Abstract
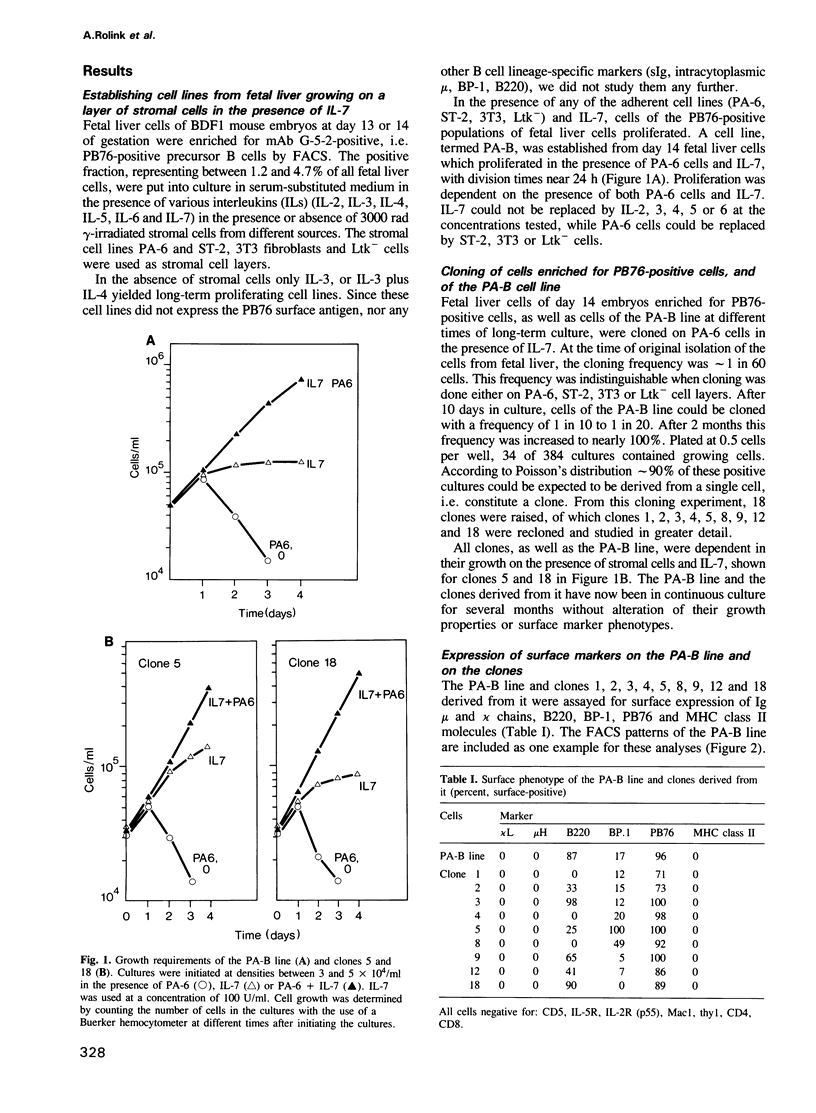
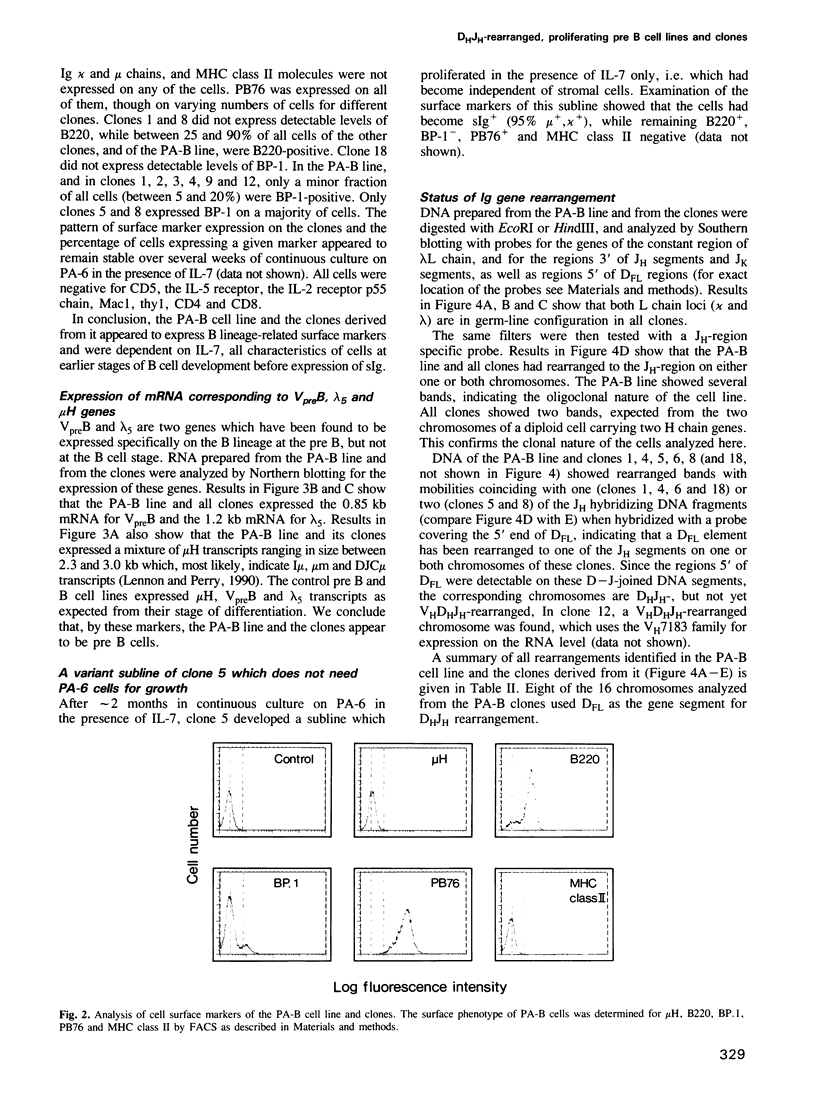
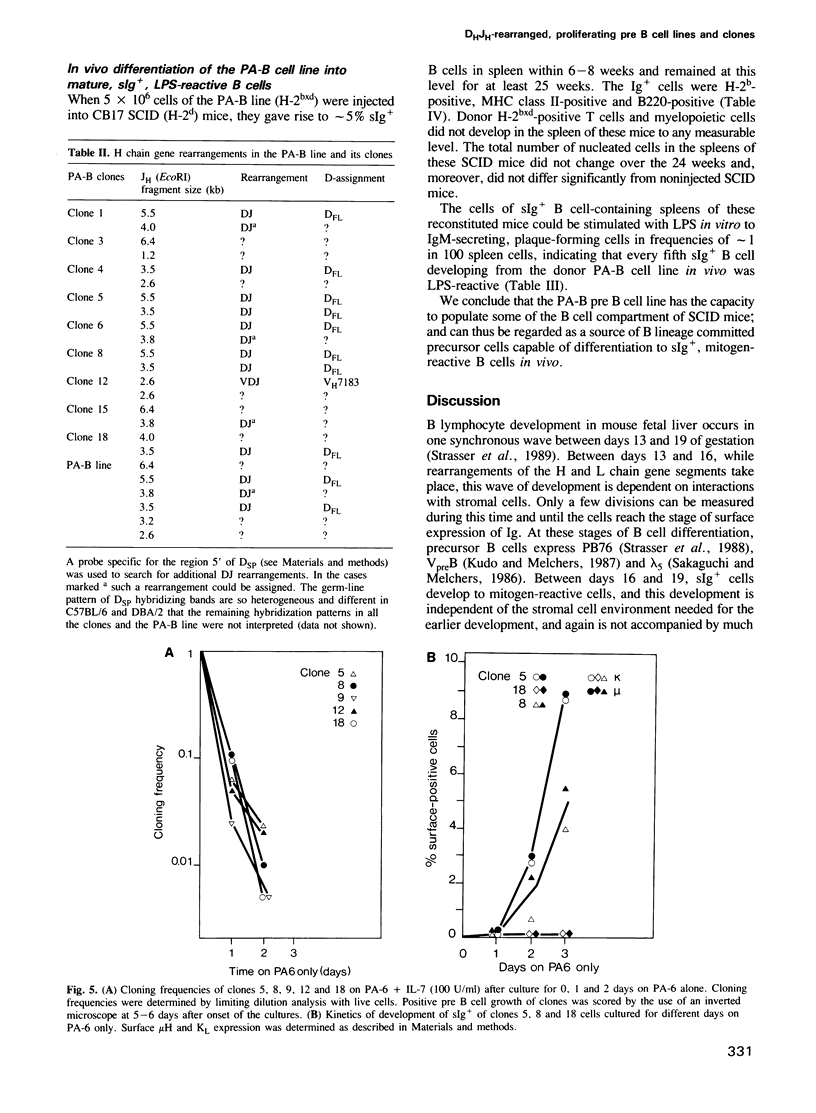
Cell lines and clones were established from PB76-positive mouse fetal liver at day 13 and 14 of gestation, which proliferated with division times of a day in serum-substituted cultures under the stimulatory influence of adherent stromal cells and the cytokine IL-7 for periods longer than half a year. These lines expressed varying levels of the B lymphocyte lineage related markers PB76, B220, BP-1, VpreB and lambda 5, but no surface Ig or MHC class II molecules. All clones expressed PB76, VpreB and lambda 5 in a high percentage of cells, while B220 and/or BP-1 expression was low or undetectable in some. A cell line, and several clones established from it, all had kappa and lambda light chain genes in germ-line configuration. Either one or both of their H-chain-gene containing chromosomes carried a DH to JH. These pre B cell lines and clones could be induced to VH to DH and VL to JL rearrangements. This resulted in the development of varying percentages of sIg-positive surface, MHC class II negative, LPS-reactive B cells within 2-3 days, in the absence of contacts with stromal cells and/or IL-7. When injected into SCID mice, the cultured pre B cells populated the spleen of these mice to 5% with surface Ig-, MHC class II-positive LPS-reactive cells for greater than 25 weeks. The long-term in vitro proliferative capacity of these DH-JH rearranged pre B cell clones makes them major candidates for committed stem cells of the B lineage.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Coutinho A., Melchers F. Frequencies of mitogen-reactive B cells in the mouse. I. Distribution in different lymphoid organs from different inbred strains of mice at different ages. J Exp Med. 1977 Jun 1;145(6):1511–1519. doi: 10.1084/jem.145.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A., Dorf M. E., Springer T. A. A shared alloantigenic determinant on Ia antigens encoded by the I-A and I-E subregions: evidence for I region gene duplication. J Immunol. 1981 Dec;127(6):2488–2495. [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Bruce J., Symington F. W., McKearn T. J., Sprent J. A monoclonal antibody discriminating between subsets of T and B cells. J Immunol. 1981 Dec;127(6):2496–2501. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collins L. S., Dorshkind K. A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol. 1987 Feb 15;138(4):1082–1087. [PubMed] [Google Scholar]
- Cooper M. D., Mulvaney D., Coutinho A., Cazenave P. A. A novel cell surface molecule on early B-lineage cells. Nature. 1986 Jun 5;321(6070):616–618. doi: 10.1038/321616a0. [DOI] [PubMed] [Google Scholar]
- Dialynas D. P., Quan Z. S., Wall K. A., Pierres A., Quintáns J., Loken M. R., Pierres M., Fitch F. W. Characterization of the murine T cell surface molecule, designated L3T4, identified by monoclonal antibody GK1.5: similarity of L3T4 to the human Leu-3/T4 molecule. J Immunol. 1983 Nov;131(5):2445–2451. [PubMed] [Google Scholar]
- Dorshkind K., Johnson A., Collins L., Keller G. M., Phillips R. A. Generation of purified stromal cell cultures that support lymphoid and myeloid precursors. J Immunol Methods. 1986 May 1;89(1):37–47. doi: 10.1016/0022-1759(86)90029-3. [DOI] [PubMed] [Google Scholar]
- Gronowicz E., Coutinho A., Melchers F. A plaque assay for all cells secreting Ig of a given type or class. Eur J Immunol. 1976 Aug;6(8):588–590. doi: 10.1002/eji.1830060812. [DOI] [PubMed] [Google Scholar]
- Hunt P., Robertson D., Weiss D., Rennick D., Lee F., Witte O. N. A single bone marrow-derived stromal cell type supports the in vitro growth of early lymphoid and myeloid cells. Cell. 1987 Mar 27;48(6):997–1007. doi: 10.1016/0092-8674(87)90708-2. [DOI] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscove N. N., Melchers F. Complete replacement of serum by albumin, transferrin, and soybean lipid in cultures of lipopolysaccharide-reactive B lymphocytes. J Exp Med. 1978 Mar 1;147(3):923–933. doi: 10.1084/jem.147.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Kudo A., Melchers F. The proteins encoded by the VpreB and lambda 5 pre-B cell-specific genes can associate with each other and with mu heavy chain. J Exp Med. 1990 Sep 1;172(3):969–972. doi: 10.1084/jem.172.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kerr W. G., Cooper M. D., Feng L., Burrows P. D., Hendershot L. M. Mu heavy chains can associate with a pseudo-light chain complex (psi L) in human pre-B cell lines. Int Immunol. 1989;1(4):355–361. doi: 10.1093/intimm/1.4.355. [DOI] [PubMed] [Google Scholar]
- Kinashi T., Inaba K., Tsubata T., Tashiro K., Palacios R., Honjo T. Differentiation of an interleukin 3-dependent precursor B-cell clone into immunoglobulin-producing cells in vitro. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4473–4477. doi: 10.1073/pnas.85.12.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Paige C. J., Scheid M. P. Cellular interactions affecting the maturation of murine B lymphocyte precursors in vitro. J Immunol. 1981 Jul;127(1):255–260. [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Kodama H., Sudo H., Koyama H., Kasai S., Yamamoto S. In vitro hemopoiesis within a microenvironment created by MC3T3-G2/PA6 preadipocytes. J Cell Physiol. 1984 Mar;118(3):233–240. doi: 10.1002/jcp.1041180303. [DOI] [PubMed] [Google Scholar]
- Kudo A., Melchers F. A second gene, VpreB in the lambda 5 locus of the mouse, which appears to be selectively expressed in pre-B lymphocytes. EMBO J. 1987 Aug;6(8):2267–2272. doi: 10.1002/j.1460-2075.1987.tb02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo A., Sakaguchi N., Melchers F. Organization of the murine Ig-related lambda 5 gene transcribed selectively in pre-B lymphocytes. EMBO J. 1987 Jan;6(1):103–107. doi: 10.1002/j.1460-2075.1987.tb04725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lennon G. G., Perry R. P. The temporal order of appearance of transcripts from unrearranged and rearranged Ig genes in murine fetal liver. J Immunol. 1990 Mar 1;144(5):1983–1987. [PubMed] [Google Scholar]
- Leptin M. Monoclonal antibodies specific for murine IgM. II. Activation of B lymphocytes by monoclonal antibodies specific for the four constant domains of IgM. Eur J Immunol. 1985 Feb;15(2):131–137. doi: 10.1002/eji.1830150206. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melchers F. B lymphocyte development in fetal liver. I. Development of reactivities to B cell mitogens "in vivo" and "in vitro". Eur J Immunol. 1977 Jul;7(7):476–481. doi: 10.1002/eji.1830070714. [DOI] [PubMed] [Google Scholar]
- Namen A. E., Lupton S., Hjerrild K., Wignall J., Mochizuki D. Y., Schmierer A., Mosley B., March C. J., Urdal D., Gillis S. Stimulation of B-cell progenitors by cloned murine interleukin-7. Nature. 1988 Jun 9;333(6173):571–573. doi: 10.1038/333571a0. [DOI] [PubMed] [Google Scholar]
- Nishikawa S. I., Hayashi S. I., Ogawa M., Kunisada T., Nishikawa S., Sudo T., Nakauchi H., Suda T. Control of cell growth and differentiation during early B-cell development by stromal cell molecules. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):171–174. doi: 10.1101/sqb.1989.054.01.021. [DOI] [PubMed] [Google Scholar]
- Nishikawa S., Ogawa M., Nishikawa S., Kunisada T., Kodama H. B lymphopoiesis on stromal cell clone: stromal cell clones acting on different stages of B cell differentiation. Eur J Immunol. 1988 Nov;18(11):1767–1771. doi: 10.1002/eji.1830181117. [DOI] [PubMed] [Google Scholar]
- Ogawa M., Nishikawa S., Ikuta K., Yamamura F., Naito M., Takahashi K., Nishikawa S. B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J. 1988 May;7(5):1337–1343. doi: 10.1002/j.1460-2075.1988.tb02949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Gisler R. H., McKearn J. P., Iscove N. N. Differentiation of murine B cell precursors in agar culture. Frequency, surface marker analysis and requirements for growth of clonable pre-B cells. Eur J Immunol. 1984 Nov;14(11):979–987. doi: 10.1002/eji.1830141104. [DOI] [PubMed] [Google Scholar]
- Palacios R., Henson G., Steinmetz M., McKearn J. P. Interleukin-3 supports growth of mouse pre-B-cell clones in vitro. Nature. 1984 May 10;309(5964):126–131. doi: 10.1038/309126a0. [DOI] [PubMed] [Google Scholar]
- Palacios R., Steinmetz M. Il-3-dependent mouse clones that express B-220 surface antigen, contain Ig genes in germ-line configuration, and generate B lymphocytes in vivo. Cell. 1985 Jul;41(3):727–734. doi: 10.1016/s0092-8674(85)80053-2. [DOI] [PubMed] [Google Scholar]
- Palacios R., Stuber S., Rolink A. The epigenetic influences of bone marrow and fetal liver stroma cells on the developmental potential of Ly-1+ pro-B lymphocyte clones. Eur J Immunol. 1989 Feb;19(2):347–356. doi: 10.1002/eji.1830190220. [DOI] [PubMed] [Google Scholar]
- Park Y. H., Osmond D. G. Phenotype and proliferation of early B lymphocyte precursor cells in mouse bone marrow. J Exp Med. 1987 Feb 1;165(2):444–458. doi: 10.1084/jem.165.2.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrangeli C. E., Hayashi S., Kincade P. W. Stromal cell lines which support lymphocyte growth: characterization, sensitivity to radiation and responsiveness to growth factors. Eur J Immunol. 1988 Jun;18(6):863–872. doi: 10.1002/eji.1830180606. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Formation of disulphide-linked mu 2 omega 2 tetramers in pre-B cells by the 18K omega-immunoglobulin light chain. Nature. 1987 Sep 10;329(6135):172–174. doi: 10.1038/329172a0. [DOI] [PubMed] [Google Scholar]
- Reed K. C., Mann D. A. Rapid transfer of DNA from agarose gels to nylon membranes. Nucleic Acids Res. 1985 Oct 25;13(20):7207–7221. doi: 10.1093/nar/13.20.7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Jackson S., Alt F. W. VHDJH formation and DJH replacement during pre-B differentiation: non-random usage of gene segments. EMBO J. 1986 Sep;5(9):2131–2138. doi: 10.1002/j.1460-2075.1986.tb04476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M., Gehrmann P., Petrac E., Wiese P. A novel VH to VHDJH joining mechanism in heavy-chain-negative (null) pre-B cells results in heavy-chain production. 1986 Aug 28-Sep 3Nature. 322(6082):840–842. doi: 10.1038/322840a0. [DOI] [PubMed] [Google Scholar]
- Rolink A. G., Melchers F., Palacios R. Monoclonal antibodies reactive with the mouse interleukin 5 receptor. J Exp Med. 1989 May 1;169(5):1693–1701. doi: 10.1084/jem.169.5.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi N., Melchers F. Lambda 5, a new light-chain-related locus selectively expressed in pre-B lymphocytes. Nature. 1986 Dec 11;324(6097):579–582. doi: 10.1038/324579a0. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Strasser A. PB76: a novel surface glycoprotein preferentially expressed on mouse pre-B cells and plasma cells detected by the monoclonal antibody G-5-2. Eur J Immunol. 1988 Nov;18(11):1803–1810. doi: 10.1002/eji.1830181123. [DOI] [PubMed] [Google Scholar]
- Strasser A., Rolink A., Melchers F. One synchronous wave of B cell development in mouse fetal liver changes at day 16 of gestation from dependence to independence of a stromal cell environment. J Exp Med. 1989 Dec 1;170(6):1973–1986. doi: 10.1084/jem.170.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S., Gillis S., Palacios R. In vitro effects of recombinant interleukin 7 on growth and differentiation of bone marrow pro-B- and pro-T-lymphocyte clones and fetal thymocyte clones. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1634–1638. doi: 10.1073/pnas.86.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tsubata T., Reth M. The products of pre-B cell-specific genes (lambda 5 and VpreB) and the immunoglobulin mu chain form a complex that is transported onto the cell surface. J Exp Med. 1990 Sep 1;172(3):973–976. doi: 10.1084/jem.172.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Tidmarsh G. F., Muller-Sieburg C., Weissman I. L. Bone marrow stromal cell lines with lymphopoietic activity express high levels of a pre-B neoplasia-associated molecule. Cell. 1987 Mar 27;48(6):1009–1021. doi: 10.1016/0092-8674(87)90709-4. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C., Denis K., Robertson D., Witte O. In vitro analysis of murine B-cell development. Annu Rev Immunol. 1985;3:213–235. doi: 10.1146/annurev.iy.03.040185.001241. [DOI] [PubMed] [Google Scholar]
- Witte P. L., Robinson M., Henley A., Low M. G., Stiers D. L., Perkins S., Fleischman R. A., Kincade P. W. Relationships between B-lineage lymphocytes and stromal cells in long-term bone marrow cultures. Eur J Immunol. 1987 Oct;17(10):1473–1484. doi: 10.1002/eji.1830171014. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Alt F. W. Developmentally controlled and tissue-specific expression of unrearranged VH gene segments. Cell. 1985 Feb;40(2):271–281. doi: 10.1016/0092-8674(85)90141-2. [DOI] [PubMed] [Google Scholar]