Abstract
It has become evident that mitral valve (MV) repair is the preferable treatment for the majority of patients presenting with severe mitral regurgitation (MR). This success clearly testifies that the surgical procedure is accessible, reproducible and is carrying excellent long-lasting results. From the pre-extracorporeal circulation’s era to the last percutaneous approaches, a large variety of techniques have been proposed to address the different features of MV diseases. This article aimed at reviewing chronologically the development of these dedicated techniques through their origins and the debates that they generated in the literature.
Keywords: Mitral valve (MV), valvuloplasty, history of medicine
Introduction
Mitral valve (MV) repair is a surgical procedure that has continuously developed over the past five decades. Progressive comprehension and evaluation of the pathology, alongside with standardization of the surgical techniques, has led to outstanding long-lasting results. This has made MV repair the most attractive approach in the treatment of severe mitral regurgitation (MR), as demonstrated by the constant increased rate of MV repairs in the United States (1,2) (Figure 1). Many surgeons have been involved with and propelled this evolution over the years, with the contributions of Carpentier certainly the most important. In 1968, he developed the concept of prosthetic ring annuloplasty which aimed to restore the shape of the deformed annulus fibrosus of the MV (3). The remodeling annuloplasty, as he called it, brought to valve repair the predictability and stability of outcomes lacking with earlier techniques. The ring annuloplasty opened a new era in valve reconstruction by allowing the development of complementary techniques in order to address the variety of the lesions encountered. It took him 10 years to develop “the French correction” technique which describes the surgical correction of a posterior leaflet (PL) prolapse by quadrangular resection, transfer of normal chordae to other prolapsing area and remodeling annuloplasty (4). It is now considered worldwide as the landmark of MV reconstructive surgery. But probably as important, was his work on clarifying the pathophysiological aspects of MV pathology so that all surgeons and all cardiologists could speak the same language. This was the real origin of MV repair success: giving to all a comprehensive valve analysis system. His pathophysiological triad classification allowed the surgeon to choose the optimal surgical technique of repair for any given pathological MV. It distinguished clearly the terms that describes the etiology (degenerative, ischemic), the valve lesions resulting from the etiology (rupture of chordae, perforation), and the valve dysfunction resulting from the lesions. The latter was addressed in the functional classification published in 1983 (5). In the present keynote lecture (Video 1), evolutions in MV reconstructive surgery over the last five decades will be followed regarding these three different classes of dysfunction. More specific approaches had been also developed through the years in each of these three classes of dysfunction with regard to the lesions and the pathologies.
Figure 1.
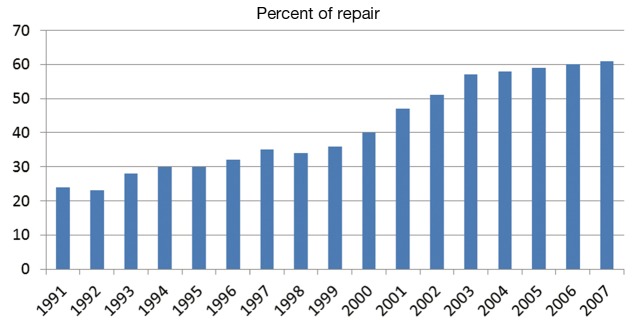
Mitral valve (MV) repair rates in the United States for primary MV intervention for 1990 to 2007 (1,2).
Video 1.

Mitral valve repair over five decades.
Technical evolution in type I MR
Annular dilatation
Since the early 1950s, annular dilatation was found to be a constant feature in MV disease. Thus, reducing the size of the mitral orifice was one of the first described techniques to correct MV diseases. In 1955, Davila et al. were the first to propose a circumferential purse-string suture of the mitral annulus, pulled out from the left atrium to be tight until the digital cardiac examination revealed quite untraceable thrill (6). With the rise of extracorporeal circulation and open-heart surgery, this reduction annuloplasty technique was refined by Kay et al. in 1960 and Belcher et al. in 1964, who proposed intra-atrial placement of incomplete purse-string suture reinforced by fabric bands on the posterior part of the annulus (7,8). A few years earlier, Nichols et al. in Philadelphia followed by Lillehei et al. in Minneapolis, and Merendino et al. in Seattle, had proposed a direct plicature of the annulus and/or the leaflets at the level of the posterior commissure (9-11). In the early 1960s, specific surgical correction of isolated mitral insufficiency was in early development. Annular dilatation was addressed by partial circumferential reduction as described above, or by symmetrical antero-posterior diameter reduction as published by Kay et al. (12). Mid-term results of all these palliative techniques showed a high proportion of recurrence either on the regurgitant or stenotic component (13,14). Moreover, by the same period, the initial promising results of prosthetic valve replacement in the mitral position reduced the appeal of narrowing annuloplasty interventions (15).
Annuloplasty techniques was revived in the late 1960s with the concept of a remodeling annuloplasty introduced by Carpentier (3,16), carrying superior predictability and long term stability of outcomes compared to MV repair surgery (17). A semi-rigid prosthetic D-shape ring was sutured to the fibrous mitral annulus, designed to restore the physiological systolic shape of a normal mitral annulus in which the postero-anterior diameter is smaller than the medio-lateral diameter. The size of the ring was required to be chosen in accordance to the amount of available leaflet tissue, to allow optimization of the coaptation surface between the anterior and the PLs. A few years later, Duran and colleagues proposed a complete flexible ring to treat mitral dilatation (18), advocating that the geometry of the annulus undergoes continuous changes during the cardiac cycle. This argument was a source of bitter debate since Carpentier’s ring has a systolic shape to assure leaflets coaptation and therefore is not supposed to contract further. By contrast, a flexible and deformable ring eases the aorto-mitral dynamics during the cardiac cycle but achieves not-imposed coaptation. Nevertheless, a recent prospective randomized study reported 62% and 55% freedom from significant MR at eight years with Carpentier ring and Duran ring respectively (P=0.17) (19). In addition, early anatomical studies have indicated that pathological deformation of the mitral annulus was not homogenous, as only the posterior part of the annulus was believed to be susceptible to dilatation. This false belief was at the core of the design of partial prosthetic rings, which were rapidly attractive because they were easier to implant (20-22). With the use of radio-opaque markers, Timek and his colleagues from Stanford proved that the anterior part of the annulus could also enlarge over time (23) and, more recently, two studies have shown increase recurrence of MR after partial ring annuloplasty (24,25).
The Classic Carpentier ring was primarily designed for rheumatic valvular diseases. In 1995, degenerative valvular diseases had become predominant and an evolution of this ring was introduced. The Physio-Ring (Edwards Lifesciences; Irvine, CA, USA) added semi-flexibility and a saddled shape to assure improved dynamics for most patients with degenerative valvular diseases (26). In order to approximate normal anatomy and dynamics of the mitral annulus, new prosthetic rings also adopted a non-flattened design. Indeed, several research studies had emphasized the 3D-saddled shape of the mitral annulus and its importance on reducing stress to the MV apparatus (27,28). Since then, several other prosthetic rings have been proposed to incorporate an elevated anterior segment in the same goal of even more stability of the repair. In the last two decades pathological-specific rings have been developed, allowing surgeons to choose the optimal annuloplasty type for any given etiology as stated by the pathophysiological triad concept.
Disease-specific evolutions in type I MR
The larger antero-posterior diameter of the Physio-Ring as well as the Physio-Ring II, specifically designed for degenerative valvular diseases, increases the valve orifice area and reduces the risk of systolic anterior motion (SAM) often present in patients with Barlow’s disease (29). Subsequently, a slight modification in the size selection of the ring was described by Carpentier and colleagues, taking into account both the intercommissural distance and the height of the anterior leaflet (AL) with a particular caution not to choose too small rings. In cases of extremely developed AL in which there is a high discrepancy between AL height and intercommissural distance, vertically enlarged prosthetic rings have been manufactured such as the Myxo Etiologix (Edwards Lifesciences; Irvine, CA, USA), which represents a new alternative to the Classic Carpentier ring manually enlarged by the operator.
In the setting of functional MR (FMR) secondary to end-stage dilated cardiomyopathy, of ischemic or non-ischemic cause, MV replacement had produced very poor mid-term results (30,31). In 1995, Bolling and his colleagues showed that annuloplasty was highly favorable at one-year for this population, since it completely preserved annular-chordal-papillary continuity (32). Nevertheless, many observers eventually found that recurrent MR after initially successful annuloplasty was frequent (33,34), thus questioning the notion that repair should be preferred to replacement with total chordal preservation (35,36). This encompasses the fact that the primum movens of FMR are left ventricular (LV) dilatation and LV dysfunction. As mentioned by Carpentier, the association of annular dilatation with a type IIIb MR is present in 90% of the cases, the tethering applied on the leaflets by the subvalvular apparatus displacement being responsible for a reduced amount of functionally available tissue to close the mitral orifice in systole. Therefore, Boling et al. proposed the concept of restrictive annuloplasty with a flexible ring (37). Later, Braun and the group from Leiden specified that the prosthetic ring should be selected two ring sizes down (38). They also showed that LV remodeling after successful annular reduction, and thus survival, were dependent upon preoperative LV dimensions. For LV end systolic diameters greater than 51 mm, additional surgical strategies specifically focused on type IIIb MR are mandatory (see below). Finally, Kwan et al. and Agricola et al. had differentiated two types of FMR based on the echocardiographic characteristics of the regurgitant jets aspects (39,40) (Figure 2). Eccentric jets are usually associated with asymmetrical posterior tethering patterns as a consequence of both posterior wall dysfunction and posterior annular dilatation. This contrasted with central-directed jets, which results predominantly from apical tethering of both leaflets due to global LV and annular dilation. Based on these clinical observations, along with the fact that restrictive annuloplasty was thought to be potentially responsible for exercise mitral stenosis (41), different innovative prosthetic annular devices have been recently released. These devices were designed to address the particular needs of FMR restrictive annuloplasty while preserving the effective MV area: the Geoform ring (Edwards Lifesciences; Irvine, CA, USA) designed for symmetrical annulo-ventricular reshaping and the IMR ETlogix (Edwards Lifesciences; Irvine, CA, USA) for posterior restrictive annuloplasty.
Figure 2.
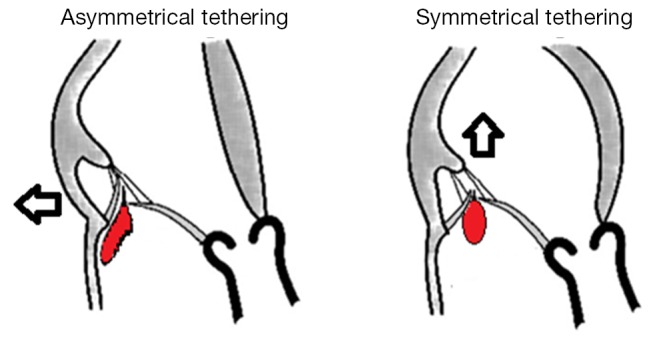
Mechanisms of the two different features of leaflet tenting in FMR according to Kwan et al. (39) and Agricola et al. (40). Asymmetrical posterior tethering is the result of both posterior wall dysfunction and posterior annular dilatation. Symmetrical tethering is the result of predominant apical tethering of both leaflets due to global LV and annular dilation. FMR, functional mitral regurgitation; LV, left ventricular.
The use of small prosthetic rings has also raised some concerns in young patients who require MV repair. Importantly, mitral annuloplasty has been shown to reduce the risk for reoperation in rheumatic valve disease which account for the majority of worldwide MV interventions in children. In 2003, Kalangos et al. introduced a biodegradable annuloplasty ring with the advantages of allowing the native valve to grow and eliminating any foreign material on the annulus, whenever a re-intervention would be eventually needed (42).
Percutaneous approaches have been more recently proposed primarily using devices inserted transvenously in the great cardiac vein via the coronary sinus, to surround the posterior part of the mitral annulus. The Carillon Mitral Contour System (Cardiac Dimensions Inc.; Kirkland, WA, USA) is currently the only percutaneous annuloplasty device which received European Union approval for commercialization and is currently investigated in the ongoing Titan II clinical trial. After successful research studies in animal models (43), current prospective trials have included patients ineligible for conventional heart surgery. However, there are several limitations of this technique, including mainly a low feasibility rate (44,45), incomplete annular reduction (46), and device-related complications by compression of diagonal/marginal coronary arteries (47) or wire fractures (48). In order to avoid these limitations, new devices have been developed proposing direct annuloplasty technology through a retrograde approach via the femoral artery. Depending on the device: Mitralign (Mitralign; Tewksbury, MA, USA) or Accucinch GDS (Guided Delivery System; Santa Clara, CA, USA), mitral annulus dimensions are reduced by placement of a various number of anchors around the annular circumference. These devices are currently the subject of experimental and clinical studies.
Technical evolutions in type II MR
As proposed by Carpentier, MV prolapse should not be referred as a pathological entity but rather corresponds to a valve dysfunction (type II) instead, and may be the result of different lesions [elongated/ruptured chordae or papillary muscles (PM)] from various etiologies (e.g., degenerative, endocarditis). McGoon was the first to report a surgical treatment of chordal rupture on PL (49). This technique, known as McGoon’s, was modified from plication to partial annular plication by Gerbode et al. 2 years later (50). Extensive annular plication described with incorporation of the whole prolapsed segment was also proposed by Kay et al. (51). In the early 1960s, other techniques described re-attachment of the leaflet free edge to the PM directly, or with the interposition of artificial chordae made of silk, mersilene or other materials (52-54). Carpentier was the first to advise resecting rather than plicating the prolapsed leaflet segment to facilitate leaflet motion (4). The resection should be triangular for limited AL or PL prolapses and quadrangular for more extensive PL prolapse. In more extensive AL prolapse, particularly due to chordal elongation, he proposed the “sliding plasty of the PM” or the “shortening plasty of chordae”, with which the latter was responsible for secondary chordal ruptures and eventually abandoned in favor of chordal transfer (55,56). With the publication of the French Correction in 1983, two additional techniques were described to treat AL prolapse due to chordal rupture: chordal transposition (or transfer) and leaflet fixation on secondary chordae (5). In the case of AL prolapse limited to one segment, a variation of this chordal transfer method was proposed by Duran with the flip-over technique (57). The principle was to anchor the prolapsed portion of AL to the corresponding non-prolapsed portion of PL, detach the latter and reconstruct PL as done for standard quadrangular resection. Noting the remarkable anatomy of the posterior PM, Dreyfus also proposed a simplification of the “papillary muscle plasty” technique for AL prolapse secondary to elongated chordae, by repositioning the anterior head downward into the ventricular cavity (58).
Two other major surgical procedures were described for type II MR. First, the revival of the old edge-to-edge approximation by Alfieri and colleagues, which involved suturing the AL and PL together at their middle portion (59). This approach has been criticized because it dramatically increases stress on both leaflets thus resulting in MV stenosis with leaflet tissue fibrosis (60), and because it compromises one of the fundamental principles of MV repair: preserving leaflet mobility. The edge-to-edge technique was initially proposed to correct complex lesions of type II MR with multiple prolapsed leaflet segments, and now has been proved over time to be a simple and quite efficient method in the treatment of type IIIb MR (61) and in the occurrence of SAM after MV repair (62,63). Indeed, the incidence of SAM had been reported as high as 14% after repair in degenerative valvulopathy (29). Thus, in the early 1990’s, Carpentier had refined his quadrangular resection technique to include PL sliding plasty. This was particularly recommended in Barlow’s disease with a considerable excess of tissue in the PL remnants once the quadrangular resection has been performed (64). Furthermore, Gillinov et al. demonstrated that the sliding plasty technique, along with ring implantation and the use of chordal transposition instead of chordal shortening, was a factor positively influencing long-term durability of MV repair in degenerative (65).
The renewal of artificial chords implantation using polytetrafluoroethylene sutures was the second major evolution of MV repair since Carpentier’s description of the French Correction (66,67). Although length adjustment of Gore-Tex chords is challenging and has been the source of many technical propositions (68-72), this procedure has gained an incredible popularity over the years. It must be said that initial concerns over the risk of secondary rupture and thrombotic complications of neochords were rapidly lifted (73,74). Despite the excellent long-term results of the “quadrangular resection”, Perier proposed an alternative technique for localized prolapse of PL using only artificial chordae without any leaflet resection (75). The objective of the “respect rather than resect” approach, as it was called, was to promote larger surface of coaptation in bringing deep in the ventricular cavity the free edge of PL which acts as a buttress for AL. This concept is very efficient in preventing SAM and also quite convenient for mini-invasive approaches of MV repair, in which extensive leaflet resection or sliding leaflet plasty could be technically challenging. On the other hand, it compromises PL mobility and results need to be confirmed on the very long-term.
These two surgical techniques have influenced the latest innovative percutaneous treatment of type II mitral insufficiency. Indeed, the MitraClip system (Abbott Laboratories; Abbott Park, IL, USA) reproduces the edge-to-edge approach by opposing both free edges of mitral leaflet with a clip delivered transvenously. This device has been used in selective patients with high-risk surgical profiles in degenerative and subsequently FMR. The EVEREST trial has established the safety and feasibility of the procedure (76). Subsequent clinical studies, including the EVEREST II trial, have proven its efficiency in term of MR amount reduction and LV reverse remodeling. However, there remain questions with regard to the duration of these results because surgical edge-to-edge procedures without concomitant ring annuloplasty, have shown a high recurrence rate of MR (77,78). The recent NeoChord system (NeoChord, Inc., Eden Prairie, MN, USA) offer to implant artificial chordae anchored to the apex via a small left thoracotomy on a beating heart and should be soon available for total percutaneous approach (79). Safety and feasibility of the NeoChord system was the subject of the initial transapical artificial chordae tendinae (TACT) trial (80).
The excellent results of MV reconstructive techniques in type II MR have recently modified the recommended indications for surgery in severe MR. Asymptomatic patients are indeed candidates for surgery if the chances of repair are high whatever their ventricular function and dimensions (81). It has also been recommended that complex type II lesions should be addressed to large experienced centers with more than 90% of repair rate (82).
Technical evolution in type IIIa MR
Type IIIa or diastolic restricted leaflet motion is most commonly seen in rheumatic valvular disease. The lesions produced are commonly annular dilatation, leaflet thickening and chordal thickening and fusion. They can be either stenotic, regurgitant or both. This pathology was the first surgically corrected by closed and subsequently opened commissurotomies. But their inconstant results along with the emergence of prosthetic valves led to the preference of MV replacement in the 1960s. The renewal of MV repair in this particular disease came again from Carpentier (16). For predominant stenotic disease, in addition to controlled commissurotomy, he described a variety of surgical maneuvers on the subvalvular apparatus leading to maximal leaflets mobilization. Resection of secondary chordae, fenestration of marginal chordae and longitudinal incision of PM should be associated in order to gain optimize leaflet mobility. In addition whenever annular deformation was present or LV cavities enlarged, ring annuloplasty was promptly recommended to avoid resultant MR, however, lesions on leaflet tissue still remained problematic.
Leaflet extension and replacement by autologous pericardium to treat the complex problem of leaflet thickening and/or calcification was initially described by Shumway et al. (83) and tested clinically by Frater et al. ten years later (84). The results were disappointing, mainly due to occurrence of retraction and calcification. The use of other material such as autologous fascia lata or glutaraldehyde-treated bovine pericardium did not alter outcomes. But, after successful experimental studies on stabilization of autologous tissue, Chauvaud et al. and Carpentier demonstrated better clinical outcomes of MV extension with the use of glutaraldehyde-preserved autologous pericardium (85). They also advocated that besides the delay obtained in the valve shrinkage process, valve extension allow the use of adult-size prosthetic ring in children. These results were maintained over the years whatever leaflet (PL or AL) was expanded (86,87). When the leaflets were thickened and calcified but without too much shrinkage, cusp thinning has also been proposed by others as an alternative (88).
The success of Gore-Tex chordae in degenerative disease finally spread in the field of rheumatic disease, and with the same goal of achieving as much leaflet mobilization as possible, Rankin proposed to simply cut the most thickened marginal chordae of those valves and replace them with artificial chords (89). Although all these techniques have sensibly improved the long-term results, MV repair in type IIIa MR remains a challenge and the rate of feasibility is still low in non-experienced centers. The real turnover in the approach of stenotic rheumatic valve treatment has been seen when percutaneous balloon valvulotomy is routinely performed. Today, it is the gold standard for treatment of pure stenotic rheumatic valves and surgery is only recommended for non-favorable anatomic lesions such as significant associated MR or important calcification.
Technical evolution in type IIIb MR
Type IIIb MR or systolic restricted leaflet motion is primarily a ventricular disease and not a valvular disease. It could have an ischemic or a non-ischemic origin and is considered as FMR, since the valve complex itself showed no particular anatomic lesion besides annular dilatation. This is the reason why the first surgical approaches consisted of isolated annuloplasties. After the publication of Phillips and Burch, PM dysfunction was thought to be the sole mechanism of ischemic FMR (90), but Miller et al. proved experimentally that in order to obtain significant MR, the dysfunction should also involve the surrounding ventricular wall (91). Since then, the literature has abundantly described the mechanisms and the physiopathology of FMR whether its origin was ischemic or not (92-95). As mentioned previously, emphasis of the reduction in functional leaflet tissue generated by leaflet tethering was the source of the restricted annuloplasty concept introduced by Bolling (see above). Nonetheless, recurrent MR after MV repair with undersized ring has remained as frequent as 10-30% (33,34). Recurrence of MR has been found in some patients despite absence of reverse LV remodeling after surgery (96). This finding has encouraged, at the beginning of this century, the development of adjunct surgical techniques directed to the level of subvalvular apparatus. Hvass et al. proposed to bring closer the two PM groups by tightening a Gore-Tex tube passed around their base (97). Other intraventricular methods of PM repositioning have been suggested such as PM relocation (98), direct approximation of both PM (99), or approximation of PM tip to the mitral annulus (100). Extension of AL by autologous pericardial patch had also been described in this indication with good immediate results (101). Severing secondary chords was also effective in reducing leaflet tenting and thus MR but the debate remains over the consequences on LV function (102,103). All these methods could be used regardless of the etiology of FMR, but they have not yet demonstrated a long term benefit on LV remodeling and MR reduction. On the contrary, external cardiac restraint using the CorCap device (Acorn CV; St Paul, MN, USA) has been studied in a randomized trial for non-ischemic dilated cardiomyopathy since its commercialization in 2002 (104). There was no demonstrated benefit in term of survival up to 5 years but there were significant reduced LV volumes (105). The Coapsys system (Myocor Inc; Minneapolis, MN, USA) shared the same concept of moving the ventricle and the annulus with an external compression applied on the ventricular wall at the level of the mitral annulus and PM. Two external pads attached to each end of a transventricular splint were progressively tightened under echocardiographic guidance. Grossi et al. has showed a survival advantage at two years and lower MR grades compare to a control group in the RESTOR-MV trial (Randomized evaluation of a surgical treatment for off-pump repair of the MV) (106). In ischemic FMR more specifically, abnormal segmental wall mobility has course been one of the main therapeutic targets, to the extent that coronary artery bypass grafting alone (without annuloplasty) was thought to be able to correct durably significant MR (107,108). But other studies, including a prospective randomized trial, have more recently shown that restrictive annuloplasty associated with coronary bypass surgery gave excellent freedom from recurrent MR rates and improved indices of LV function (109). Finally, Messas et al. and Coll have proposed autologous myoblast transplantation in an experimental ovine model of chronic ischemic MR to improve regional LV function (110).
Current trends in the development of new therapeutic for type IIIb MR are likewise engaged towards less invasive approaches, which is ideal in this population who often presents with low cardiac function and carries high surgical risk. The MitraClip system has thus been proposed in the treatment for FMR also. A recent report from the European multicenter pilot registry has shown excellent procedural success rates and equal reduction of MR amount in this indication compare to degenerative etiology (111). Nevertheless, re-hospitalizations for heart failure were more frequent in the FMR group. Similarly to type II MR, one can argue that one of the major limitations of the technique is the absence of concomitant annuloplasty being performed.
Other specific considerations
Endocarditis management
Type I MR also includes leaflet perforations which are most frequently encountered in the setting of destructive lesions produced by infective endocarditis. In the 1980s the surgical treatment of valves endocarditis benefited from the recognition that delayed intervention led to irreversible structural damages, increased risk of embolism and finally higher postoperative mortality (112-114). Until the early 1990s however, acute bacterial endocarditis was considered a contraindication for MV repair. Some authors had only reported sparse successful MV reconstruction but the attempt to repair the valve was far from a systematic approach (115). Using Carpentier’s techniques, more specifically pericardial patches to treat leaflet perforations and abscesses, Dreyfus and colleagues demonstrated on a series of 40 patients that MV repair not only was feasible in most instances but also provides low operative mortality and mid-term re-intervention rates (116). Still, the frequent complexity of lesions in MV endocarditis which often required multiple surgical manipulations and the absence of large series reports have limited its expansion during the following decade. Again, with growing experience, the good long-term results published by different institutions have pleaded for the benefit of MV repair in endocarditis (117-119) and it is now promptly recommended over MV replacement whenever possible (120). For extended destructive lesions in which type I MR is frequently associated with type II segments, Acar et al. described an interesting method of partial homograft MV replacement (121). Given the many difficulties of homograft selection and sizing, and restricted leaflet motion and prolapse observed after implantation due to lack of standardized surgical procedures, this technique was abandoned (122,123).
Minimally-invasive MV surgical approach
In the perspective of providing better care to the patients, smaller incision, faster recovery and reduce costs, minimally invasive valve surgery has been developed since the late 1990s. Starting with aortic valve replacement, surgical supplies had rapidly evolved. The development by a Stanford group of port-access methods and endo-aortic balloon occlusion led to the first performed cases of MV repair (124,125). The progression rapidly moves towards totally endoscopic techniques with comparable results to mitral operations with conventional sternotomy (126,127). In 2010, Gammie et al. reported that as much as 20% of all MV interventions in the United States were done using minimally invasive approaches (128). The results of large experienced centers are currently univocal: minimally invasive surgery does not compromise the quality and durability of repair with less pain and less bleeding complications (129-131). Nevertheless, in a meta-analysis regrouping 35 studies on minimally invasive MV surgery, Cheng et al. noted an increased 30-day risk of stroke aortic dissection and phrenic nerve injury compared to conventional sternotomy (132).
In the same period, developing technologies such as 3D-enhanced vision, computer-assisted wrist movements and motion scaling gave birth to robotic surgery. Carpentier and colleagues performed the first mitral valvuloplasty using the first generation of Da Vinci surgical system (Intuitive Surgical Inc., Sunnyvale, CA, USA) in 1998. They were followed by Nifong et al. in East Carolina Heart Institute which became today one of the leading centers in robotic MV reconstruction (133). With growing experience, more and more complex lesions were treated with this approach. The results of a phase II multicenter trial published in 2005 listed the different techniques used under robotic approach from quadrangular resection to chordal transfer and chordal replacement (134). Team learning curve is of paramount importance in robotic-assisted surgery and some highly experienced centers, in collaboration with industry, have developed programs for new robotic surgeons with in-depth training (135). Ramzy and colleagues even proved that a stepwise approach of training had only little impact on pump time and complication rate (136). Such approaches may accelerate the spread of this technology.
Conclusions
Almost 40 years after that a comprehensive system of valve analysis and related techniques has been described by Carpentier, MV reconstruction has been worldwide accepted as the reference in treatment of MR whatever the etiology and mechanism. The excellent long term results along with the technical progress of diagnosis have profoundly modified the management of patients with MR over the years. In some instances, patients are even referred to the surgeon before the occurrence of symptoms. Nevertheless, in order to further provide better care and faster recovery to the patients, techniques of repair are in constant evolution, and we must pay tribute to all surgeons who pioneered new approaches reaching that goal without compromising with Carpentier’s three fundamental principles of repair: restore or preserve leaflet mobility, create a large surface of coaptation and remodel the annulus to offer an optimal and stable orifice area.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- 1.Savage EB, Ferguson TB, DiSesa VJ. Use of mitral valve repair: analysis of contemporary United States experience reported to the Society of Thoracic Surgeons National Cardiac Database. Ann Thorac Surg 2003;75:820-5. [DOI] [PubMed] [Google Scholar]
- 2.Gammie JS, Sheng S, Griffith BP, et al. Trends in mitral valve surgery in the United States: results from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2009;87:1431-7; discussion 1437-9. [DOI] [PubMed] [Google Scholar]
- 3.Carpentier A. Reconstructive valvuloplasty. A new technique of mitral valvuloplasty. Presse Med 1969;77:251-3. [PubMed] [Google Scholar]
- 4.Carpentier A, Relland J, Deloche A, et al. Conservative management of the prolapsed mitral valve. Ann Thorac Surg 1978;26:294-302. [DOI] [PubMed] [Google Scholar]
- 5.Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323-37. [PubMed] [Google Scholar]
- 6.Davila JC, Glove R RP, Trout RG, et al. Circumferential suture of the mitral ring; a method for the surgical correction of mitral insufficiency. J Thorac Surg 1955;30:531-60; discussion, 560-3. [PubMed] [Google Scholar]
- 7.Kay EB, Nogueira C, Zimmerman HA. Correction of mitral insufficiency under direct vision. Circulation 1960;21:568-77. [DOI] [PubMed] [Google Scholar]
- 8.Belcher JR. The surgical treatment of mitral regurgitation. Br Heart J 1964;26:513-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichols HT. Mitral insufficiency: treatment by polar cross-fusion of the mitral annulus fibrosus. J Thorac Surg 1957;33:102-22. [PubMed] [Google Scholar]
- 10.Lillehei CW, Gott VL, Dewall RA, et al. The surgical treatment of stenotic or regurgitant lesions of the mitral and aortic valves by direct vision utilizing a pump-oxygenator. J Thorac Surg 1958;35:154-91. [PubMed] [Google Scholar]
- 11.Merendino KA, Thomas GI, Jesseph JE, et al. The open correction of rheumatic mitral regurgitation and/or stenosis; with special reference to regurgitation treated by posteromedial annuloplasty utilizing a pump-oxygenator. Ann Surg 1959;150:5-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kay EB, Mendelsohn D, Zimmerman HA. Evaluation of the surgical correction of mitral regurgitation. Circulation 1961;23:813-22. [DOI] [PubMed] [Google Scholar]
- 13.Bjoerk VO, Malers E. Annuloplastic procedures for mitral insufficiency: late results. J Thorac Cardiovasc Surg 1964;48:251-60. [PubMed] [Google Scholar]
- 14.Wychulis AR, Connolly DC, Ellis FH, Jr. Open mitral valve reconstruction. Review of 232 operations. Arch Surg 1970;101:332-7. [DOI] [PubMed] [Google Scholar]
- 15.Starr A, Edwards ML. Mitral replacement: clinical experience with a ball-valve prosthesis. Ann Surg 1961;154:726-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carpentier A, Deloche A, Dauptain J, et al. A new reconstructive operation for correction of mitral and tricuspid insufficiency. J Thorac Cardiovasc Surg 1971;61:1-13. [PubMed] [Google Scholar]
- 17.Oury JH, Peterson KL, Folkerth TL, et al. Mitral valve replacement versus reconstruction. An analysis of indications and results of mitral valve procedures in a consecutive series of 80 patients. J Thorac Cardiovasc Surg 1977;73:825-35. [PubMed] [Google Scholar]
- 18.Durán CM, Pomar JL, Cucchiara G. A flexible ring for atrioventricular heart valve reconstruction. J Cardiovasc Surg (Torino) 1978;19:417-20. [PubMed] [Google Scholar]
- 19.Chang BC, Youn YN, Ha JW, et al. Long-term clinical results of mitral valvuloplasty using flexible and rigid rings: a prospective and randomized study. J Thorac Cardiovasc Surg 2007;133:995-1003. [DOI] [PubMed] [Google Scholar]
- 20.Odell JA, Schaff HV, Orszulak TA. Early results of a simplified method of mitral valve annuloplasty. Circulation 1995;92:II150-4. [DOI] [PubMed] [Google Scholar]
- 21.Cooley DA, Frazier OH, Norman JC. Mitral leaflet prolapse: surgical treatment using a posterior annular collar prosthesis. Cardiovasc Dis 1976;3:438-43. [PMC free article] [PubMed] [Google Scholar]
- 22.Cosgrove DM, 3rd, Arcidi JM, Rodriguez L, et al. Initial experience with the Cosgrove-Edwards Annuloplasty System. Ann Thorac Surg 1995;60:499-503; discussion 503-4. [DOI] [PubMed] [Google Scholar]
- 23.Timek TA, Glasson JR, Lai DT, et al. Annular height-to-commissural width ratio of annulolasty rings in vivo. Circulation 2005;112:I423-8. [DOI] [PubMed] [Google Scholar]
- 24.Spiegelstein D, Moshkovitz Y, Sternik L, et al. Midterm results of mitral valve repair: closed versus open annuloplasty ring. Ann Thorac Surg 2010;90:489-95. [DOI] [PubMed] [Google Scholar]
- 25.Kwon MH, Lee LS, Cevasco M, et al. Recurrence of mitral regurgitation after partial versus complete mitral valve ring annuloplasty for functional mitral regurgitation. J Thorac Cardiovasc Surg 2013;146:616-22. [DOI] [PubMed] [Google Scholar]
- 26.Carpentier AF, Lessana A, Relland JY, et al. The “physio-ring”: an advanced concept in mitral valve annuloplasty. Ann Thorac Surg 1995;60:1177-85; discussion 1185-6. [DOI] [PubMed] [Google Scholar]
- 27.Levine RA, Handschumacher MD, Sanfilippo AJ, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989;80:589-98. [DOI] [PubMed] [Google Scholar]
- 28.Salgo IS, Gorman JH, Gorman RC, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation 2002;106:711-7. [DOI] [PubMed] [Google Scholar]
- 29.Mihaileanu S, Marino JP, Chauvaud S, et al. Left ventricular outflow obstruction after mitral valve repair (Carpentier’s technique). Proposed mechanisms of disease. Circulation 1988;78:I78-84. [PubMed] [Google Scholar]
- 30.Lee SJ, Bay KS. Mortality risk factors associated with mitral valve replacement: a survival analysis of 10 year follow-up data. Can J Cardiol 1991;7:11-8. [PubMed] [Google Scholar]
- 31.Christakis GT, Weisel RD, David TE, et al. Predictors of operative survival after valve replacement. Circulation 1988;78:I25-34. [PubMed] [Google Scholar]
- 32.Bolling SF, Deeb GM, Brunsting LA, et al. Early outcome of mitral valve reconstruction in patients with end-stage cardiomyopathy. J Thorac Cardiovasc Surg 1995;109:676-82; discussion 682-3. [DOI] [PubMed] [Google Scholar]
- 33.Tahta SA, Oury JH, Maxwell JM, et al. Outcome after mitral valve repair for functional ischemic mitral regurgitation. J Heart Valve Dis 2002;11:11-8; discussion 18-9. [PubMed] [Google Scholar]
- 34.McGee EC, Gillinov AM, Blackstone EH, et al. Recurrent mitral regurgitation after annuloplasty for functional ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2004;128:916-24. [DOI] [PubMed] [Google Scholar]
- 35.Gillinov AM, Wierup PN, Blackstone EH, et al. Is repair preferable to replacement for ischemic mitral regurgitation? J Thorac Cardiovasc Surg 2001;122:1125-41. [DOI] [PubMed] [Google Scholar]
- 36.Calafiore AM, Gallina S, Di Mauro M, et al. Mitral valve procedure in dilated cardiomyopathy: repair or replacement? Ann Thorac Surg 2001;71:1146-52; discussion 1152-3. [DOI] [PubMed] [Google Scholar]
- 37.Bolling SF, Pagani FD, Deeb GM, et al. Intermediate-term outcome of mitral reconstruction in cardiomyopathy. J Thorac Cardiovasc Surg 1998;115:381-6; discussion 387-8. [DOI] [PubMed] [Google Scholar]
- 38.Braun J, Bax JJ, Versteegh MI, et al. Preoperative left ventricular dimensions predict reverse remodeling following restrictive mitral annuloplasty in ischemic mitral regurgitation. Eur J Cardiothorac Surg 2005;27:847-53. [DOI] [PubMed] [Google Scholar]
- 39.Kwan J, Shiota T, Agler DA, et al. Geometric differences of the mitral apparatus between ischemic and dilated cardiomyopathy with significant mitral regurgitation: real-time three-dimensional echocardiography study. Circulation 2003;107:1135-40. [DOI] [PubMed] [Google Scholar]
- 40.Agricola E, Oppizzi M, Maisano F, et al. Echocardiographic classification of chronic ischemic mitral regurgitation caused by restricted motion according to tethering pattern. Eur J Echocardiogr 2004;5:326-34. [DOI] [PubMed] [Google Scholar]
- 41.Magne J, Sénéchal M, Mathieu P, et al. Restrictive annuloplasty for ischemic mitral regurgitation may induce functional mitral stenosis. J Am Coll Cardiol 2008;51:1692-701. [DOI] [PubMed] [Google Scholar]
- 42.Kalangos A, Christenson JT, Beghetti M, et al. Mitral valve repair for rheumatic valve disease in children: midterm results and impact of the use of a biodegradable mitral ring. Ann Thorac Surg 2008;86:161-8; discussion 168-9. [DOI] [PubMed] [Google Scholar]
- 43.Kaye DM, Byrne M, Alferness C, et al. Feasibility and short-term efficacy of percutaneous mitral annular reduction for the therapy of heart failure-induced mitral regurgitation. Circulation 2003;108:1795-7. [DOI] [PubMed] [Google Scholar]
- 44.Schofer J, Siminiak T, Haude M, et al. Percutaneous mitral annuloplasty for functional mitral regurgitation: results of the CARILLON Mitral Annuloplasty Device European Union Study. Circulation 2009;120:326-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siminiak T, Wu JC, Haude M, et al. Treatment of functional mitral regurgitation by percutaneous annuloplasty: results of the TITAN Trial. Eur J Heart Fail 2012;14:931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choure AJ, Garcia MJ, Hesse B, et al. In vivo analysis of the anatomical relationship of coronary sinus to mitral annulus and left circumflex coronary artery using cardiac multidetector computed tomography: implications for percutaneous coronary sinus mitral annuloplasty. J Am Coll Cardiol 2006;48:1938-45. [DOI] [PubMed] [Google Scholar]
- 47.Sponga S, Bertrand OF, Philippon F, et al. Reversible circumflex coronary artery occlusion during percutaneous transvenous mitral annuloplasty with the Viacor system. J Am Coll Cardiol 2012;59:288. [DOI] [PubMed] [Google Scholar]
- 48.Webb JG, Harnek J, Munt BI, et al. Percutaneous transvenous mitral annuloplasty: initial human experience with device implantation in the coronary sinus. Circulation 2006;113:851-5. [DOI] [PubMed] [Google Scholar]
- 49.McGoon DC. Repair of mitral insufficiency due to ruptured chordae tendineae. J Thorac Cardiovasc Surg 1960;39:357-62. [Google Scholar]
- 50.Gerbode F, Kerth WJ, Osborn JJ, et al. Correction of mitral insufficiency by open operation. Ann Surg 1962;155:846-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kay JH, Egerton WS. The repair of mitral insufficiency associated with ruptured chordae tendineae. Ann Surg 1963;157:351-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris JD, Penner DA, Brandt RL. Surgical correction of ruptured chordae tendineae. J Thorac Cardiovasc Surg 1964;48:772-80. [PubMed] [Google Scholar]
- 53.Menges H, Jr, Ankeney JL, Hellerstein HK. The clinical diagnosis and surgical management of ruptured mitral chordae tendineae. Circulation 1964;30:8-16. [DOI] [PubMed] [Google Scholar]
- 54.Sanders CA, Scannell JG, Harthorne JW, et al. Severe mitral regurgitation secondary to ruptured chordae tendineae. Circulation 1965;31:506-16. [DOI] [PubMed] [Google Scholar]
- 55.Smedira NG, Selman R, Cosgrove DM, et al. Repair of anterior leaflet prolapse: chordal transfer is superior to chordal shortening. J Thorac Cardiovasc Surg 1996;112:287-91; discussion 291-2. [DOI] [PubMed] [Google Scholar]
- 56.Gillinov AM, Cosgrove DM, Lytle BW, et al. Reoperation for failure of mitral valve repair. J Thorac Cardiovasc Surg 1997;113:467-73; discussion 473-5. [DOI] [PubMed] [Google Scholar]
- 57.Duran CG. Repair of anterior mitral leaflet chordal rupture or elongation (the flip-over technique). J Card Surg 1986;1:161-6. [DOI] [PubMed] [Google Scholar]
- 58.Dreyfus G, Al Aylé N, Dubois C, et al. Long term results of mitral valve repair: posterior papillary muscle repositioning versus chordal shortening. Eur J Cardiothorac Surg 1999;16:81-7. [DOI] [PubMed] [Google Scholar]
- 59.Fucci C, Sandrelli L, Pardini A, et al. Improved results with mitral valve repair using new surgical techniques. Eur J Cardiothorac Surg 1995;9:621-6 discuss 626-7. [DOI] [PubMed]
- 60.Nielsen SL, Timek TA, Lai DT, et al. Edge-to-edge mitral repair: tension on the approximating suture and leaflet deformation during acute ischemic mitral regurgitation in the ovine heart. Circulation 2001;104:I29-35. [PubMed] [Google Scholar]
- 61.De Bonis M, Lapenna E, La Canna G, et al. Mitral valve repair for functional mitral regurgitation in end-stage dilated cardiomyopathy: role of the “edge-to-edge” technique. Circulation 2005;112:I402-8. [DOI] [PubMed] [Google Scholar]
- 62.Mascagni R, Al Attar N, Lamarra M, et al. Edge-to-edge technique to treat post-mitral valve repair systolic anterior motion and left ventricular outflow tract obstruction. Ann Thorac Surg 2005;79:471-3; discussion 474. [DOI] [PubMed] [Google Scholar]
- 63.Myers PO, Khalpey Z, Maloney AM, et al. Edge-to-edge repair for prevention and treatment of mitral valve systolic anterior motion. J Thorac Cardiovasc Surg 2013;146:836-40. [DOI] [PubMed] [Google Scholar]
- 64.Jebara VA, Mihaileanu S, Acar C, et al. Left ventricular outflow tract obstruction after mitral valve repair. Results of the sliding leaflet technique. Circulation 1993;88:II30-4. [PubMed] [Google Scholar]
- 65.Gillinov AM, Cosgrove DM, Blackstone EH, et al. Durability of mitral valve repair for degenerative disease. J Thorac Cardiovasc Surg 1998;116:734-43. [DOI] [PubMed] [Google Scholar]
- 66.David TE. Replacement of chordae tendineae with expanded polytetrafluoroethylene sutures. J Card Surg 1989;4:286-90. [DOI] [PubMed] [Google Scholar]
- 67.Frater RW, Vetter HO, Zussa C, et al. Chordal replacement in mitral valve repair. Circulation 1990;82:IV125-30. [PubMed] [Google Scholar]
- 68.David TE, Armstrong S, Sun Z. Replacement of chordae tendineae with Gore-Tex sutures: a ten-year experience. J Heart Valve Dis 1996;5:352-5. [PubMed] [Google Scholar]
- 69.Kobayashi J, Sasako Y, Bando K, et al. Ten-year experience of chordal replacement with expanded polytetrafluoroethylene in mitral valve repair. Circulation 2000;102:III30-4. [DOI] [PubMed] [Google Scholar]
- 70.Von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [DOI] [PubMed] [Google Scholar]
- 71.Adams DH, Kadner A, Chen RH. Artificial mitral valve chordae replacement made simple. Ann Thorac Surg 2001;71:1377-8; discussion 1378-9. [DOI] [PubMed] [Google Scholar]
- 72.Duran CM, Pekar F. Techniques for ensuring the correct length of new mitral chords. J Heart Valve Dis 2003;12:156-61. [PubMed] [Google Scholar]
- 73.David TE, Omran A, Armstrong S, et al. Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1998;115:1279-85; discussion 1285-6. [DOI] [PubMed] [Google Scholar]
- 74.Lawrie GM, Earle EA, Earle NR. Feasibility and intermediate term outcome of repair of prolapsing anterior mitral leaflets with artificial chordal replacement in 152 patients. Ann Thorac Surg 2006;81:849-56; discussion 856. [DOI] [PubMed] [Google Scholar]
- 75.Perier P, Hohenberger W, Lakew F, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the “respect rather than resect” approach. Ann Thorac Surg 2008;86:718-25; discussion 718-25. [DOI] [PubMed] [Google Scholar]
- 76.Feldman T, Wasserman HS, Herrmann HC, et al. Percutaneous mitral valve repair using the edge-to-edge technique: six-month results of the EVEREST Phase I Clinical Trial. J Am Coll Cardiol 2005;46:2134-40. [DOI] [PubMed] [Google Scholar]
- 77.Umaña JP, Salehizadeh B, DeRose JJ, et al. “Bow-tie” mitral valve repair: an adjuvant technique for ischemic mitral regurgitation. Ann Thorac Surg 1998;66:1640-6. [DOI] [PubMed] [Google Scholar]
- 78.Sartipy U, Albåge A, Mattsson E, et al. Edge-to-edge mitral repair without annuloplasty in combination with surgical ventricular restoration. Ann Thorac Surg 2007;83:1303-9. [DOI] [PubMed] [Google Scholar]
- 79.Bajona P, Katz WE, Daly RC, et al. Beating-heart, off-pump mitral valve repair by implantation of artificial chordae tendineae: an acute in vivo animal study. J Thorac Cardiovasc Surg 2009;137:188-93. [DOI] [PubMed] [Google Scholar]
- 80.Seeburger J, Rinaldi M, Nielsen SL, et al. Off-pump transapical implantation of artificial neo-chordae to correct mitral regurgitation: the TACT Trial (Transapical Artificial Chordae Tendinae) proof of concept. J Am Coll Cardiol 2014;63:914-9. [DOI] [PubMed] [Google Scholar]
- 81.Kang DH, Kim JH, Rim JH, et al. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation 2009;119:797-804. [DOI] [PubMed] [Google Scholar]
- 82.Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:2440-92. [DOI] [PubMed] [Google Scholar]
- 83.Shumway NE, Lewis FJ. Experimental surgery of the mitral valve under direct vision using hypothermia. Surg Forum 1955;5:12-6. [PubMed] [Google Scholar]
- 84.Frater RW, Berghuis J, Brown AL, et al. The experimental and clinical use of autogenous pericardium for the replacement and extension of mitral and tricuspid value cusps and chordae. J Cardiovasc Surg (Torino) 1965;6:214-28. [PubMed] [Google Scholar]
- 85.Chauvaud S, Jebara V, Chachques JC, et al. Valve extension with glutaraldehyde-preserved autologous pericardium. Results in mitral valve repair. J Thorac Cardiovasc Surg 1991;102:171-7; discussion 177-8. [PubMed] [Google Scholar]
- 86.Acar C, de Ibarra JS, Lansac E. Anterior leaflet augmentation with autologous pericardium for mitral repair in rheumatic valve insufficiency. J Heart Valve Dis 2004;13:741-6. [PubMed] [Google Scholar]
- 87.Dillon J, Yakub MA, Nordin MN, et al. Leaflet extension in rheumatic mitral valve reconstruction. Eur J Cardiothorac Surg 2013;44:682-9. [DOI] [PubMed] [Google Scholar]
- 88.Talwar S, Rajesh MR, Subramanian A, et al. Mitral valve repair in children with rheumatic heart disease. J Thorac Cardiovasc Surg 2005;129:875-9. [DOI] [PubMed] [Google Scholar]
- 89.Rankin JS, Sharma MK, Teague SM, et al. A new approach to mitral valve repair for rheumatic disease: preliminary study. J Heart Valve Dis 2008;17:614-9. [PubMed] [Google Scholar]
- 90.Phillips JH, Burch GE, Depasquale NP. The syndrome of papillary muscle dysfunction. its clinical recognition. Ann Intern Med 1963;59:508-20. [DOI] [PubMed] [Google Scholar]
- 91.Miller GE, Jr, Kerth WJ, Gerbode F. Experimental papillary muscle infarction. J Thorac Cardiovasc Surg 1968;56:611-6. [PubMed] [Google Scholar]
- 92.Kaul S, Spotnitz WD, Glasheen WP, et al. Mechanism of ischemic mitral regurgitation. An experimental evaluation. Circulation 1991;84:2167-80. [DOI] [PubMed] [Google Scholar]
- 93.Gorman JH, 3rd, Gorman RC, Plappert T, et al. Infarct size and location determine development of mitral regurgitation in the sheep model. J Thorac Cardiovasc Surg 1998;115:615-22. [DOI] [PubMed] [Google Scholar]
- 94.Levine RA, Hung J, Otsuji Y, et al. Mechanistic insights into functional mitral regurgitation. Curr Cardiol Rep 2002;4:125-9. [DOI] [PubMed] [Google Scholar]
- 95.Timek TA, Lai DT, Tibayan F, et al. Ischemia in three left ventricular regions: Insights into the pathogenesis of acute ischemic mitral regurgitation. J Thorac Cardiovasc Surg 2003;125:559-69. [DOI] [PubMed] [Google Scholar]
- 96.Braun J, van de Veire NR, Klautz RJ, et al. Restrictive mitral annuloplasty cures ischemic mitral regurgitation and heart failure. Ann Thorac Surg 2008;85:430-6; discussion 436-7. [DOI] [PubMed] [Google Scholar]
- 97.Hvass U, Tapia M, Baron F, et al. Papillary muscle sling: a new functional approach to mitral repair in patients with ischemic left ventricular dysfunction and functional mitral regurgitation. Ann Thorac Surg 2003;75:809-11. [DOI] [PubMed] [Google Scholar]
- 98.Kron IL, Green GR, Cope JT. Surgical relocation of the posterior papillary muscle in chronic ischemic mitral regurgitation. Ann Thorac Surg 2002;74:600-1. [DOI] [PubMed] [Google Scholar]
- 99.Menicanti L, Di Donato M, Frigiola A, et al. Ischemic mitral regurgitation: intraventricular papillary muscle imbrication without mitral ring during left ventricular restoration. J Thorac Cardiovasc Surg 2002;123:1041-50. [DOI] [PubMed] [Google Scholar]
- 100.Ueno T, Sakata R, Iguro Y, et al. New surgical approach to reduce tethering in ischemic mitral regurgitation by relocation of separate heads of the posterior papillary muscle. Ann Thorac Surg 2006;81:2324-5. [DOI] [PubMed] [Google Scholar]
- 101.Kincaid EH, Riley RD, Hines MH, et al. Anterior leaflet augmentation for ischemic mitral regurgitation. Ann Thorac Surg 2004;78:564-8; discussion 568. [DOI] [PubMed] [Google Scholar]
- 102.Messas E, Yosefy C, Chaput M, et al. Chordal cutting does not adversely affect left ventricle contractile function. Circulation 2006;114:I524-8. [DOI] [PubMed] [Google Scholar]
- 103.Goetz WA, Lim H-S, Lansac E, et al. Anterior mitral basal “stay” chords are essential for left ventricular geometry and function. J Heart Valve Dis 2005;14:195-202; discussion 202-3. [PubMed] [Google Scholar]
- 104.Acker MA, Bolling S, Shemin R, et al. Mitral valve surgery in heart failure: insights from the Acorn Clinical Trial. J Thorac Cardiovasc Surg 2006;132:568-77, 577.e1-4. [DOI] [PubMed]
- 105.Mann DL, Kubo SH, Sabbah HN, et al. Beneficial effects of the CorCap cardiac support device: five-year results from the Acorn Trial. J Thorac Cardiovasc Surg 2012;143:1036-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grossi EA, Patel N, Woo YJ, et al. Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Am Coll Cardiol 2010;56:1984-93. [DOI] [PubMed] [Google Scholar]
- 107.Trichon BH, Glower DD, Shaw LK, et al. Survival after coronary revascularization, with and without mitral valve surgery, in patients with ischemic mitral regurgitation. Circulation 2003;108:II103-10. [DOI] [PubMed] [Google Scholar]
- 108.Benedetto U, Melina G, Roscitano A, et al. Does combined mitral valve surgery improve survival when compared to revascularization alone in patients with ischemic mitral regurgitation? A meta-analysis on 2479 patients. J Cardiovasc Med (Hagerstown) 2009;10:109-14. [DOI] [PubMed] [Google Scholar]
- 109.Grossi EA, Woo YJ, Patel N, et al. Outcomes of coronary artery bypass grafting and reduction annuloplasty for functional ischemic mitral regurgitation: a prospective multicenter study (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Thorac Cardiovasc Surg 2011;141:91-7. [DOI] [PubMed] [Google Scholar]
- 110.Messas E, Bel A, Morichetti MC, et al. Autologous myoblast transplantation for chronic ischemic mitral regurgitation. J Am Coll Cardiol 2006;47:2086-93. [DOI] [PubMed] [Google Scholar]
- 111.Nickenig G, Estevez-Loureiro R, Franzen O, et al. Percutaneous Mitral Valve Edge-to-Edge Repair: In-Hospital Results and 1-Year Follow-Up of 628 Patients of the 2011-2012 Pilot European Sentinel Registry. J Am Coll Cardiol 2014;64:875-84. [DOI] [PubMed] [Google Scholar]
- 112.Lewis BS, Agathangelou NE, Colsen PR, et al. Cardiac operation during active infective endocarditis: results of aortic, mitral, and double valve replacement in 94 patients. J Thorac Cardiovasc Surg 1982;84:579-84. [PubMed] [Google Scholar]
- 113.Cukingnan RA, Carey JS, Wittig JH, et al. Early valve replacement in active infective endocarditis. Results and late survival. J Thorac Cardiovasc Surg 1983;85:163-73. [PubMed] [Google Scholar]
- 114.Nelson RJ, Harley DP, French WJ, et al. Favorable ten-year experience with valve procedures for active infective endocarditis. J Thorac Cardiovasc Surg 1984;87:493-502. [PubMed] [Google Scholar]
- 115.Fleisher AG, David I, Mogtader A, et al. Mitral valvuloplasty and repair for infective endocarditis. J Thorac Cardiovasc Surg 1987;93:311-5. [PubMed] [Google Scholar]
- 116.Dreyfus G, Serraf A, Jebara VA, et al. Valve repair in acute endocarditis. Ann Thorac Surg 1990;49:706-11; discussion 712-3. [DOI] [PubMed] [Google Scholar]
- 117.Iung B, Rousseau-Paziaud J, Cormier B, et al. Contemporary results of mitral valve repair for infective endocarditis. J Am Coll Cardiol 2004;43:386-92. [DOI] [PubMed] [Google Scholar]
- 118.Ruttmann E, Legit C, Poelzl G, et al. Mitral valve repair provides improved outcome over replacement in active infective endocarditis. J Thorac Cardiovasc Surg 2005;130:765-71. [DOI] [PubMed] [Google Scholar]
- 119.Shimokawa T, Kasegawa H, Matsuyama S, et al. Long-term outcome of mitral valve repair for infective endocarditis. Ann Thorac Surg 2009;88:733-9; discussion 739. [DOI] [PubMed] [Google Scholar]
- 120.Nishimura RA, Carabello BA, Faxon DP, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2008;118:887-96. [DOI] [PubMed] [Google Scholar]
- 121.Acar C, Tolan M, Berrebi A, et al. Homograft replacement of the mitral valve. Graft selection, technique of implantation, and results in forty-three patients. J Thorac Cardiovasc Surg 1996;111:367-78; discussion 378-80. [DOI] [PubMed] [Google Scholar]
- 122.Kumar AS, Choudhary SK, Mathur A, et al. Homograft mitral valve replacement: five years’ results. J Thorac Cardiovasc Surg 2000;120:450-8. [DOI] [PubMed] [Google Scholar]
- 123.Ali M, Iung B, Lansac E, et al. Homograft replacement of the mitral valve: eight-year results. J Thorac Cardiovasc Surg 2004;128:529-34. [DOI] [PubMed] [Google Scholar]
- 124.Carpentier A, Loulmet D, Carpentier A, et al. Open heart operation under videosurgery and minithoracotomy. First case (mitral valvuloplasty) operated with success. C R Acad Sci III 1996;319:219-23. [PubMed] [Google Scholar]
- 125.Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74; discussion 574-6. [DOI] [PubMed] [Google Scholar]
- 126.Loulmet DF, Carpentier A, Cho PW, et al. Less invasive techniques for mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:772-9. [DOI] [PubMed] [Google Scholar]
- 127.Felger JE, Chitwood WR, Jr, Nifong LW, et al. Evolution of mitral valve surgery: toward a totally endoscopic approach. Ann Thorac Surg 2001;72:1203-8; discussion 1208-9. [DOI] [PubMed] [Google Scholar]
- 128.Gammie JS, Zhao Y, Peterson ED, et al. J. Maxwell Chamberlain Memorial Paper for adult cardiac surgery. Less-invasive mitral valve operations: trends and outcomes from the Society of Thoracic Surgeons Adult Cardiac Surgery Database. Ann Thorac Surg 2010;90:1401-8, 1410.e1; discussion 1408-10. [DOI] [PubMed]
- 129.Seeburger J, Borger MA, Falk V, et al. Minimal invasive mitral valve repair for mitral regurgitation: results of 1339 consecutive patients. Eur J Cardiothorac Surg 2008;34:760-5. [DOI] [PubMed] [Google Scholar]
- 130.Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [DOI] [PubMed] [Google Scholar]
- 131.Goldstone AB, Atluri P, Szeto WY, et al. Minimally invasive approach provides at least equivalent results for surgical correction of mitral regurgitation: a propensity-matched comparison. J Thorac Cardiovasc Surg 2013;145:748-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cheng DC, Martin J, Lal A, et al. Minimally invasive versus conventional open mitral valve surgery: a meta-analysis and systematic review. Innovations (Phila) 2011;6:84-103. [DOI] [PubMed] [Google Scholar]
- 133.Nifong LW, Rodriguez E, Chitwood WR, Jr. 540 consecutive robotic mitral valve repairs including concomitant atrial fibrillation cryoablation. Ann Thorac Surg 2012;94:38-42; discussion 43. [DOI] [PubMed] [Google Scholar]
- 134.Nifong LW, Chitwood WR, Pappas PS, et al. Robotic mitral valve surgery: a United States multicenter trial. J Thorac Cardiovasc Surg 2005;129:1395-404. [DOI] [PubMed] [Google Scholar]
- 135.Chitwood WR, Jr, Nifong LW, Chapman WH, et al. Robotic surgical training in an academic institution. Ann Surg 2001;234:475-84; discussion 484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramzy D, Trento A, Cheng W, et al. Three hundred robotic-assisted mitral valve repairs: the Cedars-Sinai experience. J Thorac Cardiovasc Surg 2014;147:228-35. [DOI] [PubMed] [Google Scholar]


