Abstract
A single-nucleotide polymorphism on the oxytocin receptor gene (OXTR), rs53576, involving a guanine (G) to adenine (A) substitution has been associated with altered prosocial features. Specifically, individuals with the GG genotype (i.e. the absence of the polymorphism) display beneficial traits including enhanced trust, empathy and self-esteem. However, because G carriers might also be more socially sensitive, this may render them more vulnerable to the adverse effects of a negative social stressor. The current investigation, conducted among 128 white female undergraduate students, demonstrated that relative to individuals with AA genotype, G carriers were more emotionally sensitive (lower self-esteem) in response to social ostracism promoted through an on-line ball tossing game (Cyberball). Furthermore, GG individuals also exhibited altered blood pressure and cortisol levels following rejection, effects not apparent among A carriers. The data support the view that the presence of the G allele not only promotes prosocial behaviors but also favors sensitivity to a negative social stressor.
Keywords: cortisol, ostracism, oxytocin, social sensitivity, SNP
INTRODUCTION
Oxytocin, a neuropeptide known for its role in childbirth, breastfeeding and infant-mother bonding (Gimpl and Fahrenholz, 2001), influences social behaviors and might thus contribute to disorders, including autism, schizophrenia, anxiety and depressive disorders, which involve social disturbances (Scantamburlo et al., 2007; Guastella, et al., 2010; Feifel et al., 2012). Several single-nucleotide polymorphisms (SNPs) have been identified on the oxytocin receptor gene (OXTR), but one in particular, rs53576, which involves a guanine (G) to adenine (A) substitution, seems particularly relevant to prosocial behaviors. Compared with A allele carriers (i.e. the polymorphism is present), individuals with two G alleles exhibit a range of favorable attributes, such as high levels of trust (Krueger et al.. 2012), self-esteem (Saphire-Bernstein et al., 2011), empathy (Rodrigues et al., 2009; Smith et al., 2014), maternal sensitivity (Bakermans-Kranenburg and van Ijzendoorn, 2008) and may be more attune to social cues (Rodrigues et al., 2009). Individuals homozygous for the G allele also exhibited lower depressive symptoms compared with A carriers (Saphire-Bernstein et al., 2011), and G carriers displayed higher positive affect (Lucht et al., 2009).
Although it is tempting to consider the G allele of the rs53576 SNP as advantageous and the A allele as a risk/vulnerability factor for negative mood states, this may be an overly simplistic view. In fact, in an African American sample comprising individuals who had experienced severe childhood maltreatment, those with the GG genotype (i.e. in the absence of the polymorphism) displayed greater disorganized attachments and increased emotional dysregulation compared with their A carrier counterparts (Bradley et al., 2011). In line with these findings, in the context of early-life maltreatment, G carriers displayed greater depressive scores than individuals with the AA genotype (McQuaid et al., 2013). Together, these findings suggest that although the G allele may be associated with beneficial prosocial features, in some contexts, in other contexts as in the case of early-life adversity, the social sensitivity associated with the G allele may render individuals more vulnerable to behavioral disturbances. From this perspective, oxytocin might not just serve as a prosocial hormone but might also influence the salience of or sensitivity to social cues, irrespective of whether these are positive or negative (Averbeck, 2010; Bartz et al., 2011).
In addition to affecting behavioral and emotional responses to stressors, the OXTR polymorphism has been associated with several physiological responses to stressors. Compared with A allele carriers, individuals with the GG genotype of the OXTR SNP displayed lower awakening salivary cortisol levels (Norman et al., 2012) and lower heart rate responses to an anticipatory startle stimulus (Rodrigues et al., 2009). However, in response to a psychosocial stressor, those with the GG genotype showed greater sympathetic reactivity (Norman et al., 2012) as well as increased sympathetic and subjective arousal when presented with stimuli showing others in distress (Smith et al., 2014). Although some of these findings are inconsistent with one another, it is possible that carrying a G allele may confer particular sensitivity to stressors involving a social component.
Ostracism is a powerful social stressor (Williams, 2001; Eisenberger, 2012) that induces strong negative emotions even when it occurs briefly (Williams et al., 2000). For instance, being ostracized within a virtual ball-tossing game, Cyberball, is accompanied by lower feelings of belonging, self-esteem, meaningful existence and control (Zadro et al., 2004). It is of particular interest that social rejection in this context activates the same neural pain networks, including the dorsal anterior cingulate cortex (dACC) and the insula, that are associated with bodily injury (Eisenberger et al., 2003, 2006). Given the contribution of oxytocin to social behaviors, it is possible that this hormone contributes to the processes underlying social rejection sensitivity. Indeed, in response to social ostracism elicited by participants being excluded from conversations, intranasal oxytocin reduced cortisol levels compared with placebo (Linnen et al., 2012) and increased self-perceived trust among those reporting negative mood (Cardoso et al., 2013).
As oxytocin administration modulates responses to social rejection, it might also be expected that OXTR rs53576 genotypes would influence reactions to social ostracism. In this study, we examined the OXTR SNP in relation to ostracism elicited by exclusion in a Cyberball game among a sample of white females. It was predicted that following rejection, G carriers would report more pronounced responses to ostracism, including lower belonging, control, self-esteem and meaningful existence, which are influenced by ostracism (Williams, 1997, 2001). Further, if G carriers are more prosocial, it would be expected that compared with their AA counterparts, G carriers would judge their Cyberball co-players less harshly following rejection. Finally, it was predicted that G carriers would be physiologically more reactive to social stressors, displaying higher blood pressure and cortisol levels upon rejection compared with individuals with two A alleles.
METHODS
Participants
This study comprised 128 white female Carleton University undergraduate students with a mean age of 19.82 (standard deviation = 3.86). The OXTR genotype could be determined for 126 individuals. A homogenous ethnic sample was used in this study as marked cultural differences have been found in association with this OXTR SNP (i.e. Caucasians who have at least one G allele are more likely to seek emotional social support, an effect not found among Asian G carriers; Kim et al., 2010). Thus, because of population stratification, data were collected from non-white participants (n = 122) but were not included in any analyses. The ethnicity of these participants included Black (32.5%, n = 38), Asian (21.4%, n = 25), other, (13.7%, n = 16), Arab (12.0%, n = 14), South Asian (10.3%, n = 12), Latin American (5.1%, n = 6) and Aboriginal (2.6%, n = 3). It would have been of interest to assess the influence of genotype across different ethnic groups, but this was precluded owing to the small number of participants in each of the ethnic groups. The distributions of the OXTR genotypes vary substantially across ethnic groups. As listed in Table 1, for example, Black individuals and Asian individuals display the complete opposite OXTR genotype distributions. Further to this issue, not all three OXTR genotypes could even be represented in each ethnic group.
Table 1.
Oxytocin receptor gene polymorphism distributions by ethnicity
| Ethnicity | G/G | A/G | A/A |
|---|---|---|---|
| Caucasian (n = 126) | 56 | 52 | 18 |
| Black (n = 38) | 25 | 13 | 0 |
| Asian (n = 25) | 3 | 12 | 10 |
| Arab/West Asian (n = 14) | 8 | 4 | 2 |
| South Asian (n = 12) | 3 | 5 | 4 |
| Latin American/Hispanic (n = 6) | 2 | 4 | 0 |
| South East Asian (n = 3) | 1 | 2 | 0 |
| Aboriginal (n = 3) | 1 | 1 | 1 |
| Other (n = 15) | 7 | 5 | 3 |
Participants were recruited from an online computerized recruitment system used by the university. Eighteen percent (n = 23) of participants reported a family income of <$45,000, whereas almost half of participants reported a family income between $45,000 and $90,000 (44.5%, n = 57) and 35.1% (n = 45) reported a family income greater than $90,000. Self-reported religion included Catholic (31.3%, n = 40), Agnostic (23.4%, n = 30), Protestant (20.3%, n = 26), Atheist (16.4%, n = 21), other (5.5%, n = 7), Buddhist (1.6%, n = 2) and Jewish (0.8%, n = 1).
General procedure
All procedures in this study were approved by the Carleton University Ethics Committee for Psychological Research. Once informed consent was signed, participants provided a saliva sample for DNA genotyping using Oragene OG-500 collection kits (DNA Genotek, Inc., Ottawa, Ontario, Canada). Participants were informed that the purpose of the study was to assess mental visualization through playing an online ball tossing game (Cyberball). Prior to beginning Cyberball, participants relaxed over a 20-min period and also completed demographic information and a trait anxiety questionnaire. Once participants finished playing Cyberball, they completed several questionnaires including those assessing feelings of rejection and judgments regarding their Cyberball co-players. Saliva samples for cortisol assays and blood pressure measurements were obtained at baseline (20 min after arrival to the laboratory), as well as 15 and 30 min following Cyberball. Participants were then fully debriefed. Each session took up to 1.25 h to complete. Additionally, two participants were excluded based on previous experience playing Cyberball.
Cyberball task
Cyberball is a well-established computerized game used to induce feelings of social rejection (Williams et al., 2000). Participants were tested individually but were led to believe that they were playing with two other university students from other laboratories connected to the same server. In actuality, the other players did not exist, and the game was computer simulated. As previously described (Williams et al., 2000), to increase the validity of Cyberball, prior to beginning, participants’ pictures were taken and they were told that their pictures were uploaded onto the on-line server, so that their two co-players would be able to see them, and photographs of two virtual players were shown to the participants throughout the game. Participants were randomly assigned to one of two conditions, inclusion or exclusion. In the included condition, participants passed and received a virtual ball an equal amount of times as other players throughout the game. In contrast, excluded participants received the ball twice at the beginning and then never again. The game lasted ∼2 min 30 s for both conditions.
Salivary cortisol
Saliva samples were collected in SalivetteR tubes, (Sarstedt, Germany), 20 min after arrival to the laboratory (baseline) as well as 15 and 30 min following Cyberball. Immediately following the test session, saliva samples were frozen at −80°C. Following the manufacturer’s protocol, a competitive radioimmunoassay, 125I kit (ICN Biomedicals Inc., Irvine, CA), was used to determine, in duplicate, salivary cortisol levels. The intra- and interassay variability was <10%. The minimum detectable of cortisol was 0.02 µg/dl and the specificity was 100% cortisol. In some instances (n = 8), participants did not have three valid cortisol measures and thus were appropriately removed from the repeated measures analyses.
Genotyping
Genomic DNA was extracted from the Oragene OG-500 collection kits according to the manufacturer’s protocol and diluted to equal concentration of 20 ng/µl. Quantitative polymerase chain reaction (qPCR) was used for genotyping. A total volume of 15 μl was used to perform the amplification reactions, which contained ∼1 μl (20 ng) of genomic template, 0.6 μl of each primer (concentration 10 μM), 1.2 μl of dNTP, 1.5 μl 10X Buffer, 1.5 μl of MgCl2, 0.3 μl of Salmon Sperm DNA, 0.15 μl of Taq polymerase, 0.015 of SYBR green and 8.135 μl of water. All qPCR plates were run in duplicate and genotypes were called blind. All qPCR products were then electrophoresed on 2% agarose gel and visualized to confirm qPCR results. The Bio-Rad Iq5 Primer sequences used for qPCR included OXTR F1 forward: TCCCTGTTTCTGTGGGACTGAGGAC, OXTR F2 forward: TCCCTGTTTCTGTGGGACTGAGGAT and OXTR reverse: ACCCAAGAGGCTGGTTTGGGGTT.
The genotype distribution for the OXTR polymorphism was 56 individuals with the GG genotype, 52 GA individuals and 18 AA individuals. These distributions met the expectations for Hardy–Weinberg Equilibrium, χ2 (1) = 1.07, P = 0.30. We were not able to confirm an OXTR genotype for two individuals who were therefore excluded from any analyses including the OXTR genotype.
Measures
Social ostracism
The Social Ostracism and Mood Scale (Williams, 2001; Zadro et al., 2004) was used to assess the effectiveness of the ostracism manipulation through questions such as ‘what percentage of the throws were directed to you?’ and ‘to what extent you currently feel accepted or rejected?’. In addition, the questionnaire contained 11 items on a 9-point scale of 1 (not at all) to 9 (very much so) that assessed participant’s levels of four fundamental needs proposed by Williams (1997, 2001). These comprised belonging (e.g. I felt like an outsider during the Cyberball game; α = 0.78), control (e.g. I felt in control during the Cyberball game; α = 0.75), self- esteem (e.g. I felt somewhat inadequate during the Cyberball game; α = 0.79) and meaningful existence (I felt non-existent during the Cyberball game and I felt that my performance had some effect on the direction of the game; α = 0.74). Mean scores for each of the four needs was calculated.
Co-player judgments
Participants reported judgments about both of their Cyberball co-players on a scale of 1 (not at all) to 9 (very much so) on 13 characteristics that included how likable, good, attractive, prejudiced, trustworthy, tolerant, arrogant, friendly, manipulative, fair, loyal, hypocritical and to what degree they believed they were sell-outs. Ratings for each co-player were calculated together to obtain a mean score on each judgment.
Anxiety symptoms
Trait anxiety levels were assessed by the Spielberger State-Trait Anxiety Inventory (Speilberger, 1983). A 20-item trait anxiety scale was used to measure general anxiety symptoms before playing Cyberball, where participants responded to statements regarding how often they generally felt each feeling (e.g. nervous and restless) on a scale of 1 (almost never) to 4 (almost always). Total scores were calculated by summing across all items (α = 0.95).
Statistical analyses
Statistical analyses were performed using SPSS for Windows 18.0 (SPSS Science, Chicago, IL). Analyses assessing initial differences on trait anxiety scores between Cyberball conditions and the Cyberball manipulation checks were performed using an independent samples t-test. Analyses assessing the social ostracism outcomes (i.e. belonging, control, self-esteem and meaningful existence) and co-player judgments were analyzed using 2 (Cyberball condition: excluded versus included) × 3 (OXTR genotype: GG, AG or AA) multivariate analysis of variance (MANOVA)s. For blood pressure scores, a 2 (Cyberball condition) × 3 (OXTR genotype) × 3 (Time: 1–3 time-points) mixed measures analysis of variance (ANOVA) with Time serving as the within-group factor was used. Further to this, a 2 (Cyberball condition) × 3 (OXTR genotype) analyses of covariance was also conducted for blood pressure, controlling for baseline levels. Cortisol was analyzed using a 2 (Cyberball condition) × 3 (OXTR genotype) × 3 (Time: 1–3 time points) mixed measures ANOVA with Time serving as the within-group factor. Follow-up comparisons comprised t-tests with a Bonferroni correction to maintain the alpha level at 0.05. Additionally, an area under the curve (AUC) analysis was performed for cortisol using a formula proposed by Pruessner et al. (2003).
RESULTS
Psychosocial measures
As expected, there were no initial differences on trait anxiety between OXTR genotype groups, F (2, 123) = 0.91, P = 0.40 or Cyberball conditions, t (1, 126) = 0.31, P = 0.76. Following Cyberball, analyses of two manipulation checks revealed that participants who were excluded reported receiving the ball less than included participants, t (1, 83.99) = 23.65, P < 0.001, and participants in the ostracism condition reported feeling more rejected relative to their included counterparts, t (1, 125) = −11.42, P < 0.001.
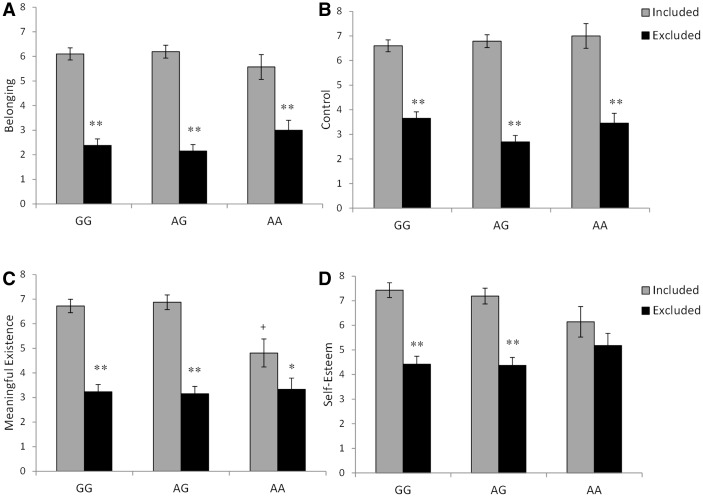
A MANOVA revealed a significant difference in the four needs as a function of the Cyberball condition, Pillai’s Trace F (4, 117) = 49.39, P < 0.001, η2 = 0.63. Furthermore, there was a significant Cyberball × OXTR genotype interaction for the four needs, Pillai’s Trace F (8, 236) = 2.82, P < 0.01, η2 = 0.09. Individual ANOVAs revealed that irrespective of OXTR genotype, Cyberball exclusion significantly reduced feelings of belonging, F (1, 126) = 236.56, P < 0.001, η2 = 0.65, and control, F (1, 126) = 171.15, P < 0.001, η2 = 0.58 (Figure 1A and B). There was a significant Cyberball × OXTR genotype interaction on meaningful existence, F (2, 120) = 3.74, P < 0.05, η2 = 0.06. As shown in the follow-up analyses of the simple effects, depicted in Figure 1C, under conditions where participants had been included in the Cyberball game, self-reports of meaningful existence were lower among the AA carriers compared with AG (P < 0.001) and GG individuals (P < 0.001). However, following exclusion in the Cyberball game, meaningful existence diminished to a greater extent in the GG (P < 0.001) and AG (P < 0.001) genotypes than in those with the AA genotype (P < 0.05), so that similar levels of meaningful existence were self-reported across the genotypes. The self-esteem profile was very much like meaningful existence but the Cyberball × OXTR genotype interaction was shy of significance, F (2, 120) = 2.67, P = .07, η2 = 0.04. Nonetheless, follow-up tests of the simple effects based on a priori predictions revealed that self-esteem was reduced among excluded individuals with the GG or AG genotype compared with their respective counterparts in the included condition, P’s < 0.001 (Figure 1D). In contrast, this difference was not evident among individuals who carried two A alleles.
Fig. 1.
Feelings of belonging (A), control (B), meaningful existence (C) and self-esteem (D) among individuals with the GG, AG or AA OXTR genotypes who were either included or excluded during the Cyberball game. Data represent means ± SEM. *P < 0.05, **P < 0.001 relative to included counterparts and +P < 0.001 relative to included GG and AG individuals.
It was of interest to examine how being excluded would affect individual judgments concerning the Cyberball co-players and to examine whether this occurred more readily in relation to a specific OXTR genotype. A MANOVA revealed a significant difference in co-player judgments between excluded and included participants irrespective of OXTR genotype, Pillai’s Trace F (13, 108) = 8.21, P < 0.001, η2 = 0.50. Individual ANOVAs revealed that excluded participants viewed their co-players as less likeable, F (1,120) = 68.42, P < 0.001, η2 = 0.36, good, F (1,120) = 35.00, P < 0.001, η2 = 0.22, trustworthy, F (1,120) = 20.44, P < 0.001, η2 = 0.15, tolerant, F (1,120) = 25.30, P < 0.001, η2 = 0.17, friendly, F (1,120) = 53.04, P < 0.001, η2 = 0.31, fair, F (1,120) = 95.38, P < 0.001, η2 = 0.44 and loyal, F (1,120) = 6.12, P < 0.05, η2 = 0.05, as well as more prejudiced, F (1,120) = 14.65, P < 0.001, η2 = 0.11, arrogant, F (1,120) = 34.59, P < 0.001, η2 = 0.22, manipulative, F (1,120) = 12.33, P < 0.01, η2 = 0.09, hypocritical, F (1,120) = 8.90, P < 0.01, η2 = 0.07 and were more likely report them as sell-outs, F (1,120) = 15.67, P < 0.001, η2 = 0.12 compared with included counterparts. Despite the negative opinion of their co-players, the ostracized participants were not more likely to describe them as less attractive compared with participants who were included, F (1, 126) = 2.90, P = .09. In effect, the participants’ negative views were limited to personality characteristics of their co-players but not their physical appearance.
Physiological measures
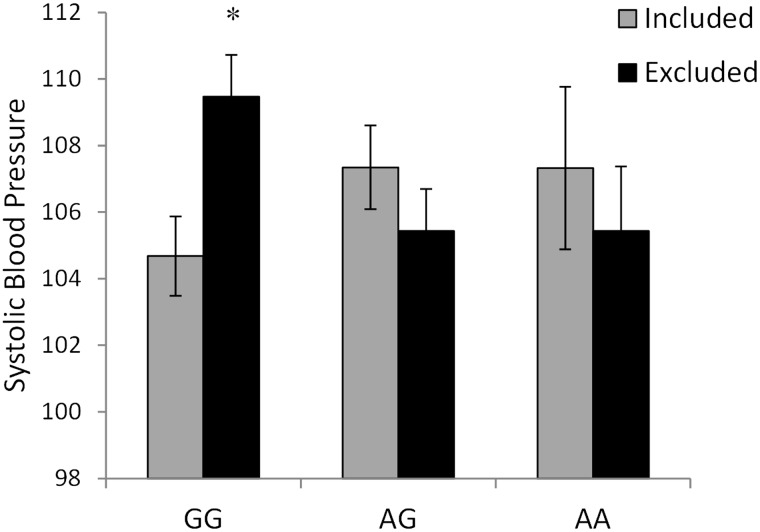
Prior to the Cyberball session, systolic blood pressure differences were not apparent as a function of OXTR genotypes, F (2,120) = 0.61, P = 0.54, or the Cyberball conditions, F (1,120) = 0.15, P = 0.70. Systolic blood pressure varied as a function of Cyberball condition × OXTR genotype × Time, F (4, 238) = 2.53, P < 0.05. Upon examining the follow-up analyses comprising this effect, blood pressure levels for included GG individuals declined across the session (P < 0.001), an effect not apparent among the AG (P = 0.13) or AA (P = 1.0) genotypes. Following exclusion, systolic blood pressure among individuals with the GG genotype remained elevated and thus did not change as a function of time, (P = 1.0). In contrast, individuals with the AG genotype had blood pressure scores that declined over the session (P < 0.01). Among individuals with the AA genotype, blood pressure declined somewhat over the session, but this effect was not significant (P = 0.14), likely owing to the limited power associated with the small number of AA individuals. A follow-up examining systolic blood pressure 30 min after Cyberball (controlling for baseline levels), varied as a function of the OXTR genotype ×Cyberball interaction, F (2,118) = 4.14, P < 0.05, η2 = 0.07. As depicted in Figure 2 and confirmed by the follow-up tests, among excluded individuals with the GG genotype, systolic blood pressure was elevated relative to that of individuals in the included condition during Cyberball (P < 0.01). In contrast to the effect of exclusion among GG individuals, a comparable effect of exclusion was not apparent among AG (P = 0.29) or AA individuals (P = 0.54). This said, among those with the AA genotype, a large amount of variability was evident, likely owing to the small number of individuals in this group. Unlike systolic blood pressure, diastolic blood pressure did not vary as a function of the OXTR genotype × Cyberball conditions.
Fig. 2.
Systolic blood pressure levels collected 30 min following either inclusion or exclusion during the Cyberball game (controlling for baseline systolic blood pressure) among individuals with the GG, AG or AA OXTR genotypes. Data represent means ± SEM. *P < 0.01 relative to included GG individuals.
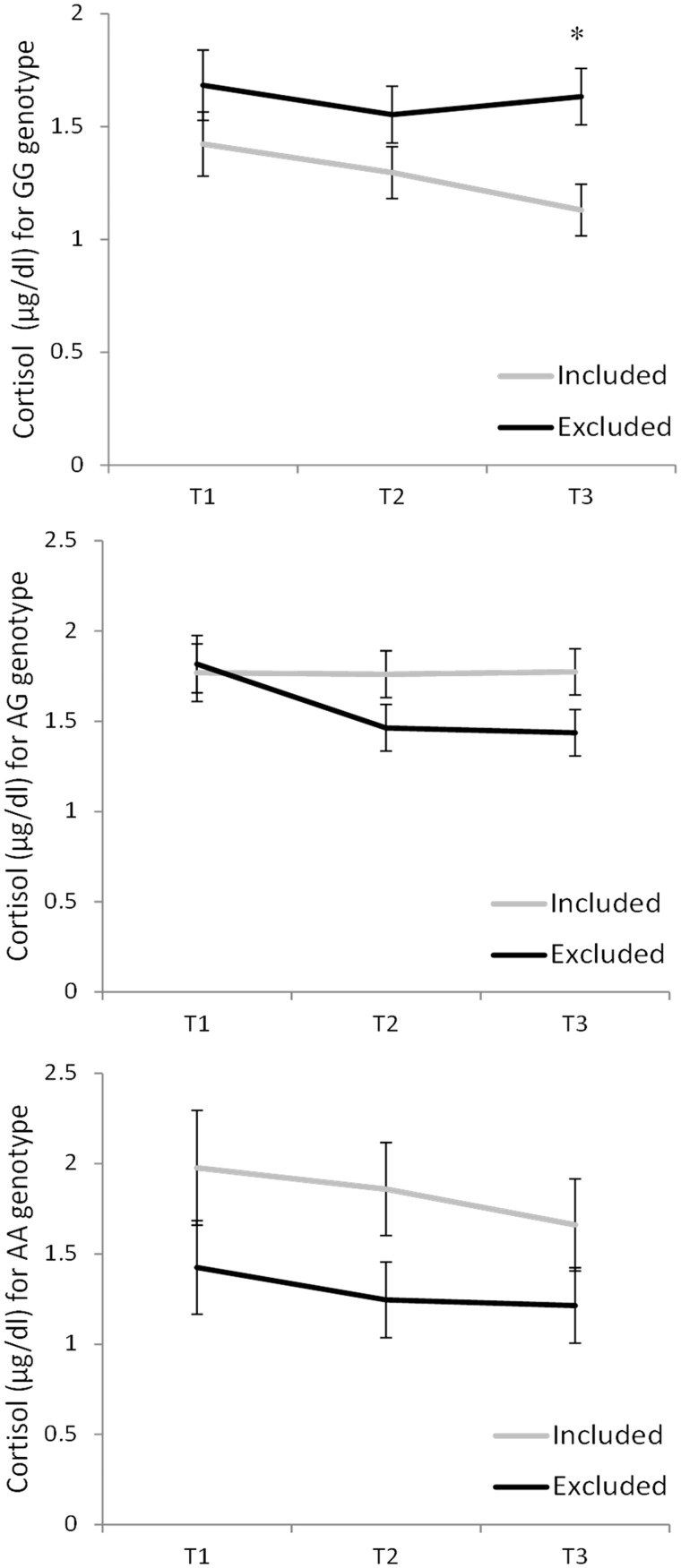
The number of cigarettes smoked, current medications including oral contraceptives, time of day and waking time did not influence cortisol and thus these variables were not controlled for in subsequent analyses. Although cortisol levels are sensitive to some laboratory stressors, such as the Trier Social Stress Test (TSST: Kirschbaum et al., 1993), which involves public speaking and mental arithmetic in front of a small audience, the levels of cortisol typically do not increase appreciably following exclusion in the Cyberball situation (Zöller et al., 2010; Zwolinski, 2012; Seidel et al., 2013). However, in this study, it was of interest to determine whether cortisol would vary with genotype. Consistent with earlier findings, relative to baseline, cortisol levels did not vary as a function of the Cyberball condition, F (2, 111) = 0.53, P = .57, but instead declined over the course of the session, F (2, 111) = 4.40, P < 0.05, η2 = 0.04. The analyses also revealed a significant Cyberball × OXTR genotype effect, F (2, 112) = 4.82, P = 0.01, η2 = 0.08, such that individuals with the GG genotype that had been excluded during Cyberball displayed cortisol levels that exceeded those of included participants (P < 0.05). In contrast, among those carrying an A allele, Cyberball exclusion did not significantly influence cortisol levels and, in fact, cortisol in those that were excluded were marginally lower than those in the included condition. Given the a priori hypothesis that the effects of the Cyberball manipulation would vary by genotype over the course of the session (i.e. baseline vs the post-testing period), follow-up tests were conducted to assess whether the effects of the Cyberball manipulation and genotype interaction further varied as a function of the time of saliva sampling. As shown in Figure 3, analyses of the simple effects revealed that among those with the GG genotype who were in the included condition within the Cyberball game, cortisol tended to decline over the course of the session. In contrast, among the GG individuals who had been in the exclusion condition, cortisol levels did not decline over the course of the session and as a result the cortisol levels in this group significantly exceeded that in the included counterparts at T3 (P < 0.01). In contrast to the effect seen in those with the GG genotype, among the AG and AA individuals, these differences between groups were not evident, and there was no indication of elevated cortisol among individuals who had been excluded in the Cyberball game relative to those individuals who were in the included condition.
Fig. 3.
Cortisol levels in saliva ( µg/dl) collected at three time points including before Cyberball (T1), 15 min following Cyberball (T2) and 30 min following Cyberball (T3). The graph represents individuals with the GG genotype (top panel), AG genotype (middle panel) and AA genotype (bottom panel) who were either included or excluded during the Cyberball game. Data represent means ± SEM. *P < 0.05 relative to included GG individuals.
To further support these analyses, and considering the small group sizes, it was also important to compute one standard measure of cortisol. As such, the AUC was calculated following a method described by Pruessner et al., (2003). There are two formulas for AUC, namely, AUC with respect to the ground (AUCG) and AUC with respect to increase (AUCI). As the current data did not display an appreciable cortisol increase following Cyberball, we used the AUCG formula. Results indicated a significant Cyberball X OXTR genotype interaction, F (2, 112) = 4.58, P < 0.05. The follow-up simple effects support the repeated measures findings that G/G individuals who were excluded displayed higher cortisol than their included counterparts (P < 0.05), an effect not apparent among AG and AA individuals.
DISCUSSION
As expected, individuals with one or two copies of the G allele could, in several ways, be distinguished from those with the AA genotype. In the absence of ostracism, individuals with the AA genotype tended to express low meaningful existence relative to G carriers. The idea that AA individuals generally feel that their presence matters less is in line with reports showing that they tend to have a more negative disposition comprising poor affect and low optimism (Saphire-Bernstein et al., 2011). When individuals were rejected in the Cyberball game, however, those carrying the G allele exhibited a more pronounced decline in their feeling that their presence in the game mattered (meaningful existence). This effect was less prominent among individuals with the AA genotype because they had lower levels of meaningful existence in the included condition in the absence of a manipulation.
As previously reported (Saphire-Bernstein et al., 2011), although individuals with the AA genotype tended to express low levels of self-esteem, they were not especially sensitive to rejection in the Cyberball game. In contrast, individuals carrying the G allele showed a decline of self-esteem upon being ostracized, potentially reflecting the elevated sensitivity of G carriers in response to a social stressor. The other two dimensions of needs described by Williams (2001), feelings of belonging and control, were also affected by ostracism, irrespective of genotype and thus all individuals perceived the rejection accurately, reflected by the lower levels of belonging and control, but the degree to which this impacted their sense of self (i.e. self-esteem) was limited in the AA individuals.
The behavioral outcomes were in line with the physiological responses, suggesting that individuals with the GG genotype were more reactive to ostracism. When individuals with the GG genotype were excluded within the Cyberball game, their systolic blood pressure was elevated relative to that of their included counterparts. This difference, however, was not apparent among AG or AA individuals who experienced ostracism, just as individuals with the GG genotype displayed greater sympathetic reactivity to a psychosocial stressor (Norman et al., 2012). However, individuals with the GG genotype also display less sympathetic reactivity in response to a non-social stressor (Rodrigues et al., 2009). Thus, it is possible that GG carriers might only be more reactive to stressors of a social nature.
As previously reported (Zöller et al., 2010; Zwolinski, 2012; Seidel et al., 2013), in the current investigation, exclusion in the Cyberball game did not elicit a cortisol rise and in the main cortisol levels declined over the course of the session. However, among ostracized individuals with the GG genotype, the decline of cortisol was not apparent, so that 30 min following Cyberball cortisol levels were greater among ostracized participants than among those in the included condition. This effect, was not apparent among ostracized AG or AA individuals, reinforcing the perspective that the GG individuals are sensitive to social insults, whereas this sensitivity may be limited in the presence of the polymorphism. These findings are very much in line with the perspective that genetic variants associated with greater interpersonal sensitivity result in increased reactions to social exclusion in the form of enhanced neural activity in the dACC and anterior insula (Eisenberger et al., 2007).
It is interesting that individuals with the AG genotype displayed psychosocial responses similar to GG carriers but physiological reactivity like that of AA carriers. Although this might seem surprising, oxytocin interacts with other hormones and neurotransmitter systems, and it is likely that different outcomes or behaviors (i.e. psychosocial responses versus physiological reactivity) involve these diverse interactions (McQuaid et al., 2014). For instance, oxytocin may interact with mesolimbic dopamine functioning, so that the rewarding attributes of particular stimuli take on greater salience (Love, 2014), and oxytocin also influences amygdala activity (Kirsch et al., 2005; Petrovic et al., 2008), possibly through actions on γ-aminobutyric acid, so that fear reactions are altered (Huber et al., 2005). The divergent outcomes related to oxytocin interactions with other hormones in the context of specific behaviors among those who are heterozygous regarding the OXTR polymorphism, speaks to the importance of examining the three OXTR genotypes separately whenever possible.
Several beneficial traits have been observed among G carriers; yet, it was also proposed that individuals with this genotype might be more sensitive to their environments (Bradley et al., 2011; McQuaid et al., 2013). In this regard, individuals with one or two copies of the G allele displayed greater emotional dysregulation (Bradley et al., 2011) and depressive symptoms (McQuaid et al., 2013) in the context of high levels of early-life maltreatment. Conversely, G carriers displayed higher positive affect and resilience if they were raised in a warm family environment (Bradley et al., 2013). These findings are congruent with the view that certain genotypes confer greater plasticity in the context of both positive and negative environmental stimuli, thereby affecting behavior ‘for better or for worse’ (Belsky et al., 2009). However, the data supporting this view have not been unanimous. For instance, youth with at least one A allele and raised with a depressed mother experienced particularly high levels of depressive symptoms at age 15 years (Thompson et al., 2014). Maternal depression certainly might offer a negative environment, although this may not necessarily be equivalent to experiencing maltreatment in the form of abuse and/or neglect, which likely constitutes a breach of trust that might have a greater impact on G carriers (McQuaid et al., 2013).
There are several limitations of this study that should be acknowledged. Although we and others have suggested that individuals with the G allele of the OXTR rs53576 SNP may be more socially sensitive, possibly owing to the oxytocin system operating differently than in AA individuals, the functionality of this particular SNP is still unknown. It has been hypothesized that this OXTR SNP, which is located on intron 3, may be involved in transcriptional suppression (Mizumoto et al., 1997), but it may also be that the effects observed in the current investigation were due to linkage(s) with other functional OXTR SNPs (Lin et al., 2007). In addition, the sample size in this study was modest, and it certainly would have been ideal to have greater power through a larger number of AA participants. Despite these limitations, the current findings suggested that individuals with the GG genotype, who are typically viewed as having many beneficial traits, were emotionally and biologically more affected by ostracism. At the same time, even in the face of this brief rejection from unknown co-players, ostracized participants tended to judge them harshly, irrespective of their oxytocin genotype. Evidently, regardless of their genotype, individuals are able to recognize slights experienced, but in line with our previous suggestion (McQuaid et al., 2013), those with the GG genotype for this OXTR SNP are more adversely affected by negative social experiences. The current findings provide support for the view that oxytocin functioning, besides promoting prosocial behaviors, might also enable higher social sensitivity or reactivity to social challenges. In this regard, it has been suggested (Cardoso et al., 2014) that treatment with an oxytocin nasal spray might enhance mood state among some individuals, but others may engender excessive sensitivity, rendering individuals more vulnerable to the negative impacts of social stressors. Knowledge of an individual’s genotype might be useful as a biomarker to determine vulnerability to adverse effects of social stressors and might be useful in predicting the efficacy of treatment options.
Acknowledgments
This work was supported by the Canadian Institutes of Health Research (CIHR) (MOP-106591). H.A. holds a Canadian Research Chair in Neuroscience. R.J.M. was supported by the CIHR Frederick Banting and Charles Best Canada Graduate Scholarship.
REFERENCES
- Averbeck BB. Oxytocin and the salience of social cues. Proceedings of the National Academy of Science of the United States of America. 2010;107:9033–4. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–34. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effect of oxytocin in humans: context and person matter. Trends in Cognitive Science. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Molecular Psychiatry. 2009;14:746–54. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Davis TA, Wingo AP, Mercer KB, Ressler KJ. Family environment and adult resilience: contributions of positive parenting and the oxytocin receptor gene. European Journal of Psychotraumatology. 2013;4:21659. doi: 10.3402/ejpt.v4i0.21659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley B, Westen D, Mercer KB, et al. Association between childhood maltreatment and adult emotional dysregulation in a low-income, urban, African American sample: moderation by oxytocin receptor gene. Development and Psychopathology. 2011;23:439–52. doi: 10.1017/S0954579411000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Linnen AM. The effect of intranasal oxytocin on perceiving and understanding emotion on the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT) Emotion. 2014;14:43–50. doi: 10.1037/a0034314. [DOI] [PubMed] [Google Scholar]
- Cardoso C, Ellenbogen MA, Serravalle L, Linnen AM. Stress-induced negative mood moderates the relation between oxytocin administration and trust: Evidence for the tend-and-befriend response to stress? Psychoneuroendocrinology. 2013;38:2800–4. doi: 10.1016/j.psyneuen.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nature Reviews Neuroscience. 2012;13:421–34. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Jarcho JM, Lieberman MD, Naliboff BD. An experimental study of shared sensitivity to physical pain and social rejection. Pain. 2006;126:132–8. doi: 10.1016/j.pain.2006.06.024. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? An FMRI study of social exclusion. Science. 2003;302:290–2. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding genetic risk for aggression: clues from the brain’s response to social exclusion. Biological Psychiatry. 2007;61:1100–8. doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Feifel D, Macdonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophrenia Research. 2012;139:207–10. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function and regulation. Physiological Reviews. 2001;81:629–83. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Einfeld SL, Gray KM, et al. Intranasal oxytocin improves emotion recognition for youth with autism spectrum disorders. Biological Psychiatry. 2010;67:692–4. doi: 10.1016/j.biopsych.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Huber D, Veinante P, Stoop R. Vasopressin and oxytocin excite distinct neuronal populations in the central amygdala. Science. 2005;308:245–8. doi: 10.1126/science.1105636. [DOI] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, et al. Oxytocin modulates neural circuitry for social cognition and fear in humans. The Journal of Neuroscience. 2005;25:11489–93. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Sherman DK, Sasaki JY, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proceedings of the National Academy of Science of the United States of America. 2010;107:15717–21. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ – a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Krueger F, Parasuraman R, Iyengar V, et al. Oxytocin receptor genetic variation promotes human trust behavior. Frontiers in Human Neuroscience. 2012;6 doi: 10.3389/fnhum.2012.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnen A-M, Ellenbogen MA, Cardoso C, Joober R. Intranasal oxytocin and salivary cortisol concentrations during social rejection in university students. Stress. 2012;15:393–402. doi: 10.3109/10253890.2011.631154. [DOI] [PubMed] [Google Scholar]
- Lin P-I, Vance JM, Pericak-Vance MA, Martin ER. No gene is an island: the flip-flop phenomenon. The American Journal of Human Genetics. 2007;80:531–8. doi: 10.1086/512133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love TM. Oxytocin, motivation and the role of dopamine. Pharmacology, Biochemistry, and Behavior. 2014;119C:49–60. doi: 10.1016/j.pbb.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucht MJ, Barnow S, Sonnenfeld C, et al. Associations between the oxytocin receptor gene (OXTR) and affect, loneliness and intelligence in normal subject. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2009;33:860–6. doi: 10.1016/j.pnpbp.2009.04.004. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, Anisman H. Making room for oxytocin in understanding depression. Neuroscience and Biobehavioral Reviews. 2014;45:305–22. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Stead JD, Matheson K, Anisman H. A paradoxical association of an oxytocin receptor gene polymorphism: Early-life adversity and vulnerability to depression. Frontiers in Neuroscience. 2013;7 doi: 10.3389/fnins.2013.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumoto Y, Kimura T, Ivell R. A genomic element within the third intron of the human oxytocin receptor gene may be involved in transcriptional suppression. Molecular Cell Endocrinology. 1997;135:129–38. doi: 10.1016/s0303-7207(97)00195-0. [DOI] [PubMed] [Google Scholar]
- Norman GJ, Hawkley L, Luhmann M, et al. Variation in the oxytocin receptor gene influences neurocardiac reactivity to social stress and HPA function: a population based study. Hormones and behavior. 2012;61:134–9. doi: 10.1016/j.yhbeh.2011.11.006. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalisch R, Singer T, Dolan RJ. Oxytocin attenuates affective evaluations of conditioned faces and amygdala activity. The Journal of Neuroscience. 2008;28:6607–15. doi: 10.1523/JNEUROSCI.4572-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–31. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation related to empathy and stress reactivity in humans. Proceedings of the National Academy of Science of the United States of America. 2009;106:21437–41. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proceedings of the National Academy of Science of the United States of America. 2011;108:15118–22. doi: 10.1073/pnas.1113137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scantamburlo G, Hansenne M, Fuchs S, et al. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32:407–10. doi: 10.1016/j.psyneuen.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Seidel EM, Silani G, Metzler H, et al. The impact of social exclusion vs. inclusion on subjective and hormonal reactions in females and males. Psychoneuroendocrinology. 2013;38:2925–32. doi: 10.1016/j.psyneuen.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KE, Porges EC, Norman GJ, Connelly JJ, Decety J. Oxytocin receptor gene variation predicts empathetic concern and autonomic arousal while perceiving harm to others. Social Neuroscience. 2014;9:1–9. doi: 10.1080/17470919.2013.863223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11–9. doi: 10.1016/j.psyneuen.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KD. Social ostracism. In: Kowalski RM, editor. Aversive Interpersonal Behaviors. New York: Plenum; 1997. pp. 133–70. [Google Scholar]
- Williams KD. Ostracism: The Power of Silence. New York: Guilford Press; 2001. [Google Scholar]
- Williams KD, Cheung CKT, Choi W. CyberOstracism: effects of being ignored over the internet. Journal of Personality and Social Psychology. 2000;79:748–62. doi: 10.1037//0022-3514.79.5.748. [DOI] [PubMed] [Google Scholar]
- Zadro L, Williams KD, Richardson R. How low can you go? Ostracism by a computer is sufficient to lower self-reported levels of belonging, control, self-esteem, and meaningful existence. Journal of Experimental Psychology. 2004;40:560–7. [Google Scholar]
- Zöller C, Maroof P, Weik U, Deinzer R. No effect of social exclusion on salivary cortisol secretion in women in a randomized controlled study. Psychoneuroendocrinology. 2010;35:1294–8. doi: 10.1016/j.psyneuen.2010.02.019. [DOI] [PubMed] [Google Scholar]
- Zwolinski J. Psychological and neuroendocrine reactivity to ostracism. Aggressive Behavior. 2012;38:108–25. doi: 10.1002/ab.21411. [DOI] [PubMed] [Google Scholar]