Abstract
Objectives
The present study is the first meta-analysis to compare the surgical outcomes of robotic vs. conventional mitral valve surgery in patients with degenerative mitral valve disease.
Methods
A systematic review of the literature was conducted to identify all relevant studies with comparative data on robotic vs. conventional mitral valve surgery. Predefined primary endpoints included mortality, stroke and reoperation for bleeding. Secondary endpoints included cross-clamp time, cardiopulmonary bypass time, length of hospitalization and duration of intensive care unit (ICU) stay. Echocardiographic outcomes were assessed when possible.
Results
Six relevant retrospective studies with comparative data for robotic vs. conventional mitral valve surgery were identified from the existing literature. Meta-analysis demonstrated a superior perioperative survival outcome for patients who underwent robotic surgery. Incidences of stroke and reoperation were not statistically different between the two treatment arms. Patients who underwent robotic surgery required a significantly longer period of cardiopulmonary bypass time and cross-clamp time. However, the lengths of hospitalization and ICU stay were not significantly different. Both surgical techniques appeared to achieve satisfactory echocardiographic outcomes in the majority of patients.
Conclusions
Current evidence on comparative outcomes of robotic vs. conventional mitral surgery is limited, and results of the present meta-analysis should be interpreted with caution due to differing patient characteristics. However, it has been demonstrated that robotic mitral valve surgery can be safely performed by expert surgeons for selected patients. A successful robotic program is dependent on a specially trained team and a sufficient volume of referrals to attain and maintain safety.
Keywords: Mini-mitral, robotic surgery, meta-analysis, mitral valve
Introduction
Over the past few decades, minimally invasive surgery has transformed the landscape of cardiothoracic surgery in a wide range of procedures (1-3). Mitral valve surgery has traditionally been performed through a conventional sternotomy approach. However, a number of studies have demonstrated that comparable repair techniques can be performed in selected patients through a minimally invasive right mini-thoracotomy approach with potentially superior perioperative outcomes (4,5). More recently, robotic surgery involving telemanipulation systems has been developed to enable surgeons to perform mitral valve surgery with increased degrees of movement and improved vision.
Despite technological improvements in miniature instrumentation and development of operative techniques through increased experience, the extent of benefits derived from robotic surgery in patients with mitral valve disease remains uncertain. Currently, robotic mitral valve surgery remains limited to specialized centers for patients with isolated mitral valve pathology. To systematically appraise the available evidence, the present study aimed to identify all relevant studies on mitral valve surgery to compare the outcomes of robotic vs. conventional approaches. Primary endpoints included mortality, stroke and re-operation for bleeding. Secondary endpoints included length of hospitalization, duration of intensive care unit (ICU) stay, cross-clamp time and cardiopulmonary bypass time. Echocardiographic outcomes before and after robotic or conventional surgery were also assessed. This study represents the first meta-analysis of robotic vs. conventional mitral valve surgery.
Methods
Literature search strategy
Electronic searches were performed using Cochrane Database of Systematic Reviews, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Heath Technology Assessment, NHS Economic Evaluation Database, Embase and Ovid Medline from 1995 to July 2013. To achieve the maximum sensitivity of the search strategy and identify all studies, we combined the terms “mini*” or “thoraco*” or “video*” or “robot*” or “laparoscop*” or “endoscop*” or “port-access” or “port access” or “partial sternotomy” or “keyhole” and “mitral*” or “Barlow*” as either keywords or MeSH terms. The reference lists of all retrieved articles were reviewed for further identification of potentially relevant studies. All relevant articles identified were assessed with application of the selection criteria.
Selection criteria
Eligible comparative studies for the present meta-analysis included those in which patients with mitral valve disease underwent surgery either through the conventional sternotomy or robotic approach. When institutions reported duplicated trials with accumulating numbers of patients or increased lengths of follow-up, only the most complete studies were included. All publications were limited to human subjects and English language. Abstracts, case studies, conference presentations, editorials and letters were excluded.
Data extraction and critical appraisal
All data were extracted from article texts, tables and figures. When insufficient data were available from publications, corresponding authors were contacted to provide additional records. Three investigators (S. G., T. A. N., and D. C.) independently reviewed each retrieved article. Discrepancies between the reviewers were resolved by discussion and consensus. The final results were reviewed by the senior investigators (C. C. and T. D. Y.).
Statistical analysis
Meta-analysis was performed by combining the results of reported incidences of mortality, stroke, re-operation for bleeding and durations of hospitalization, ICU stay, cross-clamp time and cardiopulmonary bypass time when comparable outcomes were available. The relative risk (RR) was used as a summary statistic and the random effects model was tested, as it was assumed there were variations between studies and the calculated ratios had a more conservative value (6). χ2 tests were used to study heterogeneity between trials. I2 statistic was used to estimate the percentage of total variation across studies, due to heterogeneity rather than chance. An I2 value of greater than 50% was considered substantial heterogeneity. If there was substantial heterogeneity, the possible clinical and methodological reasons for this were explored qualitatively. All P values were two-sided. All statistical analysis was conducted with Review Manager Version 5.1.2 (Cochrane Collaboration, Software Update, Oxford, United Kingdom).
Results
Quantity and quality of trials
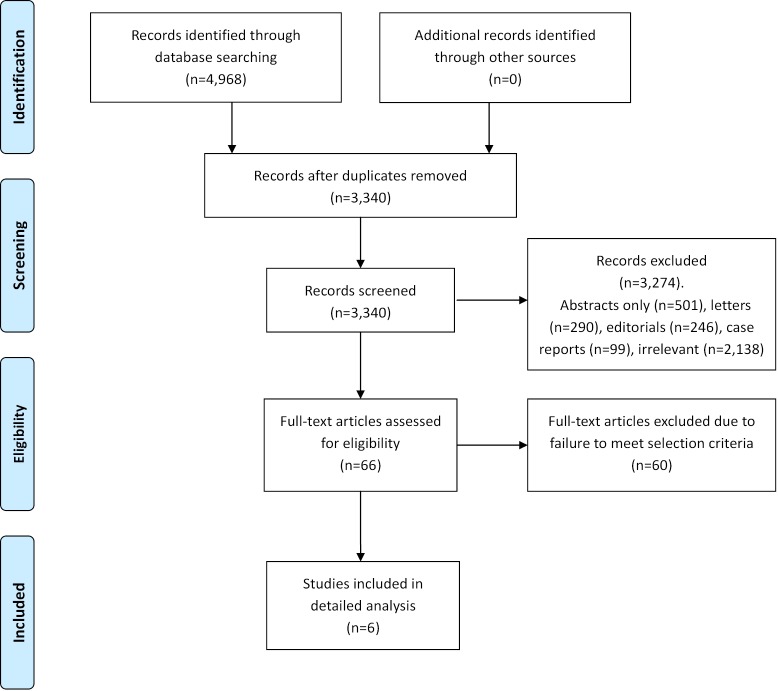
A total of 4,968 references were identified through the nine electronic database searches. After exclusion of duplicate or irrelevant references, 66 potentially relevant articles were retrieved for more detailed evaluation. After applying the selection criteria, six comparative studies remained for assessment. Manual search of the reference lists did not identify any additional relevant studies. A summary of the search strategy is presented in Figure 1. All of selected studies were retrospective observational studies, as summarized in Table 1 (7-12). In these six studies, 1,650 patients who underwent mitral valve surgery were compared, including 960 patients who underwent the robotic approach and 690 patients who underwent the conventional sternotomy approach.
Figure 1.
PRISMA chart summarizing the search strategy performed to identify relevant comparative studies on robotic vs. conventional mitral valve surgery.
Table 1. Study characteristics of relevant articles identified for meta-analysis comparing robotic vs. conventional sternotomy approaches for patients undergoing mitral valve surgery.
| Author | Year | Institution | Study period | Robotic (n) | Sternotomy (n) | Follow-up period (months) |
|---|---|---|---|---|---|---|
| Stevens (7) | 2012 | East Carolina University, USA | 1992-2009 | 447 | 377 | 77±54 |
| Mihaljevic (8) | 2011 | Cleveland Clinic, USA | 2006-2009 | 261 | 114 | NR |
| Suri (9) | 2011 | Mayo Clinic, USA | 2007-2010 | 95 | 95 | NR |
| Kam (10) | 2010 | Epworth HealthCare Network, Australia | 2005-2008 | 107 | 40 | NR |
| Folliguet (11) | 2006 | Institut Mutualiste Montsouris, France | 2000-2005 | 25 | 25 | At least 24 |
| Woo (12) | 2006 | University of Pennsylvania, USA | 2002-2005 | 25 | 39 | NR |
NR, not reported.
Patient characteristics
Four out of the six selected studies reported that all patients in both the robotic and conventional groups had isolated myxomatous valve pathology (8-11). One study did not provide sufficient pathological details (12) and another study included a significant proportion of patients who had other mitral valve pathologies (7). Two retrospective studies matched patient cohorts according to prognostic factors (8,9). However, patients in the robotic group tended to be younger, with fewer incidences of diabetes and hypertension. In addition, there was a trend towards better functional status for patients included in the robotic surgical arm. A summary of these patient baseline characteristics is presented in Tables 2,3.
Table 2. A summary of patient baseline characteristics in comparative studies on robotic vs. conventional mitral valve surgery.
| Author | Age (mean ± SD) |
Male gender, n [%] |
DM, n [%] |
HTN, n [%] |
PVD, n [%] |
COPD, n [%] |
Previous stroke, n [%] |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | |||||||
| Stevens (7) | 57±13 | 60±14 | 279 [62] | 180 [48] | 25 [6] | 58 [15] | 188 [42] | 184 [49] | 3 [1]* | 14 [4]* | 35 [8] | 33 [9] | NR | NR | ||||||
| Mihaljevic (8) | 56±11 | 61±11 | 204 [78] | 85 [75] | 5 [2] | 6 [5] | 102 [40] | 52 [46] | 29 [11] | 3 [3] | 10 [4]^ | 10 [9]^ | 3 [1] | 2 [2] | ||||||
| Suri (9) | 55±11 | 56±14 | 73 [77] | 76 [80] | 1 [1] | 2 [2] | 32 [34] | 29 [31] | 0 | 0 | 6 [6] | 5 [5] | 1 [1] | 0 | ||||||
| Kam (10) | 58±14 | 62±11 | 76 [71] | 33 [83] | 1 [1] | 1 [3] | 32 [30] | 15 [38] | 0 | 0 | NR | NR | 4 [4] | 2 [5] | ||||||
| Folliguet (11) | 59±11 | 60±11 | 16 [64] | 17 [68] | NR | NR | NR | NR | NR | NR | 0 | 0 | NR | NR | ||||||
| Woo (12) | 60±3 | 60±2 | 17 [68] | 24 [62] | 1 [4] | 3 [8] | NR | NR | 3 [12] | 4 [10] | NR | NR | 4 [16] | 5 [13] | ||||||
*, patients with severe PVD were omitted; ^, specifically mentions COPD only. NR, not reported; SD, standard deviation; DM, diabetes mellitus; HTN, hypertension; PVD, peripheral vascular disease; COPD, chronic obstructive pulmonary disease.
Table 3. A summary of patient baseline functional status and underlying valvular pathology.
| Author | NYHA, n [%] |
Valvular pathology, n [%] |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robotic |
Sternotomy |
Robotic |
Sternotomy |
||||||||||||||||||
| I | II | III | IV | I | II | III | IV | Myxo. Degen. | Isch. | Infec. | Rheum. | Funct. | Other | Myxo. Degen. | Isch. | Infec. | Rheum. | Funct. | Other | ||
| Stevens (7) | 339 [76] | 108 [24] | 177 [47] | 200 [53] | 358 [80] | 4 [1] | 20 [4] | 16 [4] | 38 [9] | 11 [2] | 165 [44] | 24 [6] | 32 [8] | 56 [15] | 23 [6] | 77 [20] | |||||
| Mihaljevic (8) | 131 [50] | 97 [37] | 31 [12] | 2 [1] | 37 [33] | 54 [47] | 22 [19] | 1 [1] | 261 [100] | 0 | 0 | 0 | 0 | 0 | 114 [100] | 0 | 0 | 0 | 0 | 0 | |
| Suri (9) | 85 [89] | 10 [11] | 86 [91] | 9 [9] | 95 [100] | 0 | 0 | 0 | 0 | 0 | 95 [100] | 0 | 0 | 0 | 0 | 0 | |||||
| Kam (10) | NR | NR | NR | NR | NR | NR | NR | NR | 107 [100] | 0 | 0 | 0 | 0 | 0 | 40 [100] | 0 | 0 | 0 | 0 | 0 | |
| Folliguet (11) | 17 [68] | 6 [24] | 2 [4] | 0 | 16 [64] | 5 [20] | 4 [16] | 0 | 25 [100] | 0 | 0 | 0 | 0 | 0 | 25 [100] | 0 | 0 | 0 | 0 | 0 | |
| Woo (12) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
NYHA, New York Heart Association functional classification; myxo degen, myxomatous degeneration; isch, ischaemic; infec, infective; rheum, rheumatic; funct, functional; NR, not reported.
Surgical techniques
All of the reported robotic mitral valve surgeries were performed through two or three ports and a 3-5 cm right mini-thoracotomy using the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, USA). Three out of the six studies reported using the endoaortic balloon occlusion device for patients who underwent robotic surgery (7,8,12). Concomitant surgical procedures such as atrial fibrillation ablation and atrial septal defect closures were reported in three studies (7-9). A summary of procedural details, including the cardioplegia strategy and repair techniques, is presented in Table 4.
Table 4. A summary of surgical techniques performed during mitral valve surgery through robotic or sternotomy approaches.
| Author | Robotic access approach | Robotic clamp technique | Cardioplegia |
Mitral-valve repair details |
Concomitant surgery |
Conversion to sternotomy | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Robotic | Sternotomy | Robotic | Sternotomy | Robotic | Sternotomy | |||||||
| Stevens (7) | 3 ports & 3-4 cm incision right infra-mammary fold | Aortic cross-clamp or endoballoon | AG & RG | Isolated annuloplasty (54/442); chordal procedure (223/442); post. leaflet resection (245/442) |
Isolated annuloplasty (78/208); chordal procedure (46/208); post. leaflet resection (74/208) |
Leaflet sliding plasty (103/442); AF ablation (84/447) |
Leaflet sliding plasty (16/208); AF ablation (22/377) |
NR | ||||
| Mihaljevic (8) | 3 ports & mini-thoracotomy in 4th right ICS (mid-axillary) | Aortic cross-clamp or endoballoon | AG & RG | Annuloplasty (261/261); leaflet resection (243/261); chordal procedure (8/261); edge-to-edge (26/261) |
Annuloplasty (113/114); leaflet resection (105/114); chordal procedure (5/114); edge-to-edge (4/114) |
ASD/PFO closure (34/261); AF ablation (22/261) |
ASD/PFO closure (7/114); AF ablation (31/114) |
24/261 | ||||
| Suri (9) | 3 ports & 4 cm incision lateral to camera port | Aortic cross-clamp | AG | NR | Annuloplasty and leaflet correction |
Annuloplasty and leaflet correction | ASD/PFO closure; AF ablation (MAZE procedures) |
ASD/PFO closure; AF ablation (MAZE procedures) |
0/95 | |||
| Kam (10) | 2 ports & 4 cm right thoracotomy (<4 cm) | NR | NR | NR | NR | NR | NR | NR | NR | |||
| Folliguet (11) | 2 ports & 4-5 cm 5th right ICS lateral incision | Aortic cross-clamp | AG | NR | Quadrangular resection; open band annuloplasty |
Quadrangular resection; open band or closed ring |
NR | NR | 1/25 (extended thoracotomy) | |||
| Woo (12) | 3 ports & 4th right ICS incision | Aortic cross-clamp or endoballoon | AG & RG | Sternotomy | Leaflet, chordal and annular reconstruction as needed | NR | NR | NR | ||||
AG, antegrade; RG, retrograde; NR, not reported; ICS, intercostal space; AF, atrial fibrillation; ASD; atrial septal defect; PFO, patent foramen ovale.
Assessment of primary endpoints
Mortality
Although all six selected studies reported the incidences of all-cause perioperative mortality, only two studies reported deaths that occurred. From the available data, patients who underwent mitral surgery through the robotic approach had a significantly lower incidence of mortality compared to the conventional sternotomy approach [0.5% vs. 2.2%; RR, 0.32; 95% confidence interval (CI), 0.12-0.83; P=0.02; I2=0%]. These results are presented in Figure 2.
Figure 2.
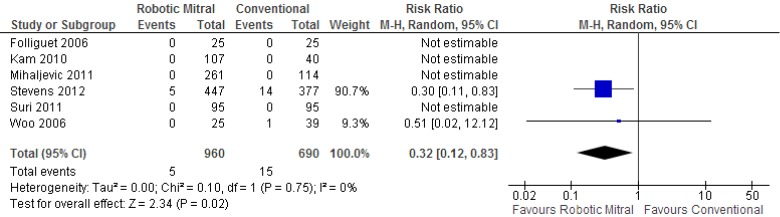
Forest plot of the relative risk (RR) of perioperative mortality after robotic vs. conventional mitral valve surgery. The estimate of the RR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary RR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials is given below the summary statistics.
Stroke
Three studies reported on the incidences of perioperative stroke, and two studies recorded events. From the available data, there was no significant difference between the robotic vs. conventional approaches (0.8% vs. 2.4%; RR, 0.50; 95% CI, 0.05-4.65; P=0.54; I2=65%). These results are presented in Figure 3.
Figure 3.
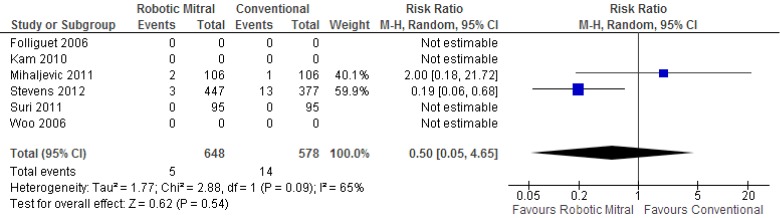
Forest plot of the relative risk (RR) of perioperative stroke after robotic vs. conventional mitral valve surgery. The estimate of the RR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary RR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials is given below the summary statistics.
Re-operation
Re-operation for bleeding was reported in all studies, and there were no significant differences between the robotic vs. conventional approaches (3.0% vs. 3.7%; RR, 0.82; 95% CI, 0.47-1.42; P=0.47; I2=0%). These results are presented in Figure 4.
Figure 4.
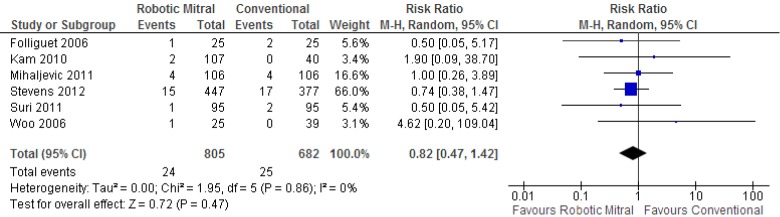
Forest plot of the relative risk (RR) of re-operation for bleeding after robotic vs. conventional mitral valve surgery. The estimate of the RR of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). On each line, the numbers of events as a fraction of the total number randomized are shown for both treatment groups. For each subgroup, the sum of the statistics, along with the summary RR, is represented by the middle of the solid diamonds. A test of heterogeneity between the trials is given below the summary statistics.
Assessment of secondary endpoints
Meta-analysis identified a significantly longer duration of cross-clamp time [standardized mean difference (SMD), 2.05; 95% CI, 1.23-2.87; P<0.00001; I2=94%] and cardiopulmonary bypass time (SMD, 3.03; 95% CI, 0.84-5.23; P=0.007; I2=98%) for patients who underwent robotic surgery vs. conventional approach. These results are presented in Figures 5,6, respectively. The lengths of hospitalization (SMD, –1.07; 95% CI, –2.83-0.70; P=0.24; I2=96%) and ICU stay (SMD, –1.58; 95% CI, –3.45-0.29; P=0.10; I2=97%) were not significantly different between the two approaches.
Figure 5.
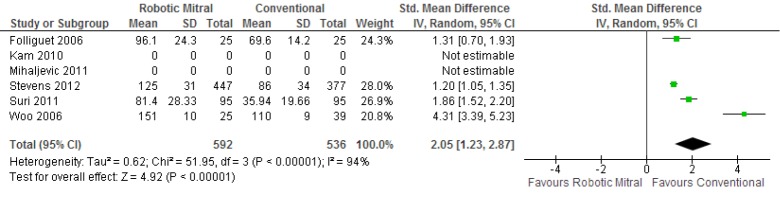
Forest plot of the standardized mean difference (SMD) of duration of cross-clamp time after robotic vs. conventional mitral valve surgery. The estimate of the SMD of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). A test of heterogeneity between the trials is given below the summary statistics.
Figure 6.
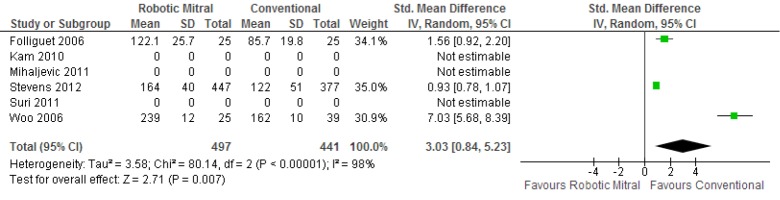
Forest plot of the standardized mean difference (SMD) of duration of cardiopulmonary bypass time after robotic vs. conventional mitral valve surgery. The estimate of the SMD of each trial corresponds to the middle of the squares, and the horizontal line shows the 95% confidence interval (CI). A test of heterogeneity between the trials is given below the summary statistics.
Sensitivity analysis
Stevens et al. reported a number of significant differences between the baseline patient characteristics of the robotic and conventional treatment groups (7). In addition, unlike other studies included in the present meta-analysis, a significant proportion of patients had ischaemic, rheumatic, infective or functional mitral valve pathology. Hence, a sensitivity analysis was performed by excluding data from this study. By doing so, the perioperative mortality outcome was no longer statistically significant between the robotic vs. conventional treatment arms, as only one other study reported any incidence of deaths.
Echocardiography outcomes
Available echocardiographic findings were categorized into predefined severities of mitral regurgitation. Preoperatively, 443/502 patients undergoing robotic surgery had severe regurgitation and 59/502 had moderate regurgitation. The respective figures were 264/297 and 33/297 for patients undergoing conventional sternotomy. Postoperatively, 4/346 patients who underwent robotic surgery had moderate regurgitation, 24/346 had mild regurgitation and 318/346 had trivial or no residual regurgitation. The corresponding figures were 4/285, 18/285 and 263/285 for patients who underwent conventional sternotomy. A summary of these echocardiographic findings before and after surgery is presented in Figure 7A,B.
Figure 7.
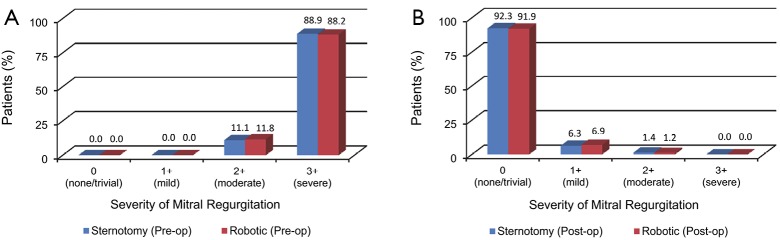
A summary of echocardiographic findings of the severity of mitral valve regurgitation. (A) Before robotic or conventional mitral valve surgery; and (B), after robotic or conventional mitral valve surgery.
Discussion
Mitral valve surgery through the robotic approach enables the surgeon minimal access and 7 degrees of freedom of movement, with additional benefits of three-dimensional visualization, tremor minimization and avoidance of the fulcrum effect associated with long-shafted endoscopic instruments. Proponents of robotic mitral surgery propose that similar surgical repairs can be performed through the minimally invasive approach as conventional sternotomy, with potential benefits of reduced trauma and associated advantages of reduced blood product requirement and shorter hospitalization (12). Expert robotic surgeons from specialized institutions have demonstrated the feasibility of performing complicated mitral repair techniques through the robotic approach without compromising surgical outcomes (7). Recently, Nifong et al. reported excellent results in a group of 540 patients who underwent robotic mitral valve repairs including concomitant biatrial cryoablation procedure (13).
To systematically evaluate the outcomes of robotic vs. conventional mitral surgery, the present meta-analysis identified all comparative studies in the existing literature and found that robotic mitral valve surgery was associated with a statistically significant survival benefit over conventional surgery. Perioperatively, there were no significant differences in regards to the incidences of stroke or re-operation for bleeding. Cross-clamp and cardiopulmonary bypass times were significantly longer for robotic surgery, but the durations of hospitalization and ICU stay were not statistically different between the two surgical techniques.
A number of limitations to our study should be acknowledged and the results should be interpreted with caution. Firstly, our systematic review of the current literature has demonstrated that the quantity and quality of the existing evidence for robotic vs. conventional surgery is very limited. All six studies included in the meta-analysis were retrospective case-series reports with differences between the robotic and conventional patient cohorts. Without randomization, surgical outcomes may reflect the patient characteristics rather than the surgical intervention. However, attempts were made in two studies to match patients according to propensity scores to minimize selection bias (8,9). The large study by Stevens et al. reported a heterogenous cohort of patients with various mitral valve pathologies and unbalanced patient baseline characteristics. When data from this study was excluded from analysis, the mortality difference between the two surgical techniques was no longer significant. Secondly, as with any novel surgical or medical intervention, there is the possibility for publication bias, with potentially more favorable outcomes being reported from large volume expert centers that may not be representative of all institutions. Finally, significant heterogeneity was detected for the analysis of stroke and secondary outcomes such as cross-clamp time, cardiopulmonary bypass duration and length of hospitalization. This may reflect the limited number of studies and different surgical techniques and clinical practices, respectively.
In conclusion, the present meta-analysis identified a statistical survival benefit for robotic mitral valve surgery vs. the conventional sternotomy approach. However, this was largely a reflection of the heterogenous patient cohort between the two treatment arms within the identified retrospective studies. Without randomization and adequate patient matching, the existing evidence for robotic surgery is not robust, and limited conclusions can be drawn from the current literature. However, it has been demonstrated that robotic mitral valve surgery can be feasibly performed by expert surgeons for selected patients. These centers have recognized that a successful robotic surgical program is dependent on the development of a highly specialized and well-trained team including anesthesiologists, perfusionists, operating room staff, and surgeons (14). In addition, they believe that surgical units must be able to maintain a sufficient volume of referrals to attain and maintain safety. As there is currently limited robust clinical data on the surgical outcomes of robotic mitral valve surgery, this novel procedure should be limited to specialized centers that fulfil the above criteria. Ultimately, surgical outcomes of mitral valve surgery will be dependent on choosing the right procedure by the right surgeon for the right patient, rather than by the length of the skin incision (15).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Cao C, Ang SC, Indraratna P, et al. Systematic review and meta-analysis of transcatheter aortic valve implantation versus surgical aortic valve replacement for severe aortic stenosis. Ann Cardiothorac Surg 2013;2:10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao C, Manganas C, Ang SC, et al. A meta-analysis of unmatched and matched patients comparing video-assisted thoracoscopic lobectomy and conventional open lobectomy. Ann Cardiothorac Surg 2012;1:16-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: a randomized trial. J Thorac Cardiovasc Surg 2011;142:77-83. [DOI] [PubMed] [Google Scholar]
- 5.Ryan WH, Brinkman WT, Dewey TM, et al. Mitral valve surgery: comparison of outcomes in matched sternotomy and port access groups. J Heart Valve Dis 2010;19:51-8; discussion 59. [PubMed] [Google Scholar]
- 6.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [DOI] [PubMed] [Google Scholar]
- 7.Stevens LM, Rodriguez E, Lehr EJ, et al. Impact of timing and surgical approach on outcomes after mitral valve regurgitation operations. Ann Thorac Surg 2012;93:1462-8. [DOI] [PubMed] [Google Scholar]
- 8.Mihaljevic T, Jarrett CM, Gillinov AM, et al. Robotic repair of posterior mitral valve prolapse versus conventional approaches: potential realized. J Thorac Cardiovasc Surg 2011;141:72-80.e1-4. [DOI] [PubMed]
- 9.Suri RM, Burkhart HM, Daly RC, et al. Robotic mitral valve repair for all prolapse subsets using techniques identical to open valvuloplasty: establishing the benchmark against which percutaneous interventions should be judged. J Thorac Cardiovasc Surg 2011;142:970-9. [DOI] [PubMed] [Google Scholar]
- 10.Kam JK, Cooray SD, Kam JK, et al. A cost-analysis study of robotic versus conventional mitral valve repair. Heart Lung Circ 2010;19:413-8. [DOI] [PubMed] [Google Scholar]
- 11.Folliguet T, Vanhuyse F, Constantino X, et al. Mitral valve repair robotic versus sternotomy. Eur J Cardiothorac Surg 2006;29:362-6. [DOI] [PubMed] [Google Scholar]
- 12.Woo YJ, Nacke EA. Robotic minimally invasive mitral valve reconstruction yields less blood product transfusion and shorter length of stay. Surgery 2006;140:263-7. [DOI] [PubMed] [Google Scholar]
- 13.Nifong LW, Rodriguez E, Chitwood WR, Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [DOI] [PubMed] [Google Scholar]
- 14.Bush B, Nifong LW, Chitwood WR, Jr. Robotics in cardiac surgery: past, present, and future. Rambam Maimonides Med J 2013;4:e0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mihaljevic T, Gillinov M. Invited commentary. Ann Thorac Surg 2012;93:1468. [DOI] [PubMed] [Google Scholar]