct and/or interactive effects on cognitive control in the absence of emotional stimuli, this study manipulated affective context immediately prior to a non-emotional task involving top-down control of attention. A measure of trait affect was included in analyses to determine its separate and interacting effects with state affect. A version of the color-word Stroop task (see Silton et al., 2010, 2011) was performed during an affective context manipulation (ACM) created using a guided imagery task (Miller et al., 1987). Because the effects of induced affective contexts can dissipate quickly (Chartier and Ranieri, 1989), the color-word Stroop task was interspersed with cue words between some trials to help maintain the affective context more robustly throughout the task.
Hypotheses were based on previous work on emotion–attention interactions. First, it was expected that behavioral performance would be impaired during the negative ACM and improved during the positive ACM. Trait affect might modulate these effects. Specifically, the impairing effect of negative ACM might be exacerbated as trait NA increases. Second, suppressed activity in dorsal regions (e.g. PFC and parietal regions) and increased activity in ventral regions (e.g. amygdala, OFC and ventral striatum) were expected in response to state affect (induced by both negative and positive ACM). Trait affect was predicted to modulate the pattern of activity in associated brain regions.
METHODS
Participants
Participants (N = 40, 50% female, 60% Caucasian) were paid volunteers recruited from undergraduate psychology classes, ranging in age from 18 to 28 years (M = 19.2, standard deviation = 1.8). All were native speakers of English, right handed and screened for abnormal color vision, claustrophobia, history of a loss of consciousness longer than 10 min, recent drug or alcohol use, excessive caffeine intake, lack of sleep and any contraindications for MRI scanning.
Materials and procedure
Trait affect
Participants completed the General Temperament Survey (GTS) to assess trait affect using the Negative Temperament and Positive Temperament subscales (Watson and Clark, 1993). Participants were instructed to decide whether statements mostly described them and to rate each item as true/false.
ACM
Before the experiment, participants filled out a survey in which they described three memories—one ‘happy’, one ‘neutral’ and one ‘sad’. Participants then provided five cue words for each memory. Each cue was a single word, eight letters or less, with no colors or abbreviations and no overlap among the three sets of five words.
During the experiment, each of the three memories provided an ACM in a within-subject manipulation. For each ACM, participants listened to taped audio instructions [adapted from Miller et al. (1987) and recorded by W.H.] encouraging them to focus on their memory (e.g. ‘imagine the situation as vividly as you can’). Participants rated how they felt before and after each ACM using the Valence and Arousal scales of the Self-Assessment Manikin (SAM), a non-verbal pictorial scale that measures affect (Bradley and Lang, 1994). Participants completed a 6.4-min color-word Stroop task after each ACM, counterbalanced for order of ACM type.
Color-word stroop task
The task contained three types of words, congruent (e.g. the word ‘blue’ presented in blue letters), incongruent (e.g. the word ‘blue’ presented in green letters) and neutral (e.g. the word ‘hour’ presented in green letters), presented in blocks of 16 trials and 32 s per block. Neutral trials were intermixed 50:50 in congruent and incongruent blocks to prevent the development of word-reading strategies. Each run of the task consisted of 12 blocks (192 trials total) presented in one of six counterbalanced block orders designed such that congruent, incongruent and neutral blocks preceded each other equally often. Stimulus presentation and response recording were controlled by Psychophysics Toolbox 2 in MATLAB (Brainard, 1997). Each trial (2000 ms ± 225 ms onset to onset) began with the presentation of one word for 1500 ms, followed by either a fixation cross or a cue word presented for an average of 500 ms (±225 ms). Cue words appeared pseudorandomly on two-thirds of trials, counterbalanced to ensure that they would not convey any information about the upcoming Stroop word condition or the length of time between trial onsets. There were 10 cue words for each ACM. Five of the cue words were generated by the participants. The other five were standardized, ACM-congruent words selected from the set used by Gilboa-Schechtman et al. (2000). Positive and negative words were matched on mean frequency and arousal per the Affective Norms for English Words (ANEW; Bradley and Lang, 1999). Neutral words were drawn from the ANEW word set and matched with the positive and negative words on frequency, length and ANEW arousal rating.
fMRI data acquisition
MR data were collected using a Siemens Magnetom Trio 3T scanner. A total of 128 functional images were acquired for each of the three task blocks using a gradient-echo echo-planar imaging sequence (TR 3000 ms, TE 25 ms, flip angle 90°, FOV = 256 mm). Each image consisted of 50 oblique axial slices (slice thickness 2.40 mm, in-plane voxel size: 2.13 mm × 2.13 mm) acquired parallel to the anterior and posterior commissures. Three volumes at the beginning of each task block were omitted to allow the scanner to reach steady state. Prior to the EPI sequence, a 512-slice MPRAGE structural sequence was acquired (slice thickness 0.45 mm, in-plane voxel size 0.9 mm × 0.45 mm) for registering each subject’s functional data to standard space and gradient field maps were collected for correction of geometric distortions in the EPI data caused by magnetic field inhomogeneity.
fMRI data reduction and analyses
fMRI data processing and statistical analysis were implemented primarily using the FSL analysis package (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/). After motion correction via MCFLIRT (Jenkinson et al., 2002), each time series was corrected for geometric distortions caused by magnetic field inhomogeneity. Slice-timing correction was then applied, followed by spatial smoothing using a 3D Gaussian kernel (FWHM 5 mm). Each time series was then temporally filtered with a non-linear high-pass filter (to remove drift in signal intensity) and mean-based intensity-normalized by the same single scaling factor prior to analysis.
Level 1 regression analyses were then performed for each ACM block of each participant's preprocessed functional time series data using FILM (Woolrich et al., 2001). Statistical maps were generated via multiple regression computed for each intracerebral voxel. A separate predictor [explanatory variable (EV)] was entered for congruent, incongruent and neutral trial types. Each EV was convolved with a gamma function to approximate the temporal course of the blood-oxygen-level-dependent hemodynamic response function. A per-voxel effect-size parameter estimate (β) map representing the magnitude of activation was created for each EV. Three comparisons of interest were created by contrasting the β values of the relevant parameters to examine the effect of congruence: (i) incongruent vs neutral trials, (ii) congruent vs neutral trials and (iii) incongruent vs congruent trials. Previous research (e.g. Spielberg et al., 2011) and preliminary data analysis indicated that the neural effects of congruence in the Stroop task were most evident when comparing incongruent with congruent trials (I-C contrast); this report will, therefore, focus on this contrast for both behavioral and fMRI results.
Functional activation maps for each participant were then warped into a common stereotaxic space [the 2009 Montreal Neurological Institute 152 symmetrical 1 mm × 1 mm × 1 mm template, resampled to 2 mm × 2 mm × 2 mm; Fonov et al., 2009] using FMRIB's Non-Linear Image Registration Tool (FNIRT; Andersson et al., 2007).
Level 2 analyses were performed to compare the I-C contrast in different ACMs within each participant. Two ACM contrasts were computed using a fixed-effects model: (i) negative ACM vs neutral ACM and (ii) positive ACM vs neutral ACM. Level 3 statistical analyses were carried out using FLAME (FMRIB’s local analysis of mixed effects; Woolrich et al., 2004). Means for each comparison were computed across participants. Each third-level regression analysis produced a single β map, the mean contrast level across participants. In addition to the main effect of ACM type, analyses of the moderating effect of trait affect for each contrast were conducted. Brain activation captured in each contrast from the Level 2 analysis was entered as a dependent variable in a multiple regression analysis with trait NA and trait PA scores as simultaneous predictor variables. The resulting β map for each predictor reflected the unique variance associated with that predictor. An alternative analysis with each trait affect variable (trait NA and trait PA) as the only predictor confirmed the findings reported below as still significant.
Significantly activated voxels were identified via thresholding of per-voxel, two-tailed t-tests conducted on contrast βs maps that were converted to z scores. Monte Carlo simulations via AFNI’s AlphaSim program (Ward, 2000) were used to estimate the appropriate cluster size at an overall family-wise error rate of 0.05. To limit the number of voxels under consideration, a priori regions of interest were examined using masks of frontal cortex, parietal cortex, ACC, OFC, amygdala and nucleus accumbens (NAc) created using the FSL Harvard-Oxford probabilistic atlas. For each of these masks, a cluster size threshold was computed and used only for voxels within the mask. An individual-voxel threshold z value of 2.05 was used for all masks unless otherwise specified. The minimum cluster sizes for the masks were frontal cortex = 1536 mm3, parietal cortex = 1240 mm3, ACC = 584 mm3, OFC = 1016 mm3, amygdala = 336 mm3 and NAc = 152 mm3.
RESULTS
Sample characteristics
Descriptive statistics for trait NA and trait PA measured by two subscales of the General Temperament Scale (GTS) is provided in Table 1. Values were similar to those reported for college samples (e.g. Watson et al., 1995).
Table 1.
Descriptive statistics for negative and positive temperament subscales of GTS
| Subscale | Mean (s.d.) | Min. | Max. | Skewness | Kurtosis |
|---|---|---|---|---|---|
| Negative temperament | 13.6 (6.9) | 0 | 28 | −0.18 | −0.63 |
| Positive temperament | 17.9 (7.0) | 3 | 27 | −0.65 | −0.79 |
Notes: s.d., standard deviation; GTS, General Temperament Scale. Possible minimum and maximum score is 0 and 28 for negative temperament scale, and 0 and 27 for positive temperament scale. s.d. values are in parentheses. A skewness and kurtosis value of ±1 is considered good for most psychometric uses.
Behavioral performance
Validity of ACM
Separate ACM (positive vs neutral vs negative) × time (pre vs post) analyses of variance (ANOVAs) were conducted for SAM valence and arousal ratings. For SAM valence ratings, the expected ACM × time interaction effect emerged, F(2,78) = 104.7, P < 0.001, ε = 1.0, which was further explored with paired t-tests comparing pre- and post-ratings for each ACM. Changes in pre- and post-ratings for both positive ACM, t(39) = 8.3, P < 0.001, and negative ACM, t(39) = −10.2, P < 0.001, were in the expected direction, with no change for the neutral ACM, t(39) = −0.3, P > 0.7. Thus, self-reported affect changed as intended for all three ACM conditions (Table 2).
Table 2.
SAM valence and arousal ratings pre- and post-ACMs
| Positive ACM |
Neutral ACM |
Negative ACM |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Mean change | Pre | Post | Mean change | Pre | Post | Mean change | |
| Valence | 5.1 | 2.7 | 2.4** | 4.9 | 4.9 | 0.0 | 4.7 | 6.9 | −2.3** |
| (1.7) | (1.0) | (1.4) | (1.1) | (1.3) | (1.2) | ||||
| Arousal | 5.7 | 4.9 | 0.8* | 5.7 | 6.4 | −0.7** | 5.5 | 5.9 | −0.5 |
| (1.9) | (2.1) | (1.7) | (1.7) | (1.8) | (1.9) | ||||
Notes: s.d., standard deviation. Rating scales are from 1 (positive valence or high arousal) to 9 (negative valence or low arousal). s.d. values are in parentheses.
*P < 0.05.
**P < 0.01 (two tailed).
A parallel analysis was conducted with SAM arousal ratings. An ACM × time interaction effect, F(2,78) = 5.5, P = 0.01, ε = 1.0, was explored with paired t-tests for pre- and post-ratings of each ACM. Ratings did not change as a function of the negative ACM, t(39) = −1.2, P > 0.2. Participants reported increased arousal after the positive ACM, t(39) = 2.1, P = 0.04, and decreased arousal after the neutral ACM, t(39) = −2.8, P = 0.007 (Table 2).
Separate ACM × time (pre vs post) analyses of covariance (ANCOVAs) for valence ratings were conducted with trait NA or trait PA as a covariate. The three-way interaction of Trait NA × ACM × time was not significant, F(2,76) = 1.1, P > 0.3, ε = 0.95, indicating that pre- and post-valence ratings for ACMs did not differ as a function of trait NA. The Trait PA × ACM × time interaction was also not significant, F(2,76) = 0.05, P > 0.9, ε = 0.95. Parallel analyses were conducted for arousal ratings. Pre- and post-ACM arousal ratings did not differ as a function of trait NA, F(2,76) = 0.8, P > 0.4, ε = 0.86, or trait PA, F(2,76) = 0.2, P > 0.8, ε = 0.85.
Color-word stroop task
Mean color-naming accuracy across participants was 93%. An ACM (positive vs neutral vs negative) × Congruence (incongruent vs congruent vs neutral trials) ANOVA confirmed the expected main effect of Congruence on accuracy, with an average of 3.6 more errors (out of 192 trials) on incongruent trials than on congruent trials, F(2,78) = 6.4, P = 0.004, ε = 0.9. ACM did not affect number of errors (P > 0.5) or interact with Congruence (P > 0.2).
A parallel ANOVA analysis on RT also showed the expected main effect of Congruence, F(2,78) = 81.4, P < 0.001, ε = 0.85. RT for incongruent trials was slower than for neutral trials, F(1,39) = 42.1, P < 0.001, and RT for neutral trials was slower than for congruent trials, F(1,39) = 59.6, P < 0.001. A marginal main effect of ACM on RT, F(2,78) = 2.8, P = 0.06, ε = 1.00, revealed a slightly longer RT overall in negative ACM than neutral ACM, F(1,39) = 4.2, P = 0.046. However, there was no ACM × Congruence interaction, F(4,156) = 0.48, P > 0.7, ε = 0.89. Thus, ACM condition alone did not influence interference.1,2
ACM and trait affect
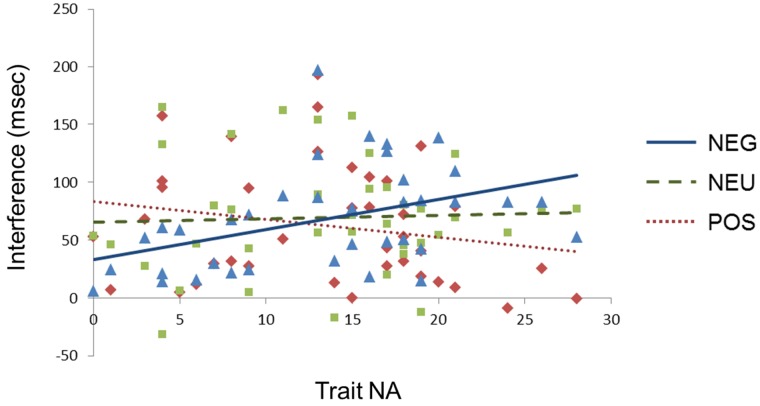
To investigate the interactive effect of ACM and trait affect on RT, separate ACM × Congruence ANCOVAs for RT were conducted with trait NA or trait PA as a covariate. With trait NA as a covariate, the Congruence effect emerged again and will not be considered further. An ACM × Congruence × Trait NA interaction, F(4,152) = 2.7, P = 0.036, ε = 0.95, was explored with contrasts that revealed slower RT for incongruent vs congruent trials (more interference) after negative ACM than after neutral ACM as a function of increasing trait NA, F(1,38) = 4.5, P = 0.04 (Figure 1).
Fig. 1.
Stroop interference in ACMs as a function of Trait NA (plotted over the observed range of trait NA on the x axis). Stroop interference = RTs of incongruent trials – RTs of congruent trials. NEG, negative ACM; NEU, neutral ACM; POS, positive ACM. Each color markers indicate the data points for the following ACMs: blue, negative ACM; green, neutral ACM; red, positive ACM.
Regression analyses were conducted separately for each ACM, with trait NA predicting interference. Trait NA predicted interference after the negative ACM, R2 = 0.179, t(38) = 2.9, P = 0.007, but not after positive ACM, R2 = 0.028, t(38) = −1.1, P > 0.2 or neutral ACM, R2 = 0.001, t(38) = −0.14, P > 0.8. With trait PA as the covariate, no effects involving Congruence emerged, F(4,152) = 0.30, P > 0.8, ε = 0.9.
Separate ACM × Congruence ANCOVAs for accuracy were conducted with trait NA or trait PA as a covariate. There was no modulation by either trait NA, F(4,152) = 0.36, P = > 0.8, ε = 1.0, or trait PA, F(4,152) = 0.63, P = > 0.6, ε = 1.0.
fMRI Results
Interference effect as a function of ACM conditions
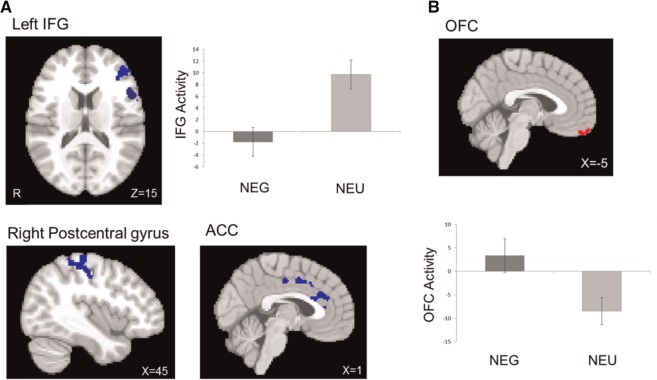
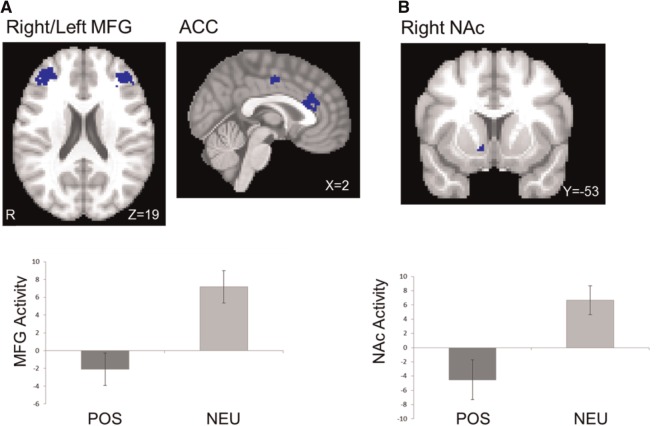
Table 3 lists brain regions that were differentially activated during incongruent vs congruent trials (I-C contrast) in the negative and positive ACM conditions (in contrast to neutral) and moderated by trait affect. The I-C contrast in the negative ACM showed decreased brain activation in left inferior and middle frontal gyrus, dACC and post-central gyrus and increased brain activation in OFC (Figure 2). The I-C contrast in the positive ACM showed decreased brain activation in left and right middle frontal gyrus, dACC and NAc (Figure 3).
Table 3.
Summary of significant activation clusters
| Condition | Region | Cluster size (mm3) | Mean z value | Location |
|||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Negative ACM | Main effect | L IFG | 220 | −2.36 | −59 | 20 | 15 |
| L Middle frontal gyrus | 291 | −2.54 | −32 | 34 | 41 | ||
| dACC | 194 | −2.44 | 0 | 29 | 21 | ||
| Posterior cingulate cortex | 239 | −2.48 | 1 | 0 | 42 | ||
| R Postcentral gyrus | 602 | −2.44 | 45 | −22 | 51 | ||
| OFC | 155 | 2.42 | −5 | 56 | −25 | ||
| Moderated by Trait NA | L IFG | 355 | −2.49 | −47 | 41 | 11 | |
| L Superior frontal gyrus | 225 | −2.43 | −6 | 2 | 73 | ||
| L Supramarginal gyrus | 466 | −2.46 | −61 | −22 | 33 | ||
| L Postcentral gyrus | 213 | −2.71 | −48 | −21 | 60 | ||
| Moderated by Trait PA | L IFG | 716 | −2.49 | −53 | 22 | 5 | |
| R Supramarginal gyrus | 123 | 2.55 | 51 | −29 | 39 | ||
| OFC | 131 | −2.48 | −27 | 15 | −20 | ||
| R Amygdala | 69 | −2.51 | 25 | −4 | −16 | ||
| Positive ACM | Main effect | L Middle frontal gyrus | 321 | −2.70 | −46 | 44 | 21 |
| R Middle frontal gyrus | 466 | −2.51 | 31 | 47 | 19 | ||
| dACC | 304 | −2.50 | 2 | 28 | 21 | ||
| Posterior cingulate cortex | 88 | −2.48 | −1 | −8 | 44 | ||
| NAca | 228 | −2.35 | 3 | −53 | 49 | ||
| Moderated by Trait NA | L Inferior/Middle frontal gyrus | 1082 | −2.43 | −46 | 34 | 13 | |
| R Postcentral gyrus | 272 | −2.47 | 50 | −25 | 55 | ||
Notes: L, left; R, right; Location, coordinates are for the maximum z-stat in MNI152 2009a symmetrical space. Brain activation during incongruent vs congruent trials (I-C contrast) in negative ACM and positive ACM was each contrasted by neutral ACM. An individual-voxel threshold z value of 2.05 was used for all masks except for NAc.
aNAc was thresholded with z = 1.96.
Fig. 2.
Brain activation during the I-C contrast in negative ACM (in contrast to neutral ACM). (A) Decreased activation in dorsal areas (negative ACM < neutral ACM, in blue) and (B) increased activation in ventral area (negative ACM > neutral ACM, in red). ACC, anterior cingulate cortex. Bars refer to 1 standard error of the mean.
Fig. 3.
Brain activation during the I-C contrast in positive ACM (in contrast to neutral ACM). (A) Decreased activation in dorsal areas (positive ACM < neutral ACM, in blue) and (B) decreased activity in ventral area (positive ACM < neutral ACM, in blue). MFG, middle frontal gyrus; ACC, anterior cingulate cortex. Bars refer to 1 standard error of the mean.
The interactive effects of ACM and trait affect
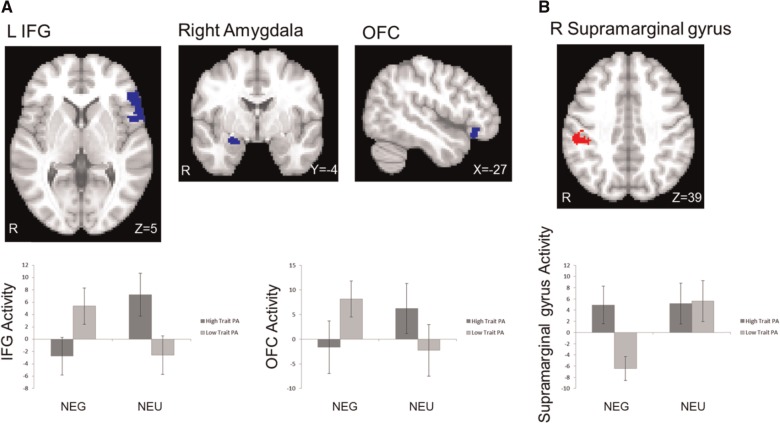
Analyses were conducted to determine whether and how the interference effect (I-C contrast) in the negative and positive ACM conditions (in contrast to neutral) was moderated by either trait NA or trait PA. In the negative ACM, high levels of trait NA were associated with less activation in left IFG and left supramarginal gyrus (Figure 4). Trait PA also moderated brain activation during the negative ACM. High trait PA was associated with less activation in left IFG. High trait PA was also associated with more activation in right supramarginal gyrus and less activation in OFC and amygdala in the negative ACM (Figure 5). In the positive ACM, high levels of trait NA were again associated with decreased activation in left IFG and right post-central gyrus. Trait PA had no moderating effect in the positive ACM.
Fig. 4.
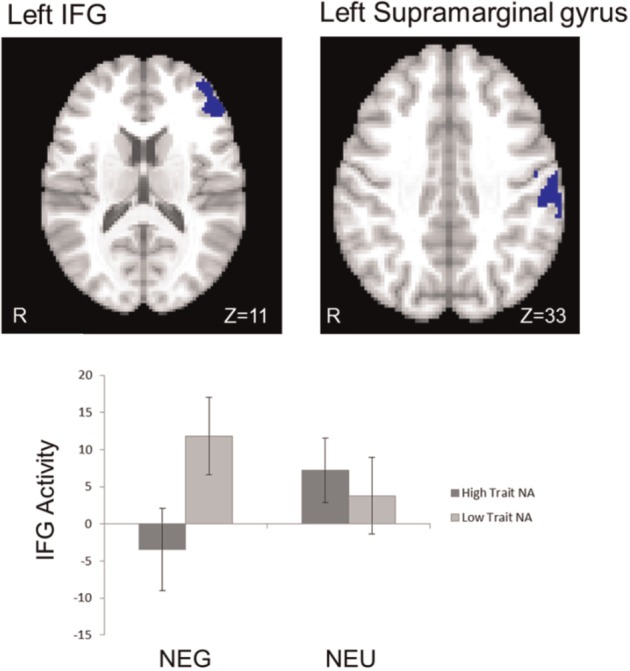
Interaction of negative ACM and Trait NA. High trait NA was associated with lower activation in dorsal regions (IFG, supramarginal gyrus) during the I-C contrast in the negative ACM (in contrast to neutral ACM) (in blue). NEG, negative ACM; NEU, neutral ACM. Bars refer to 1 standard error of the mean.
Fig. 5.
Interaction of negative ACM and Trait PA. (A) Trait PA was associated with lower activation in left IFG and ventral regions (amygdala and OFC) during the I-C contrast in the negative ACM (in contrast to neutral ACM) (in blue). (B) Trait PA was associated with greater activation in right supramarginal gyrus during the I-C contrast in the negative ACM (in red). NEG, negative ACM; NEU, neutral ACM. Bars refer to 1 standard error of the mean.
Correlations between brain activation and behavioral performance
A priori directional hypotheses were tested using one-tailed tests. Brain-behavior correlations emerged only for the accuracy measure. Specifically, in the negative ACM (in contrast to the neutral ACM), greater activation in IFG was associated with more accurate responses, r = 0.283, P = 0.04, one-tailed (Figure 6). Trait NA further moderated the effect. The higher the trait NA score, the stronger the relationship between accuracy and IFG activation (Table 4). On the other hand, OFC activation was negatively correlated with accuracy. In the negative ACM (in contrast to the neutral ACM), greater activation in OFC was associated with less accurate responses, r = .−0.339, P = 0.02, one tailed (Figure 6). None of the clusters was significantly correlated with RT.
Fig. 6.
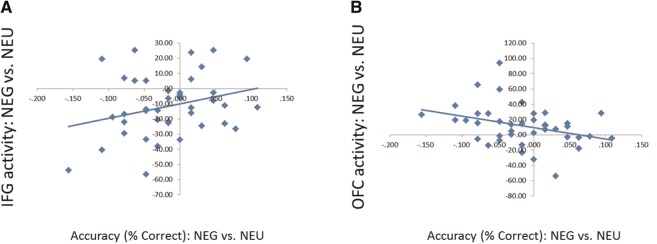
Correlation between brain activation and accuracy in negative ACM vs neutral ACM. During the negative ACM (in contrast to neutral ACM) (A) more accurate responses were associated with increased IFG activation and (B) less accurate responses were associated with increased OFC activation.
Table 4.
Interaction of IFG activation and trait NA predicting the accuracy level
| Variable | DV = accuracy level in NEG vs NEU |
||
|---|---|---|---|
| Betaa | P | ΔR2 | |
| Entered separately | |||
| Trait NA | −0.08 | 0.60 | 0.01 |
| IFG activity in NEG vs NEU | 0.18 | 0.28 | 0.03 |
| Entered simultaneously | |||
| Step 1 | |||
| Trait NA | −0.04 | 0.82 | 0.03 |
| IFG activation in NEG vs NEU | 0.17 | 0.33 | |
| Step 2 | |||
| Interaction (Trait NA × IFG activation) | 1.11 | 0.004** | 0.23 |
Notes: NEG, negative ACM; NEU, neutral ACM.
aStandardized betas.
*P < 0.05.
**P < 0.01.
DISCUSSION
This study elucidates circumstances under which cognitive control is impaired by or protected from the influence of emotion and identifies neural mechanisms that play a role in implementing these relationships. The findings demonstrate an interactive effect of state and trait affect on cognitive control at both behavioral and neural levels. Behaviorally, although there was no main effect of state affect, Stroop interference in the presence of state NA was increased by trait NA, showing that only the combination of state and trait NA sufficed to influence performance.
Consistent with behavioral results, neuroimaging data revealed an interactive effect of state and trait affect. Activity in primarily top-down control regions (IFG, dACC) was suppressed in the presence of emotional arousal (both state NA and state PA) during the cognitive task, which is consistent with evidence from relevant work on emotion–cognition interactions (e.g. Drevets and Raichle, 1998; Dolcos et al., 2011). Importantly, this effect appears to be exacerbated or offset by co-occurring activity in other top-down control regions (parietal) and emotion processing regions (e.g. OFC, amygdala and NAc) as a function of valence and state vs trait affect.
Specifically, state NA was associated with less activation in top-down control regions and more activation in an emotion processing region (OFC), which in turn was associated with impaired performance as the need for top-down control increased (incongruent vs congruent condition). These findings suggest that the emotional processing associated with the state NA condition was accompanied by a reduction in the top-down effortful control required for optimal task performance and a greater emphasis or prioritization of emotion processing. Trait NA further moderated activity in top-down control regions during the state NA condition. The higher the trait NA, the lower the activity in these regions as task demands increased, which was also manifested behaviorally; as trait NA increased, the relationship between impaired performance and suppressed IFG activity was stronger. Results are supported by findings that high trait anxiety (a construct related to trait NA) was associated with deficient recruitment of DLPFC in a response-conflict task in the absence of threat-related stimuli (Bishop, 2009). Present results further suggest that trait NA can exacerbate a pattern in which task-irrelevant negative emotion processing (here, induced by state NA) interrupts top-down attentional control.
Cognitive theories of emotional disorders often posit a vulnerability-stress model in which a pre-existing psychological vulnerability needs to be primed or activated by a precipitating stressor (e.g. a negative life event or environment factors) to exert effects on information processing (McCabe et al., 2000). According to cognitive reactivity theory, people with negative cognitive schema show increased reactivity to stressors (Ingram et al., 1998). This increased reactivity results in disruptive effects on cognitive function with persistent negative thinking and ultimately, increased risk for depression or anxiety disorders (Scher et al., 2005). Consistent with this formulation, elevated trait NA in the present paradigm allowed the presence of a negative affective context, a prototypical stressor, to activate a vulnerability to disrupted cognitive control. Inconsistent results in previous literature with regard to the effects of state NA on cognitive control can be resolved by the present findings, as they indicate that simply inducing or manipulating state NA in non-clinical populations may not be sufficient to disrupt cognitive control. To summarize, the likelihood of observing impaired cognitive control in negative contexts appears to depend on a pre-existing vulnerability conferred by high trait NA.
In this study, the combination of state NA and trait PA differed from state NA and trait NA in the observed pattern of interaction between dorsal and ventral systems during the task. Specifically, under state NA, the higher the trait PA, the lower the activity in IFG as task demands increased, a pattern similar to that described for trait NA. In this case, however, suppressed activity in IFG was offset by increased activity in parietal regions associated with attentional control (supramarginal gyrus) and decreased activity in emotion processing regions (OFC and amygdala). This suggests that, when coincident with state NA, trait PA has a protective effect for cognitive control, preserving activity in regions associated with a dorsal executive system and suppressing activity in regions associated with a ventral affective system.
Trait PA has been associated with enhanced ability to regulate negative emotions and the use of adaptive emotion regulation strategies, such as reappraisal (Nes and Segerstrom, 2006). During active reappraisal, increased activity of top-down control regions and decreased activation of emotional processing regions such as amygdala and OFC was observed (Ochsner et al., 2002; Banks et al., 2007). As suggested by the cascade-of-control model and studies examining temporal relationships between top-down control regions (Milham et al., 2002; Banich, 2009; Silton et al., 2011), increased activity in parietal regions may indicate a compensatory response to decreased activity in frontal control regions, maintaining attention while suppressing emotional processing (Peterson et al., 1999).
Similar to state NA, state PA was associated with less activity in top-down control regions (IFG and dACC) but was accompanied by less activity in emotion processing regions (NAc), which suggests that differential cognitive control mechanisms are involved depending on the valence of the to-be-ignored emotional information. As suggested for trait PA, state PA may also provide a protective mechanism for maintenance of cognitive control. Any detrimental effect of a disturbance in top-down control regions (IFG and dACC) elicited by the salience of affective information irrelevant to the task may be offset by the accompanying decreased activity in the ventral system (here, NAc).
By employing an experimental design capable of dissociating the influence of state and trait affect on cognitive control, this study demonstrates that a more dynamic interaction between dorsal and ventral systems is involved as a function of state and trait affect than the dichotomous relationships suggested by existing literature (i.e. deactivation in dorsal regions and activation in ventral regions). Specifically, findings suggest that different emotional states elicit differential patterns of neural activity and performance as task demands increase as a function of different levels of trait affect. For example, an intensified imbalance in top-down and stimulus-driven attentional control regions was observed when state NA and trait NA were combined. On the other hand, the imbalance between dorsal and ventral systems under state NA was offset by boosting activity in another executive area (supramarginal gyrus) and suppressing activity in affective regions (OFC, amygdala) when combined with trait PA.
This study has some limitations. There are many other components of executive function than those probed by the color-word Stroop task. With few exceptions, most have not yet been systematically examined in affective contexts while individual differences in trait affect are considered. More work is needed to understand the relationship between everyday affective states and different components of executive function, as well as how trait affect moderates this relationship.
Present findings may have implications for the clinical literature. Negative affective vulnerability (trait) and negative affective context (state) in interaction, not in isolation, were associated with impairment of cognitive control. Thus, a negative context may act as a catalyst in individuals with high trait NA to exert an impairing influence on top-down attentional control. It is conceivable that disrupted cognitive control serves to promote or maintain depression and anxiety. Further investigation of trait NA and its role in stress responses in clinical populations is needed to explicate whether and how the relationship between state and trait NA and its effects on cognitive control contribute to the onset and maintenance of emotional disorders.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by the National Institute of Mental Health (P50 MH079485, R01 MH61358, T32 MH19554). The authors thank Katherine Mimnaugh and Marie T. Banich for their contributions to this project.
Footnotes
1 For behavioral data analysis, ANOVA was conducted with ACM (positive, neutral and negative) and Congruence (congruent, incongruent and neutral) as within-subject independent variables and accuracy (or RT) as the dependent variable. Mauchly’s test indicated that the assumption of sphericity had been violated in effects for Word type, χ2 = 11.1, P = 0.004, and Word type × ACM, χ2 = 21.8, P = 0.01. Therefore, degrees of freedom were corrected using Huynh–Feldt estimates of sphericity for all analyses.
2 There were no interactions of ACM order with any combination of emotion variables for RT, F(4,152) = 0.38, P > 0.8 or accuracy, F(4,152) = 0.38, P > 0.3. Therefore, ACM order was not considered in the analyses.
REFERENCES
- Andersson JL, Jenkinson M, Smith S. FMRIB Technical Report TR07JA2. FMRIB Analysis Group of the University of Oxford; 2007. Non-linear registration, aka spatial normalisation. [Google Scholar]
- Ashby FG, Isen AM, Turken U. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT. Executive function the search for an integrated account. Current Directions in Psychological Science. 2009;18:89–94. [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer A, Miller GA, Heller W. Cognitive control mechanisms, emotion, & memory: a neural perspective with implications for psychopathology. Neuroscience and Biobehavioral Reviews. 2009;33:613–30. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:92–8. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ. Intensely pleasurable responses to music correlate with activity in brain regions implicated in reward and emotion. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:11818–23. doi: 10.1073/pnas.191355898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of behavior therapy and experimental psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Technical Report C-1. The Center for Research in Psychophysiology, University of Florida; 1999. Affective norms for English words (ANEW): instruction manual and affective ratings. [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial Vision. 1997;10:433–6. [PubMed] [Google Scholar]
- Canli T. Toward a neurogenetic theory of neuroticism. Annals of the New York Academy of Sciences. 2008;1129:153–74. doi: 10.1196/annals.1417.022. [DOI] [PubMed] [Google Scholar]
- Chartier GM, Ranieri DJ. Comparison of two mood induction procedures. Cognitive Therapy and Research. 1989;13:275–82. [Google Scholar]
- Chepenik LG, Cornew LA, Farah MJ. The influence of sad mood on cognition. Emotion. 2007;7:802–11. doi: 10.1037/1528-3542.7.4.802. [DOI] [PubMed] [Google Scholar]
- Crocker LD, Heller W, Spielberg JM, et al. Neural mechanisms of attentional control differentiate trait and state negative affect. Frontiers in Psychology. 2012;3:298. doi: 10.3389/fpsyg.2012.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Grabowski TJ, Bechara A, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–56. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends in Cognitive Sciences. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Pizzagalli D, Nitschke JB, Putnam K. Depression: perspectives from affective neuroscience. Annual Review of Psychology. 2002;53:545–74. doi: 10.1146/annurev.psych.53.100901.135148. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Anxiety, processing efficiency, and cognitive performance: new developments from attentional control theory. European Psychologist. 2009;14:168–76. [Google Scholar]
- Dolcos F, Iordan AD, Dolcos S. Neural correlates of emotion–cognition interactions: a review of evidence from brain imaging investigations. Journal of Cognitive Psychology. 2011;23:669–94. doi: 10.1080/20445911.2011.594433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2004;30:343. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Raichle ME. Reciprocal suppression of regional cerebral blood flow during emotional versus higher cognitive processes: implications for interactions between emotion and cognition. Cognition and Emotion. 1998;12:353–85. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44:1043–55. doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Fales CL, Barch DM, Rundle MM, et al. Altered emotional interference processing in affective and cognitive-control brain circuitry in major depression. Biological Psychiatry. 2008;63:377–84. doi: 10.1016/j.biopsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonov VS, Evans AC, McKinstry RC, Almli CR, Collins DL. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47:S102. [Google Scholar]
- Gilboa-Schechtman E, Revelle W, Gotlib IH. Stroop interference following mood induction: emotionality, mood congruence, and concern relevance. Cognitive Therapy and Research. 2000;24:491–502. [Google Scholar]
- Herrington JD, Heller W, Mohanty A, et al. Localization of asymmetric brain function in emotion and depression. Psychophysiology. 2010;47:442–54. doi: 10.1111/j.1469-8986.2009.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrington JD, Mohanty A, Koven NS, et al. Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion. 2005;5:200–7. doi: 10.1037/1528-3542.5.2.200. [DOI] [PubMed] [Google Scholar]
- Ingram RE, Miranda J, Segal ZV. Cognitive Vulnerability to Depression. New York: Guilford Press; 1998. [Google Scholar]
- Isen AM. A role for neuropsychology in understanding the facilitating influence of positive affect on social behavior and cognitive processes. In: Lopez SJ, Snyder CR, editors. Oxford Handbook of Positive Psychology. 2nd edn. New York: Oxford University Press; 2009. pp. 503–18. [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–41. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- McCabe SB, Gotlib IH, Martin RA. Cognitive vulnerability for depression: deployment of attention as a function of history of depression and current mood state. Cognitive Therapy and Research. 2000;24:427–44. [Google Scholar]
- Milham MP, Erickson KI, Banich MT, et al. Attentional control in the aging brain: insights from an fMRI study of the stroop task. Brain and Cognition. 2002;49:277–96. doi: 10.1006/brcg.2001.1501. [DOI] [PubMed] [Google Scholar]
- Miller GA, Levin DN, Kozak MJ, Cook EW, III, McLean A, Jr, Lang PJ. Individual differences in imagery and the psychophysiology of emotion. Cognition and Emotion. 1987;1:367–90. [Google Scholar]
- Nes LS, Segerstrom SC. Dispositional optimism and coping: a meta-analytic review. Personality and Social Psychology Review. 2006;10:235–51. doi: 10.1207/s15327957pspr1003_3. [DOI] [PubMed] [Google Scholar]
- Öhman A, Lundqvist D, Esteves F. The face in the crowd revisited: a threat advantage with schematic stimuli. Journal of Personality and Social Psychology. 2001;80:381–96. doi: 10.1037/0022-3514.80.3.381. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Pessoa L. How do emotion and motivation direct executive control? Trends in cognitive sciences. 2009;13:160–6. doi: 10.1016/j.tics.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45:1237–58. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Rowe G, Hirsh JB, Anderson AK. Positive affect increases the breadth of attentional selection. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:383–8. doi: 10.1073/pnas.0605198104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diathesis in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Silton RL, Heller W, Engels AS, et al. Depression and anxious apprehension distinguish frontocingulate cortical activity during top-down attentional control. Journal of Abnormal Psychology. 2011;120:272. doi: 10.1037/a0023204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silton RL, Heller W, Towers DN, et al. The time course of activity in dorsolateral prefrontal cortex and anterior cingulate cortex during top-down attentional control. Neuroimage. 2010;50:1292–302. doi: 10.1016/j.neuroimage.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR. Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: a meta-analysis and review. Psychological Bulletin. 2013;139:81. doi: 10.1037/a0028727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg JM, Miller GA, Engels AS, et al. Trait approach and avoidance motivation: lateralized neural activity associated with executive function. Neuroimage. 2011;54:661–70. doi: 10.1016/j.neuroimage.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. 2000. Simultaneous inference for fMRI data. Available: http://afni.nimh.nih.gov/pub/dist/doc/manual. AlphaSim. pdf; accessed July 27, 2006. [Google Scholar]
- Watson D, Clark LA. Behavioral disinhibition versus constraint: a dispositional perspective. In: Wegner DM, Pennebaker JW, editors. Handbook of Mental Control. New York: Prentice Hall; 1993. pp. 506–27. [Google Scholar]
- Watson D, Clark LA. Extraversion and its positive emotional core. In: Hogan R, Johnson J, Briggs S, editors. Handbook of Personality Psychology. San Diego: Academic Press; 1997. pp. 767–93. [Google Scholar]
- Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. Journal of Abnormal Psychology. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. Neuroimage. 2004;21:1732–47. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady M, Smith SM. Temporal autocorrelation in univariate linear modeling of fMRI data. Neuroimage. 2001;14:1370–86. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]