Abstract
Adolescence is the time of peak onset for many anxiety disorders, particularly Social Anxiety Disorder. Research using simulated social interactions consistently finds differential activation in several brain regions in anxious (vs non-anxious) youth, including amygdala, striatum and medial prefrontal cortex. However, few studies examined the anticipation of peer interactions, a key component in the etiology and maintenance of anxiety disorders. Youth completed the Chatroom Task while undergoing functional magnetic resonance imaging. Patterns of neural activation were assessed in anxious and non-anxious youth as they were cued to anticipate social feedback from peers. Anxious participants evidenced greater amygdala activation and rostral anterior cingulate (rACC)↔amygdala coupling than non-anxious participants during anticipation of feedback from peers they had previously rejected; anxious participants also evidenced less nucleus accumbens activation during anticipation of feedback from selected peers. Finally, anxiety interacted with age in rACC: in anxious participants, age was positively associated with activation to anticipated feedback from rejected peers and negatively for selected peers, whereas the opposite pattern emerged for non-anxious youth. Overall, anxious youth showed greater reactivity in anticipation of feedback from rejected peers and thus may ascribe greater salience to these potential interactions and increase the likelihood of avoidance behavior.
Keywords: anxiety, adolescence, anticipation, amygdala, nucleus accumbens
INTRODUCTION
Adolescence is a period of rapid development in the neural circuits involved in motivation, emotion and social interaction (Steinberg and Morris, 2001; Dahl, 2004). This maturational window is also the time of peak onset for many anxiety disorders, particularly Social Anxiety Disorder (Pine et al., 1998; Kessler et al., 2005; Rapee et al., 2009). Delineating the manner in which neural engagement to social events differs in anxious and non-anxious adolescents may inform how we understand processes that maintain adolescent anxiety. This study compares responses to anticipating social events in anxious and non-anxious adolescents.
Several recent studies have documented anxiety- and mood-related correlates using novel neuroimaging paradigms that simulate social interactions (Jarcho et al., 2013). Although findings vary across studies, activation in amygdala, striatum and medial prefrontal cortex (mPFC) consistently differentiate anxious/depressed from healthy individuals (Guyer et al., 2008; Gunther Moor et al., 2010; Davey et al., 2011; Masten et al., 2011; Lau et al., 2012; Silk et al., 2014; Somerville et al., 2013; Guyer et al., 2014). These regions are thought to function interactively to ascribe salience, generate predictions and create flexible patterns of behavior within a motivated social context (Phelps et al., 2004; Schiller et al., 2008; Haber and Knutson, 2010; Pessoa and Adolphs, 2010).
The complexity of the social interactions modeled in prior studies creates a challenge when trying to interpret past findings. This complexity has led some researchers to parse social interchange into smaller components, including the evaluation/selection of potential peers (Guyer et al., 2009; Somerville et al., 2013), receipt of peer feedback (Guyer et al., 2012; Somerville et al., 2013), learning from outcomes (Jarcho et al., unpublished data) and anticipation of interaction with peers (Guyer et al., 2014). Although the first three components have received increasing attention, the anticipation phase has been relatively neglected. This is surprising given research suggesting that the anticipation of unknown, salient outcomes is a key component in the etiology and maintenance of a number of anxiety disorders (Grupe and Nitschke, 2013). Grupe and Nitschke (2013) have argued that many features of anxiety manifest during anticipation of unpredictable outcomes, as opposed to the experience of fear, wherein the threat is clear and present (Barlow, 2004).
In a social context, events that provoke anticipatory anxiety occur in the period after a decision has been made to socially engage but prior to knowing the consequences of that engagement. For example, anticipatory anxiety may emerge after asking someone out on a date or inviting friends to a party. These are typically highly provocative situations for individuals with social anxiety, and many regions previously implicated in social anxiety and simulated social tasks (e.g. amygdala, striatum and mPFC) are thought to play an important role in the experience of anxiety during such anticipatory periods (Grupe and Nitschke, 2013).
It is likely that anticipation in social contexts is particularly provocative for adolescents, because reactivity to many motivationally relevant stimuli peaks during adolescence (Galvan, 2010; Crone and Dahl, 2012), particularly those related to peer acceptance (Steinberg and Morris, 2001; Nelson et al., 2005). Furthermore, the salience of anticipating peer interaction may be amplified during adolescence, because (i) subcortical structures (e.g. amygdala and striatum) are particularly responsive to social stimuli during this time (Casey et al., 2008; Guyer et al., 2008; Galvan, 2010) and (ii) functional connections with medial prefrontal regions, which contextualize and inhibit emotional responding, are immature (Ernst et al., 2006). Thus, divergent patterns of maturation in amygdala, striatum and medial prefrontal circuits may promote the development of anxiety in adolescence and predict continued risk of chronic anxiety in adulthood.
This study examined developmental trajectories in this circuitry in anxious and non-anxious youth while they completed the Chatroom Task, an established paradigm modified here to isolate anticipatory anxiety to impending social feedback. Specifically, we compared brain activation during the anticipation of social feedback from peers previously selected by the participant as socially desirable (selected) to those deemed not desirable (rejected). Given that coupling between mPFC and amygdala appears to be a key link in networks involved in motivation and emotion (Etkin et al., 2011; Kim et al., 2011; Zorrilla and Koob, 2013), we also examined condition-dependent connectivity between these regions.
It is crucial to examine trajectories of developmental maturation, because emotional and social mechanisms undergo tremendous change during adolescence (Blakemore, 2008). Indeed, both the importance of social concerns and the intensity of emotional responses increase across development, reach a peak during adolescence and decrease by the mid-20 s (Blakemore and Robbins, 2012; Crone and Dahl, 2012). Moreover, understanding the developmental course of anxiety during adolescence can provide greater insight into the mechanisms that promote and maintain these processes (Dahl, 2004). As a first step toward understanding the interplay between anxiety and maturation, this study tested whether anxiety is related to differential patterns of neural activation to social stimuli across age.
Based on previous findings, we predicted two specific differences would emerge in motivational brain networks in this study. Among non-anxious adolescents, we believed that anticipation of feedback from previously selected peers was likely to be more salient and motivationally engaging than anticipation for previously rejected peers. However, among anxious adolescents, who tend to focus more on pending negative outcomes (Pine et al., 2009), feedback from previously rejected individuals is likely to be more salient and anticipation of rejected peers is likely to generate greater activity in motivational networks. Therefore, our primary hypothesis was that a direct comparison of anxious and non-anxious groups would reveal greater activity in amygdala and ventral striatum among non-anxious adolescents when anticipating feedback from selected relative to rejected peers, whereas anxious adolescents would demonstrate the reverse pattern.
On the basis of findings of prefrontal regulation of subcortical circuitry (Sotres-Bayon and Quirk, 2010) and recent work indicating important developmental changes in this relationship during adolescence (Gee et al., 2013a,b), we hypothesized that non-anxious youth would demonstrate stronger negative mPFC↔amygdala coupling when anticipating feedback from rejected (vs selected) peers, reflecting greater inhibitory control. We also hypothesized that this pattern would be attenuated in anxious participants. Finally, we predicted that the pattern of stronger negative mPFC↔amygdala coupling for rejected peers would increase with age in healthy but not anxious adolescents. This pattern might reflect impairment of normative developmental increases in the engagement of regulatory mechanisms in anxious adolescents.
Although this study examined the entire mPFC, particular sub-regions may exhibit unique response profiles. Specifically, ventral portions of mPFC appear to be particularly important for the regulation (vs appraisal) of emotion (Etkin et al., 2011). Within ventral mPFC, rostral anterior cingulate (rACC), including genual/subgenual cingulate, has high levels of connectivity with amygdala (Beckmann et al., 2009). Therefore, we expected this region to be the most likely to exhibit the hypothesized relationships with amygdala.
METHOD
Participants
The sample consisted of 42 participants (52% female, age mean = 13.3, SD = 2.8, range = 8–17) (see Supplementary Material for recruitment/inclusion details, including discussion of the age range). The anxiety group consisted of 16 participants (63% female, age mean = 12.7, SD = 3.3, range = 8–17) who met criteria for Social Phobia (n = 13) and/or Generalized Anxiety Disorder (GAD; n = 9). Comorbidities were Specific Phobia (n = 6), Separation Anxiety Disorder (n = 5), Attention Deficit Hyperactivity Disorder (n = 2) and Major Depressive Disorder (n = 1). Diagnoses were made with the Kiddie Schedule for Affective Disorders, administered by a clinician (reliability κ > 0.70) and diagnoses were confirmed by a clinical interview with a senior psychiatrist. The three participants without Social Phobia diagnoses expressed clinically significant fear of social situations during diagnostic interviews and on the Fear of Negative Evaluation (FNE) scale (Watson and Friend, 1969). Specifically, the FNE distributions of the GAD (range = 20–30) and non-anxious (range = 0-19) groups did not overlap. Despite the tiny sample size of the GAD group, these groups significantly differed on mean FNE (t2.4 = 4.9, P = 0.03), whereas the two anxiety groups did not (t5 = 0.9, P = 0.40). We opted to maintain a single anxiety group, because of the similarity of symptom profiles. The healthy group consisted of 26 participants (46% female, age mean = 13.7, SD = 2.5, range = 9–17) who did not meet criteria for any psychiatric disorder. Groups did not differ in age or gender (P-values > 0.10).
Neuroimaging task
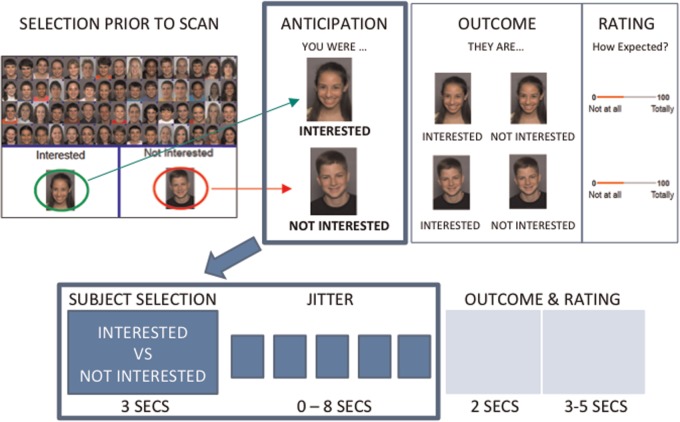
Participants performed a variant of the Chatroom Task (Guyer et al., 2012, 2014) across two visits. Participants were told that the study was designed to learn about internet-based social interactions and were shown photographs of 60 age-matched individuals whom they were led to believe were other participants in the study. On the initial visit, participants were asked to sort the photographs into 30 peers with whom they wanted to chat on line (selected) and 30 peers with whom they did not want to chat (rejected) (Figure 1). Participants were told that, although personal information (e.g. likes and dislikes) was collected on all participants, technical factors prevented this information from being displayed prior to selection. They were therefore asked to sort peers ‘based upon looks alone’; this was done to minimize confounds (see Supplementary Material for detail). Separate stimulus sets were used to ensure that participants evaluated similarly aged peers (see Supplementary Material for detail). Participants then had their own photograph taken and were told that both the photograph and their personal information would be shared with other participants in the study who would rate them in a similar manner.
Fig. 1.
Overview of the chatroom task. The paradigm was completed in two visits. During the initial visit (top left), participants were asked to decide which peers they were interested in chatting with online (selected) and which they were not interested in (rejected). Approximately 2 weeks later, participants returned for a scanning session. Typical trials for each condition are presented (top right, bottom). During this scan, participants were shown a picture of a peer and reminded of whether they had selected (‘INTERESTED’) or rejected (‘NOT INTERESTED’) that peer, after which there was a period of 0–8 seconds in which a fixation cross was presented (jitter). This is the anticipation period analyzed in this study. After the anticipation period, participants were informed of whether the peer had selected or rejected the participant, after which they rated how expected/unexpected this rating (of the participant by the peer) was (not analyzed here).
On a subsequent visit, ∼2 weeks later, participants were brought back to the lab to undergo two runs of functional magnetic resonance imaging. In the first functional run, participants were shown all previously sorted photographs and asked to guess how each depicted individual had rated them. During the second run, all trials consisted of two events: anticipation and feedback. Each trial started with a ‘cued’ anticipation event (3 s) in which a peer photograph was displayed (cue) and the participant was reminded of how they initially sorted the peer. This was followed by feedback in which the words ‘interested’ or ‘not interested’ appeared below the photograph to indicate whether the depicted peer was interested in chatting with the participant. After feedback, participants were asked to rate the degree to which the feedback was expected. To facilitate dissociation of anticipation from feedback events, they were separated by a variable duration jitter (0–8000 ms) during which a fixation cross was displayed. This study focused only on the cue portion of the task (see Supplementary Material for more detail).
After completing the scanning session, participants underwent a structured one-on-one debriefing in which the actual study procedures were revealed and participants were asked whether they had been deceived. A key consideration is the potential for age-related differences to manifest as differential motivation to engage with the task. Importantly, all participants reported that they found the task to be interesting and engaging and they attended/responded on all trials.
Functional magnetic resonance imaging data processing
See Supplementary Material for detail regarding data acquisition. Image processing and analysis were implemented using FSL (Jenkinson et al., 2012). Data were motion-corrected, temporally high-pass filtered (0.0125 Hz), spatially smoothed (full-width, half-max = 5 mm), slice-timing corrected and intensity-normalized (across participants).
Regression analyses were performed on the processed functional time series of each participant using FILM. Six task predictors were included, along with a variable number of nuisance predictors modeling outlying timepoints (via the fsl_motion_outliers tool). Two task predictors modeled the anticipation period (starting with cue onset and ending when feedback was presented), corresponding to peers selected or rejected by the participant. Four task predictors modeled the feedback period, corresponding to the participant selection/rejection factor crossed with the feedback factor (i.e. whether the peer selected/did not select the participant). Findings from the feedback period are reported elsewhere (Jarcho et al., unpublished data). For each predictor, the vector of assigned weights was convolved with a double gamma function.
To create the anticipation comparison of interest, β-values for the selected anticipation condition were contrasted against β-values for the rejected anticipation condition. β-maps were non-linearly warped into MNI152 space via FNIRT with BBR.
Group inferential analyses were conducted via FLAME. Three second-level hierarchical linear models (HLMs) were calculated and the dependent variable in all models was the within-participant selected vs rejected contrast. The first HLM modeled mean task effects, the second modeled main effects of age (continuous) and anxiety diagnosis and the third modeled the interaction between age and anxiety. In all analyses, a nuisance covariate was included that modeled the scanner on which data were collected. Two-tailed t-tests were conducted on the Level 2 βs and converted to z scores. Based on a priori hypotheses, three masks were used independently to constrain the number of voxels under consideration to bilateral amygdala, bilateral nucleus accumbens (NAcc) (both created via participant-specific probabilistic atlases) and medial PFC (i.e. anterior cingulate, paracingulate, subgenual, medial orbital/gyrus rectus from FSL’s Harvard-Oxford atlas). Gaussian-random-field theory was used to correct for multiple comparisons (via Cluster) with an individual voxel-level threshold of z ≥ 2.241 and an overall error rate of P ≤ 0.05. See Supplementary Material for analyses conducted to rule out potential confounds,
Psychophysiological interaction analyses
Psychophysiological interaction analyses were conducted using clusters identified in the primary group analyses. For each cluster, the mean (across voxels) for each timepoint was extracted to create timeseries predictors. To model brain-wide signal fluctuations that could confound estimates of connectivity (Fox et al., 2009), the mean across all intra-cerebral voxels was calculated for each timepoint. HLMs were computed in SPSS using the MIXED procedure, with participant as nesting variable and time as repeated factor. The Level 1 covariance matrix was modeled with a lag-1 autoregressive function. Level 1 task predictors were the same as the analyses described above, except that anticipation predictors modeled the difference and sum of the selected and rejected conditions (rather than individual conditions). Three additional predictors were included: one modeled the timeseries of the seed cluster of interest, one modeled the interaction between the seed-cluster timeseries and the condition-difference task predictor and a nuisance covariate modeling brain-wide signal fluctuations. At Level 2, age (continuous), anxiety diagnosis, and the age × anxiety interaction were included as predictors (main effects examined in a model without the interaction). In addition, a nuisance covariate modeled the scanner on which data were collected. Significant three-way interactions were decomposed by splitting by anxiety, then age (median split), then condition (fixation as baseline) and significant two-way interactions were decomposed similarly, except split only by the relevant effect (anxiety/age).
RESULT
Main effect of anticipation
One cluster emerged, largely in anterior cingulate sulcus (Figure 2), in which activation was greater (across all participants) for anticipating feedback from selected, relative to rejected, peers (Table 1).
Fig. 2.
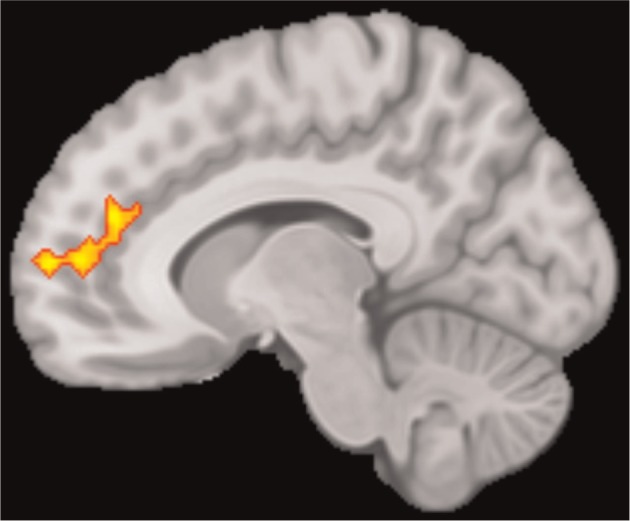
Main effect of anticipation of selected > rejected. Sagittal slice at x = −10 showing the cingulate sulcus cluster in which a main effect of condition (selected >rejected) was observed.
Table 1.
fMRI findings
| Region | Cluster size (mm3) | Max z value | Cluster P value | Location |
||
|---|---|---|---|---|---|---|
| X | Y | Z | ||||
| Main effect of anticipation | ||||||
| Cingulate sulcus (BA 9/10/32) | 21 296 | 3.33 | 0.05 | 14 | 50 | 8 |
| Effect of anxiety diagnosis on anticipation | ||||||
| Left amygdala | 488 | 3.34 | 0.02 | −18 | −2 | −20 |
| Left nucleus accumbens | 144 | −2.70 | 0.02 | −6 | 12 | −2 |
| Left nucleus accumbens | 8 | −2.26 | 0.02 | −8 | 8 | −10 |
| Right nucleus accumbens | 136 | −3.30 | 0.02 | 6 | 10 | −4 |
| Right nucleus accumbens | 8 | −2.40 | 0.02 | 12 | 18 | −4 |
| Interactive effect of anxiety diagnosis and age on anticipation | ||||||
| Rostral ACC (BA 10/24/32) | 1,784 | −3.24 | 0.03 | −8 | 40 | −4 |
Notes: ACC, anterior cingulate cortex; BA, Brodmann’s area, coordinates are for max z value.
Main effects of anxiety diagnosis and maturation on anticipation
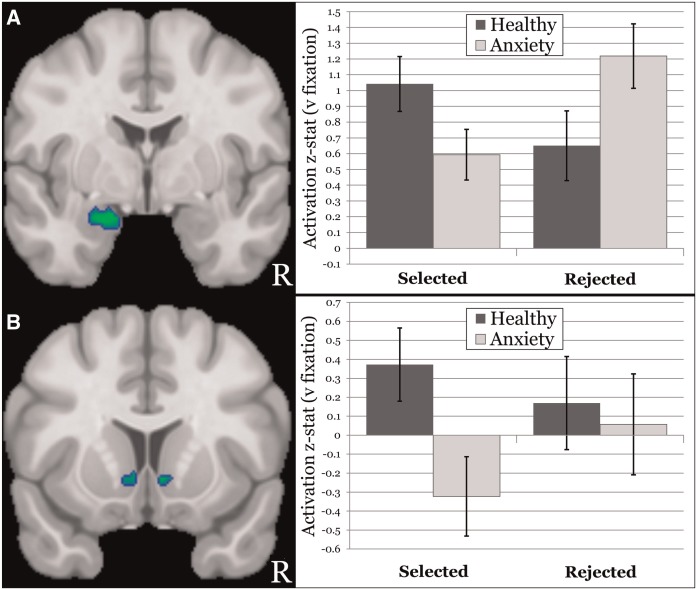
Five clusters emerged in which anxiety group moderated anticipatory activation to selected versus rejected peers (Table 1). Supporting hypotheses, the left amygdala response to anticipating feedback from rejected peers was stronger in anxious compared to healthy participants, whereas the converse pattern was found for anticipating selected peers. This effect is broken down further by anxiety group and condition (vs baseline) in Figure 3 for ease of interpretation. Four clusters also emerged in bilateral NAcc. As demonstrated in Figure 3, NAcc was activated (vs baseline) when healthy participants anticipated feedback from selected peers, whereas consistent with hypotheses, anxious participants evidenced deactivation (vs baseline). Neither group activated NAcc when anticipating feedback from rejected peers.
Fig. 3.
Moderation of anticipation by anxiety diagnosis. (A) (left) Coronal slice at y = −2 showing the amygdala cluster in which an anxiety group × task condition interaction was observed; (right) graph of the interaction in amygdala, with the x-axis representing condition, the y-axis representing mean (across amygdala cluster voxels) activation z-stat and separate bars representing the anxious (lighter fill) and healthy (darker fill) groups. (B) (left) Coronal slice at y = 12 showing the nucleus accumbens (NAcc) clusters in which an anxiety group × task condition interaction was observed; (right) graph of the interaction in the larger left NAcc cluster, with the x-axis representing condition, the y-axis representing mean (across NAcc cluster voxels) activation z-stat and separate bars representing the anxious (lighter fill) and healthy (darker fill) groups.
Interactive effect of anxiety diagnosis and maturation on anticipation
One cluster emerged in rACC in which anxiety moderated the relationship between age and task activation (Table 1). In healthy participants, age was positively and negatively associated with rACC activation (vs baseline) to selected and rejected peers, respectively [see Figure 4 (age is broken down by median split for visualization purposes)]. In contrast, anxious participants evidenced the opposite developmental pattern.
Fig. 4.
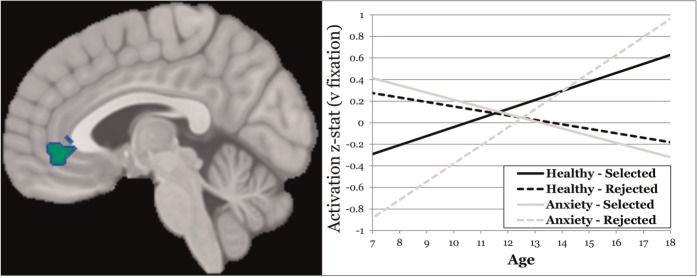
Interaction of age and anxiety diagnosis moderating anticipation. (left) Sagittal slice at y = −4 showing the rACC cluster in which an anxiety group × age × task condition interaction was observed; (right) plot of the interaction in rACC, with the x-axis representing age, the y-axis representing mean (across rACC cluster voxels) activation z-stat and separate lines for the anxious group/selected condition (lighter/solid), anxious group/rejected condition (lighter/dashed), health group/selected condition (darker/solid) and healthy group/rejected condition (darker/dashed).
Connectivity analyses
Given research indicating that rACC→amygdala coupling plays a key role in emotional processing (Etkin et al., 2011; Kim et al., 2011; Zorrilla and Koob, 2013), we examined condition-dependent connectivity between the observed clusters. Specifically, we tested whether age, diagnosis and the age × diagnosis interaction moderated condition-dependent (selected vs rejected) connectivity between the rACC cluster identified above (independent variable) and left amygdala (dependent variable). This analysis revealed significant main effects for age and anxiety and a significant age × anxiety interaction. The main effect for age (γ = −0.069, P < 0.01) was driven by a dynamic response among older participants (negative coupling for selected, positive for rejected) and a relatively low (and equal across conditions) level of coupling among younger participants. Older participants demonstrated strong negative rACC→left amygdala coupling for selected peers and positive coupling for rejected peers (age effect for selected: P = 0.04; rejected: P = 0.01). Note, negative (coupling) in this context refers to the direction, rather than the magnitude, of the relationship. Specifically, positive coupling between regions X and Y means that, when activity in X increases, activity in Y also increases. Conversely, negative coupling between regions X and Y means that, when activity in region X increases, activity in region Y decreases.
Anxiety diagnosis also moderated rACC→left amygdala coupling (γ = 0.250, P = 0.05). As hypothesized, anxiety was associated with stronger negative and positive rACC→amygdala coupling when anticipating feedback from selected and rejected peers, respectively (anxiety factor for selected: P = 0.22; rejected: P = 0.04), whereas this response was relatively muted among non-anxious participants. However, both main effects of age and anxiety are qualified by the age by anxiety interaction.
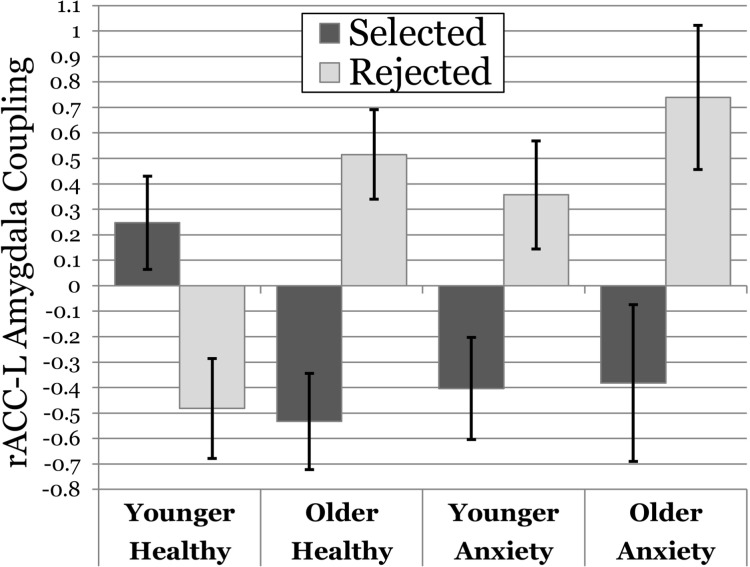
The interaction between age and anxiety (γ = −0.099, P = 0.02) revealed a fundamentally different developmental pattern between anxious and healthy adolescents. As illustrated in Figure 5 (age is broken down by median split for visualization purposes), younger, non-anxious participants demonstrated a pattern of positive rACC→amygdala coupling during anticipation of feedback from selected peers and negative coupling for rejected peers (task condition effect γ = 0.273, P = 0.014). Older non-anxious adolescents engaged the opposite pattern: negative coupling when anticipating feedback from selected peers and positive coupling for rejected peers (task condition effect γ = −0.348, P = 0.002). Older anxious participants demonstrated a pattern of rACC→amygdala coupling that was remarkably similar to healthy older adolescents (task condition effect γ = −0.417, P = 0.019). Younger anxious adolescents were notably different from younger healthy participants and displayed a pattern that was more similar to their older counterparts—although the positive coupling when anticipating feedback from rejected was blunted relative to both older groups (task condition effect γ = −0.213, P = 0.083).
Fig. 5.
Moderation of condition-dependent coupling by age and anxiety diagnosis. Graph of coupling between the rACC and left amygdala clusters observed, with the x-axis representing age (median split for visualization purposes only) and anxiety group, the y-axis representing coupling β and separate columns for the selected (darker fill) and rejected (lighter fill) conditions.
DISCUSSION
The goals of this study were 2-fold: (i) to examine whether activity in key neural circuits related to motivation would differ in adolescents with and without anxiety when anticipating social feedback and (ii) to investigate anxiety-related differences in the maturational trajectories of these regions across adolescence. Using a peer interaction paradigm, we found that anticipation-related activation within, and coupling between, key regions differed between anxious and non-anxious youth. We also found divergent patterns of age differences in these two groups across adolescence.
Anxiety and social anticipation
Adolescents with anxiety evidenced greater amygdala activation when anticipating feedback from previously rejected (relative to selected) peers, whereas healthy youth showed the converse pattern (Figure 2). This result is similar to a previous finding with an earlier version of this task wherein anxious participants displayed greater amygdala activity than non-anxious participants when evaluating the responses of rejected relative to selected peers (Guyer et al., 2008). In the present investigation, we focused on the period immediately preceding feedback, during which participants were shown a photo of a peer and reminded of whether they had selected or rejected the peer at their initial visit. During this period, participants passively anticipated the receipt of evaluative feedback from the peer. The earlier version of the task did not contain this sub-event, and the present version was modified to isolate this anticipatory-anxiety period.
Given the prominent role of amygdala for salience identification (Phelps, 2006), the present findings suggest that both anxious and non-anxious youth find social feedback to be highly salient, but the relative salience ascribed to different aspects of peer feedback appears to differ. Anxious youth appear to ascribe greater salience to potential feedback from rejected peers, whereas non-anxious youth ascribe greater salience to potential feedback from selected peers. This may reflect a greater focus in anxious than non-anxious adolescents on impending negative outcomes rather than potential benefit for future outcomes (Pine et al., 2009).
In addition to amygdala, activation in NAcc also differentiated anxious from non-anxious youth. Specifically, healthy adolescents evidenced activation in NAcc when anticipating feedback from selected peers, whereas anxious youth demonstrated decreased activation in the same condition and neither group showed activation when anticipating outcomes from rejected peers (Figure 2). The NAcc has long been associated with anticipation of rewarding events (Bartra et al., 2013), and this may reflect a diminution of reward expectation in the anxious group. However, other functions have also been attributed to this region including learning (Schultz, 2007) and aversive anticipation (Levita et al., 2009; Choi et al., 2014). This makes the functional significance of diminished NAcc activation in this context difficult to pinpoint. However, the fact that anxious youth have diminished activity in both amygdala and NAcc when anticipating feedback from selected peers suggests the presence of an aberrant pattern of motivational engagement in this potentially rewarding social context. Yet, NAcc deactivations were only present when anticipating feedback from selected peers, suggesting that anxious adolescents had a diminished expectation of reward or a mixed motivational response (Choi et al., 2013). This diminished anticipatory response may impact neural responses to reward receipt and consequent social learning (Guyer et al., 2014; Jarcho et al., unpublished data).
Although the present results were focused on clinically anxious youth, a similar pattern of striatal activity has been observed in other socially compromised populations when engaging with social stimuli. In a recent study using this paradigm, behaviorally inhibited adolescents displayed diminished activity in putamen when anticipating feedback and blunted activity in ventral striatum when receiving positive feedback from selected peers (Guyer et al., 2014). Similarly, Goff et al. (2013) recently reported that adolescents who experienced early life stress showed deactivation in NAcc when viewing faces, whereas the comparison group exhibited increases. Consequently, one possibility is that early stressful events or genetic predisposition may lead to diminished NAcc activation under conditions of potential or actual positive social outcomes. This may then lead to decreased social engagement and attenuated incorporation of social experience into the behavioral repertoire.
Anxiety-related divergence in age effects
Participants with and without an anxiety diagnosis exhibited opposite age effects in rACC (Figure 3), which may reflect differential maturational trajectories, and a differential pattern of coupling between rACC and amygdala across development. In healthy youth, increasing age was positively associated with rACC activation for selected peers and negatively associated with age for rejected peers, whereas the converse pattern was evident in anxious participants. As with the striatum and amygdala, it is difficult to clearly define the role of rACC. Evidence suggests that the region of rACC observed in this study is important for regulating emotional associations. For example, research implicates this region in the resolution of emotionally conflicting information (Etkin et al., 2006, Mohanty et al., 2007), including the extinction of threat associations (Phelps et al., 2004; Quirk and Beer, 2006; Etkin et al., 2011).
When considered in light of other findings from this study, the observed pattern of results for rACC suggests that regulatory resources are increasingly devoted to potentially adverse and rewarding outcomes as anxious and non-anxious adolescents, respectively, mature. Given that this pattern was evident during anticipation of social feedback, one intriguing possibility is that divergent maturation may reflect differences in the ability to rapidly regulate emotional responses to likely outcomes. For example, as anxious adolescents mature, they may devote increasing an proportion of the top-down control instantiated in rACC to regulating (likely aversive) emotional responses to peers they may believe they have offended (i.e. rejected peers) to minimize potential social signals given by their emotional responses (e.g. fear, Quirk and Beer, 2006). Thus, as adolescents become increasingly adept at reading social cues, it may be increasingly important for anxious individuals to mask their anxiety, leading them to devote a greater proportion of rACC resources toward emotion regulation related to potential aversive outcomes. Alternatively, as this region has been implicated in responses to both positively and negatively valenced stimuli, this finding may represent the development of an emergent bias for negatively valenced information in anxious youth and positively valenced information in healthy youth (Laxton et al., 2013; Price and Drevets, 2013).
Finally, examination of functional connectivity among these regions revealed differential maturational trajectories in rACC↔amygdala coupling in the two groups. When anticipating feedback from selected peers, younger, non-anxious participants demonstrated positive rACC↔amygdala coupling, whereas younger, anxious participants and both groups of older participants displayed negative rACC↔amygdala coupling. Conversely, when anticipating feedback from rejected peers, young healthy adolescents displayed negative rACC↔amygdala coupling, whereas the other three groups displayed positive coupling. In other words, under both conditions, young healthy participants displayed coupling opposite to that of either young anxious participants or the two older groups.
This finding resonates with recent reports indicating that normative adolescent development is associated with a switch from positive to negative mPFC↔amygdala coupling during threat processing (Gee et al., 2013b). Gee et al. argue that this switch may represent a key transition point in emotional development. The positive relationship between amygdala and medial prefrontal activity early in development may reflect a teaching signal whereby amygdala responses to salient stimuli shape response tendencies in mPFC. However, the direction of this connectivity shifts in later development, such that mPFC is inversely related to amygdala activity. This may reflect a ‘mature’ relationship between salience and regulatory systems whereby mPFC is actively inhibiting excessive amygdala activation—hence a shift from bottom-up learning to top-down control (Gee et al., 2013b).
Gee also observed that this connectivity switch was not found in children exposed to extreme, stressful conditions early in life via rearing in an institutionalized setting. In this population, negative mPFC↔amygdala coupling was evident early in development, possibly indicating accelerated maturation of regulatory circuits due to excessive stress in early life (Gee et al., 2013a,b). In our data, a similar switch from positive to negative coupling was observed in healthy participants in the selected peer condition. Although this is not a ‘fear’ condition per se, feedback from selected peers is likely extremely salient and potentially aversive (due to possible rejection by the peer). In contrast, healthy controls exhibited the converse pattern during anticipation of feedback from rejected peers; negative rACC↔amygdala coupling was evident in younger adolescents and positive coupling was found for older adolescents. It is unclear what may underlie this shift, although it appears to be normative.
The anxious group, in contrast, did not display this developmental shift. Similar to the institutionalized youth in Gee et al. (2013a), participants with anxiety exhibited the ‘mature’ pattern of mPFC↔amygdala coupling across development. Thus, it appears that even anxious youth who have not endured the extreme adversity of early institutionalization may exhibit an accelerated pattern of early maturation. Although the analyses that revealed this developmental pattern are exploratory and this finding requires replication, our data provide insight into the impact of anxiety on brain network development.
This study benefits from a number of strengths, including a powerful paradigm in which phases of social interchange are separated and examination of multiple facets of the fMRI data (within-region activation, inter-regional coupling). However, several limitations must be considered when making inferences about the data. First, examination of development was cross-sectional rather than longitudinal, which would allow for more accurate and powerful detection of the impact of maturation on brain function. For example, the age × anxiety interaction in rACC may be due to the duration of anxiety rather than age. Thus, what appears to be a maturational change may actually reflect individual differences related to earlier vs later onset of anxiety. Longitudinal designs are needed to differentiate between these possibilities. In addition, although the chatroom task is state of the art for this area, it does not fully reflect the nuances of peer interaction. For example, information on shared interests with peers was deliberately not given to participants to minimize potential confounds. However, this information will often be present in real-world interactions (even virtual ones) and the inclusion of shared interest information may have had an important impact on the present findings. Finally, the sample of each diagnostic group remains relatively small. More research is needed with this paradigm in a larger sample of both anxious and non-anxious youth. This could determine the degree to which replicable associations arise specifically with an anxiety-disorder diagnosis as opposed to broader arrays of psychopathology.
Despite these limitations, this study provides key insights into anxiety-related divergence in the maturational trajectory of circuitry engaged by anticipating social interactions. Our focus on the anticipation phase of social interchange is significant, given evidence that anticipation is particularly crucial in the expression of anxiety (Grupe and Nitschke, 2013). Overall, our findings indicate that anxious youth display a pattern of brain engagement when anticipating social feedback from peers that suggest differences in motivation and alterations in developmental trajectories of regulatory systems. These neurobiological differences are likely to contribute to differences in emotion processing and behavior across adolescent development.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This study was supported by the intramural research program of the National Institute of Mental Health.
REFERENCES
- Barlow DH. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. New York, NY: Guilford Press; 2004. [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–27. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MF. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–90. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9:267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ, Robbins TW. Decision-making in the adolescent brain. Nature Neuroscience. 2012;15:1184–91. doi: 10.1038/nn.3177. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of the New York Academy of Sciences. 2008;1124:111–26. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JM, Padmala S, Spechler P, Pessoa L. Pervasive competition between threat and reward in the brain. Social Cognitive and Affective Neuroscience. 2014;9:737–50. doi: 10.1093/scan/nst053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13:636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dahl RE. Adolescent brain development: a period of vulnerabilities and opportunities. Keynote address. Annals of the New York Academy of Sciences. 2004;1021:1–22. doi: 10.1196/annals.1308.001. [DOI] [PubMed] [Google Scholar]
- Davey CG, Allen NB, Harrison BJ, Yucel M. Increased amygdala response to positive social feedback in young people with major depressive disorder. Biological Psychiatry. 2011;69:734–41. doi: 10.1016/j.biopsych.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fox MD, Zhang D, Snyder AZ, Raichle ME. The global signal and observed anticorrelated resting state brain networks. Journal of Neurophysiology. 2009;101:3270–83. doi: 10.1152/jn.90777.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4:6. doi: 10.3389/neuro.09.006.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences of the United States of America. 2013a;110:15638–43. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, et al. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience. 2013b;33:4584–93. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience. 2013;249:129–38. doi: 10.1016/j.neuroscience.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nature Reviews Neuroscience. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor B, van Leijenhorst L, Rombouts SA, Crone EA, Van der Molen MW. Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience. 2010;5:461–82. doi: 10.1080/17470910903526155. [DOI] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, et al. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Development and Psychopathology. 2014;26:229–43. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Choate VR, Pine DS, Nelson EE. Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience. 2012;7:81–92. doi: 10.1093/scan/nsr043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JY, McClure-Tone EB, et al. Amygdala and ventrolateral prefrontal cortex function during anticipated peer evaluation in pediatric social anxiety. Archives of General Psychiatry. 2008;65:1303–12. doi: 10.1001/archpsyc.65.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure-Tone EB, Shiffrin ND, Pine DS, Nelson EE. Probing the neural correlates of anticipated peer evaluation in adolescence. Child Development. 2009;80:1000–15. doi: 10.1111/j.1467-8624.2009.01313.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Leibenluft E, Walker OL, Fox NA, Pine DS, Nelson EE. Neuroimaging studies of pediatric social anxiety: paradigms, pitfalls and a new direction for investigating the neural mechanisms. Biology of Mood and Anxiety Disorders. 2013;3:14. doi: 10.1186/2045-5380-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–90. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behavioural Brain Research. 2011;223:403–10. doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY, Guyer AE, Tone EB, et al. Neural responses to peer rejection in anxious adolescents Contributions from the amygdala-hippocampal complex. International Journal of Behavioral Development. 2012;36:36–44. doi: 10.1177/0165025411406854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxton AW, Neimat JS, Davis KD, et al. Neuronal coding of implicit emotion categories in the subcallosal cortex in patients with depression. Biological Psychiatry. 2013;74:714–9. doi: 10.1016/j.biopsych.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–87. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, McNealy K, Pfeifer JH, Dapretto M. Subgenual anterior cingulate responses to peer rejection: a marker of adolescents’ risk for depression. Development and Psychopathology. 2011;23:283–92. doi: 10.1017/S0954579410000799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanty A, Engels AS, Herrington JD, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44:343–51. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–74. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Adolphs R. Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews Neuroscience. 2010;11:773–83. doi: 10.1038/nrn2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pine DS, Cohen P, Gurley D, Brook J, Ma Y. The risk for early-adulthood anxiety and depressive disorders in adolescents with anxiety and depressive disorders. Archives of General Psychiatry. 1998;55:56–64. doi: 10.1001/archpsyc.55.1.56. [DOI] [PubMed] [Google Scholar]
- Pine DS, Helfinstein SM, Bar-Haim Y, Nelson E, Fox NA. Challenges in developing novel treatments for childhood disorders: lessons from research on anxiety. Neuropsychopharmacology. 2009;34:213–28. doi: 10.1038/npp.2008.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neural circuits underlying the pathophysiology of mood disorders. Trends in Cognitive Science. 2013;16:61–71. doi: 10.1016/j.tics.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Schniering CA, Hudson JL. Anxiety disorders during childhood and adolescence: origins and treatment. Annual Review of Clinical Psychology. 2009;5:311–41. doi: 10.1146/annurev.clinpsy.032408.153628. [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. Journal of Neuroscience. 2008;28:11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral dopamine signals. Trends in Neuroscience. 2007;30:203–10. doi: 10.1016/j.tins.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, Dahl RE. Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience. 2014;9:1798–807. doi: 10.1093/scan/nst175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Wagner DD, Wig GS, Moran JM, Whalen PJ, Kelley WM. Interactions between transient and sustained neural signals support the generation and regulation of anxious emotion. Cerebral Cortex. 2013;23:49–60. doi: 10.1093/cercor/bhr373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Watson D, Friend R. Measurement of social-evaluation anxiety. Journal of Consulting and Clinical Psychology. 1969;33:448–57. doi: 10.1037/h0027806. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. Amygdalostriatal projections in the neurocircuitry for motivation: a neuroanatomical thread through the career of Ann Kelley. Neuroscience and Biobehavioral Review. 2013;37:1932–45. doi: 10.1016/j.neubiorev.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.