Abstract
Introduction
To prospectively evaluate if the inclusion of a clinical sexologist in a penile and sexual rehabilitation program improves sexual function one year after prostate cancer surgery.
Material and methods
Twelve months after da Vinci Radical Prostatectomy (dVRP) for prostate cancer, 28 fully potent (IIEF-5 >21) and sexually active men (ages 47-69 years, mean 61) who, in 2008, were enrolled in a prospectively monitored penile rehabilitation program (reference group) were compared with 79 fully potent (IIEF-5 >21) and sexually active men (ages 45-74 years, mean 61) enrolled in 2009 (study group); whose program differed by the inclusion of evaluation and treatment by a clinical sexologist.
Results
Twelve months after dVRP, seventeen patients in the reference group (61%) were sexually active with regular penetrating sexual activity compared to sixty-six (84%) in the study group (p = 0.02). These findings were independent of whether they had undergone a nerve sparing or non-nerve sparing procedure. Almost 94% (74 patients) in the study group had at some time been able to perform penetrating sexual activity; 14 patients required additional visits to the clinical sexologist beyond the routine follow-up, 9 for short-term cognitive behavior therapy.
Conclusions
Inclusion of a clinical sexologist in a penile and sexual rehabilitation program appears to improve the ability to have regular sexual activity with penetrating sex one year after da Vinci Robotic Radical Prostatectomy.
Keywords: prostate cancer, postoperative erectile dysfunction, sexual rehabilitation, clinical sexologist
INTRODUCTION
Prostate cancer is the most common type of cancer among men in Sweden. Almost 10 000 new cases are diagnosed every year [1]. Approximately half of these men will receive curative treatment either with radiation or surgery [1]. The surgical procedure, radical prostatectomy, generally means a complete removal of the prostate gland, seminal vesicles and parts of the vas deferens. The procedure can be performed either by conventional open technique, conventional laparoscopic or robot-assisted laparoscopic technique (da Vinci Radical Prostatectomy). The major common side effects associated with surgery are incontinence and erectile dysfunction (ED). The latter results from damage to the neurovascular bundles (NVB) that mediate the normal spontaneous erectile response, susceptible because of their anatomical position [2, 3]. In selected cases, at the time of surgery, an attempt may be made to preserve these bundles to minimize the risk of postoperative ED. Despite such attempts, loss of erection or various degrees of ED still remain the most common side effect of the operation [3, 4].
Preoperative erectile function (EF), the patient's age, the possibility of preservation of the NVB, and the experience of the surgeon are important factors for the postoperative outcome of EF [4].
The ability to have a satisfactory erection and sexual function plays a significant role in the overall quality of life; not only for the patient but also for the one with whom he has a relationship [5, 6]. In modern medicine, rehabilitation is one of the cornerstones for successful management of an ailment (e.g. in orthopaedics and after neurovascular disasters), hence sexual rehabilitation ought to be a part of the postoperative management of prostate cancer surgery.
Today, the rehabilitation (so called “penile rehabilitation”) after prostate cancer surgery is predominantly focused on restoring EF alone. It is attempted with pharmacological therapy: phosphodiesterase type 5 (PDE-5) inhibitors, intraurethral prostaglandin E1 (PGE-1) gel or intracavernous PGE-1 injection; mechanical devices: vacuum pumps; surgery: penile implants; or combinations of these modalities [7, 8, 9]. Early postoperative penile rehabilitation/stimulation of EF appears to optimize the final outcome [10, 11].
In sexual medicine, it is well known that restoring EF alone does not always solve all the sexual problems associated with ED [12]. Up to 60% will discontinue their ED treatment within 2 years, even if it is pharmacologically successful [13–16]. In our opinion, the aim of rehabilitation after radical prostatectomy should not be focused on penile function alone, but, instead, aim to establish a satisfactory postoperative sexual life, as assessed by the patient (and his partner), with the ability of having penetrating sex regardless of whether there is residual spontaneous EF or not. Instead of simple “penile rehabilitation,” a more comprehensive “sexual rehabilitation” should be included that also addresses other side effects of the surgery; such as loss of ejaculate, penile shortening, change of orgasmic feeling, alterations in body image, stress incontinence, disturbances in partner relationships and various types of anxiety [17, 18, 19].
The aim of the study was to evaluate the potential benefit of a combined penile and sexual rehabilitation program with a clinical sexologist when compared to a penile rehabilitation program alone; with the intended outcome being the improved possibility of having regular sexual activity with penetrating sex, one year after robot-assisted radical prostatectomy.
MATERIAL AND METHODS
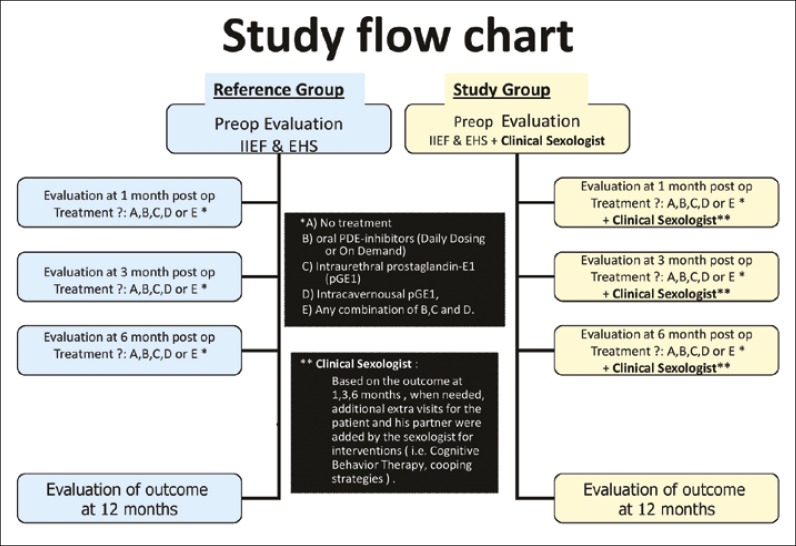
Since 2007 in the Urology department of our hospital, we have an established “penile” rehabilitation program. The aim of the program is to restore postoperative EF for all men that were preoperatively fully potent, regardless of whether we had been able to preserve the NVB or not. In this program the patients EF was evaluated preoperatively and at 12 months postoperatively with two questionnaires: the International Index of Erectile Function-short version (IIEF-5), and the Erection Hardness Score (EHS) [20, 21]. At other visits, EF was assessed using the EHS alone. Based on the outcome of the procedure: bilateral nerve-sparing (BLNS), unilateral nerve-sparing (UNLS) or non nerve-sparing (NoN NS); and the recorded EHS at one month, the patients received either:
No treatment,
Oral PDE-5 inhibitors: daily dosing (DD) Tadalafil 5 mg or on demand (OD) either Sildenafil, Vardenafil or Tadalafil in maximum dose,
Intraurethral PGE-1 gel (500-1000 micrograms),
Intracavernous PGE-1 injections (5-20 micrograms) or
Any combination of B, C and D
All patients with an EHS grade <3 at the time of the first follow-up visit were qualified for and received ED treatment (options B-E) according to patient preference. Patients with EHS 3 were recommended to initiate ED treatment with PDE-5 inhibitors daily or on demand according to patients’ preference (option B) and patients with an EHS grade 4 qualified to option A – no treatment. The patients were then followed up at 3, 6 and 12 months. Adjustments to the treatment were done, when needed, according to the outcome of the treatment and, to some degree, the patients’ preference (Figure 1).
Figure 1.
Study flow chart.
In 2009 we included a Clinical Sexologist (CS) in the rehabilitation process and the program was extended to become a combined penile and sexual medicine rehabilitation program. The CS saw the patient and his partner before surgery and at 1, 3, 6 and 12 months postoperatively and on additional visits when needed during the first postoperative year (Figure 1).
Various counselling methods were used in the sexual rehabilitation process, usually psycho-dynamic therapies for a relation perspective (i.e.; Motivation Interviewing, Cognitive Behaviour Therapy, Coaching, Coping, and Biofeedback). It is important to stress that the counselling with the CS did not involve any “hands-on” training whatsoever. All instructions on how to use vacuum devices, administering intraurethral gel or intracavernous injections were given by nurses or urotherapists. During the study period, only one CS was involved in the management of all the patients.
Statistics
Due to the small sample sizes, Fisher's exact test (two sided) was used for all statistical calculations.
The reference group consisted of 28 preoperatively potent (IIEF-5 >21) and sexually active Caucasian men (age 47-69, mean age 61 years), who, during 2008, had da Vinci Robot-assisted Radical Prostatectomy (dVRP) performed for localized prostate cancer (tumour stage: T1c, T2, pT3, PSA <10 and Gleason score <8) with or without preservation of the neurovascular bundles. In 16 cases the bundles were preserved bilaterally (BLNS), in 9 unilaterally (ULNS) and in 3 no preservation was possible (NonNS). All operations were performed by one surgeon alone. The patients were followed according to the penile rehabilitation program (Figure 1).
The study group consisted of 79 preoperatively potent (IIEF-5 >21) and sexually active Caucasian men (age 45-74, mean age 61 years), who, during 2009, had dVRP performed for localized prostate cancer (tumour stage: T1c, T2, pT3, PSA <10 and Gleason score <8) with or without preservation of the neurovascular bundles. In 36 cases the bundles were preserved bilaterally (BLNS), in 34 unilaterally (ULNS) and in 9 no preservation was possible (NonNS). All operations were performed by one surgeon alone. The patients were followed according to the combined penile and sexual rehabilitation program (Figure 1).
RESULTS
In the reference group, 17 patients (61%) were regularly sexually active (at least 1-2 times/month) with penetrating sexual activity one year after surgery. None of them reported return of completely normal erections although 8 patients were active using oral PDE-5 inhibitors alone. The remaining 9 used either intraurethral or intracavernous PGE-1 for their sexual activity. The majority of BLNS (12/16) were sexually active, but less than half of the UNLS (4/9) and only one of three with NonNS were sexually active one year after surgery (Tables 1 and 2). The reasons for not having penetrating sexual active one year postoperatively were: lack of interest from patient or partner (n = 4); lack of efficacy or side effects of treatment (n = 5); urine incontinence (n = 1) and additional cancer treatment (n = 1) (Table 3). One patient had a PSA relapse (0.18) at 12 months.
Table 1.
Sexual penetrating activity and function 12 months postoperatively
| Reference group (11-15 months median= 12.5) N = 28 | Study group (11-17 months median = 13) N = 79 | |
|---|---|---|
| Sexually active with penetrating sex (all) | 61% (17/28) | 84% (66/79) |
| Sexually active with penetrating sex and normal erections | 0% (0/17) | 15% (10/66) |
| Sexually active with penetrating sex and erections with PDE-5 inhibitors | 47% (8/17) | 32% (21/66) |
| Sexually active with penetrating sex and erections with intrauretral gel or intracavernous injection of PGE1 | 53% (9/17) | 53% (35/66) |
| Not having any penetrating sexual activity | 39% (11/28) | 16% (13/79) |
Table 3.
Reasons for not having penetrating sexual activity 12 months postoperatively
| Reference group | Study group | |
|---|---|---|
| Percentage of patients not having penetrating sex | 39% (11/28) | 16% (13/79) |
| Lack of interest by patient/partner | 36% (4/11) | 38% (5/13) |
| Loss of partner | 0% (0/11) | 15% (2/13) |
| Additional cancer treatment | 9% (1/11) | 0% (0/13) |
| Ineffective treatment and/or side effects | 45% (5/11) | 31% (4/13) |
| Urine incontinence | 9% (1/11) | 8% (1/13) |
| Reason unknown | 0% (0/11) | 8% (1/13) |
In the study group, 66 patients (84%) were regularly sexually active with penetrating sexual activity one year after surgery, 15% (10/66) of them reported return of completely normal erections and 32% (21/66) were active using oral PDE-5 inhibitors alone. The remaining 53% (35/66) of the regularly sexually active patients used either intraurethral or intracavernous PGE-1 for their sexual activity. The majority of BLNS (34/36) were sexually active, two thirds of the UNLS (24/34) and almost all (8/9) of the NonNS were sexually active one year after surgery (Tables 1 and 2). An additional 10% (8/79) of the patients had, at some time during the first postoperative year, been able to perform penetrating sexual activity, but were not sexually active regularly. The reasons for not having penetrating sexual activity one year postoperatively were: lack of interest from patient or partner (n = 5); lack of efficacy or side effects of treatment (n = 4); loss of partner (n = 2); urine incontinence (n = 1) and reason unknown (n = 1) (Table 3). 14 patients (18%) needed, on average, 3.2 (1-7) additional visits to the sexologist outside the program during the first postoperative year. 9 patients received short-term cognitive behavioural therapy. In 6 (42%) of the 14 cases, the additional visits were, to a substantial degree, related to the partner's inability to cope with the patient's dysfunction. One patient had severe stress incontinence (450 gr/24/h) at 12 months. None had a PSA relapse.
Table 2.
Sexual penetrating activity 12 months postoperatively according to type of the procedure
| Reference group N = 28 | Study group N = 79 | p value | |
|---|---|---|---|
| Sexually active with penetrating sex all type of procedures | 61% (17/28) | 84% (66/79) | p = 0.02 |
| Sexually active with penetrating sex BLNS | 75% (12/16) | 94% (34/36) | p = 0.06 (ns) |
| Sexually active with penetrating sex and incomplete preservation NVB (ULNS and Non NS) | 41% (5/12) | 74%(32/43) | p= 0.04 |
| a) Sexually active with penetrating sex ULNS | 44% (4/9) | 71% (24/34) | p = 0.24(ns) |
| b) Sexually active with penetrating sex Non NS | 33% (1/3) | 89% (8/9) | p = 0.13 (ns) |
BLNS – Bi-Lateral Nerve Sparing Procedure; NVB – Neuro Vascular Bundles; ULNS - Uni-Lateral Nerve Sparing Procedure; Non NS – Non Nerve Sparing Procedure
Overall, there is a statistically significant (p <0.02) better outcome in the study group as compared to the reference group, with regards to the ability to have regular penetrating sexual activity 12 months after surgery. Particularly, patients with incomplete perioperative preservation of the neurovascular bundles (UNLS and NonNS) seems to benefit more (p <0.04) than the BLNS (p <0.06).
DISCUSSION
Currently, very little is reported on the management of the sexual disability and overall sexual rehabilitation after radical prostatectomy [22]. The data presented is predominantly focused on how many preoperatively fully potent men have residual sufficient EF (with or without oral PDE-5 inhibitors) postoperatively [17]. Best results are seen after BLNS where 50-90% is functional within 1 year after surgery. Patients with ULNS or NonNS are doing far worse; only 10-30% have an acceptable EF [4, 7, 23, 24]. The (early) penile rehabilitation is more or less exclusively studied in patients with BLNS surgery [25–28] and its additional value is still under debate and yet to be proven [29].
The reality is that more than half of all the preoperatively potent men that undergo a radical prostatectomy will postoperatively have lost their spontaneous EF. The majority of these patients will not benefit from oral PDE-5 inhibitor treatment alone. They will, instead, require additional treatment (intraurethral prostaglandin E-1 gel, intracavernous PGE-1 injection, vacuum pumps or penile implants) and therapeutic strategies (i.e. psycho-sexual counselling) to be able to return to penetrating sexual activity after surgery.
The strength of this observational study is that both the penile and the combined rehabilitation program have been prospectively monitored and conducted according to a similar and structured follow up regime. In addition, the groups studied are equal in age, tumor pathology, and distribution between types of nerve sparing surgical approaches. Finally, all procedures were performed by one surgeon alone with one surgical method (dVRP).
However, the sample size is small and there is no randomization. Neither can the improvement of the surgeon's skill over time [4], nor can the specific individual skills of the clinical sexologist be neglected, the latter being extremely difficult to quantify. The results might also reflect the benefit of having a clinical sexologist at the clinic with specialist knowledge and insight in the specifics of prostate cancer disease and treatment. This, together with a surgeon with a specific interest in sexual medicine, and in-depth knowledge of the impact and consequences of erectile dysfunction, is a combination that is not applicable to all urological facilities.
The observation time of one year is most likely too short to draw conclusions. Age, disease progression and the evidence that many patients will discontinue their ED treatment within 2 years, even if it is pharmacologically successful [13–16, 19, 30], are factors that might negatively affect the long-term results. The return of spontaneous erection over time [19, 30], and coping with and accepting a different sexual life, are factors that might improve the long-term outcome. A long term follow-up is warranted to see if the initial results are sustainable over time. It appears that increased sexual rehabilitation efforts postoperatively improve a patients’ ability to have sexual penetrating activity one year after dVRP, particularly in patients without complete perseveration of NVB (ULNS and Non NS). This group of patients is more likely to have less residual EF and more often will have to use intracavernosal injection therapy to achieve an erection hard enough for penetration. With this altered expression of their sexual function, it is very likely that they will benefit from the increased support provided by a CS [31, 32]. As other authors have reported [33, 34], the rehabilitation efforts are resource and time consuming. It is very important, preoperatively, to assess the importance of sexual function, and what role a sexual life plays for the patient and his partner; as the motivation for postoperative sexual rehabilitation is to a large extent dependent on this [19, 29]. The partners’ involvement, participation and understanding of the rehabilitation process are also important for the outcome. Most of the visits to the CS have been conducted with both the patient and his partner present. For many of the patients that did not return to sexual activity postoperatively, partner participation or interest was absent (Table 3).
The sexual medicine rehabilitation process after prostate cancer surgery means applying behavioural science methods and tools in addition to the standard medical/surgical care, which are not normally a part of the urologists’ training. Adding this knowledge to daily clinical urological practice, together with the involvement of a CS, should optimize and contribute to an improvement in the overall quality of the management of these patients.
Based on the current knowledge of the importance of EF for the wellbeing of the male [5, 6], and the possibility of successfully treating ED medically or surgically [35], it seems reasonable that all patients who are exposed to a treatment that will affect their sexual function should be offered the possibility of rehabilitation to an acceptable sexual life. In our opinion, at centres performing prostate cancer surgery, or any treatment causing iatrogenic sexual dysfunction, a clinical sexologist should have the same indispensable position as the physiotherapist at an orthopaedic centre.
CONCLUSIONS
Involvement of a clinical sexologist in a postoperative combined penile and sexual rehabilitation program after da Vinci robot-assisted radical prostatectomy appears to improve the ability to have regular sexual activity with penetrating sex one year after surgery, when compared to a penile rehabilitation program alone, particularly in patients without perioperative complete preservation of the neurovascular bundles.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.National Board of Health and Welfare. Stockholm: National Board of Health and Welfare; 2012. Cancer Incidence in Sweden 2012. [Google Scholar]
- 2.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 3.Kundu SD, Roehl KA, Eggener SE, Antenor JA, Han M, Catalona WJ. Potency, continence and complications in 3,477 consecutive radical retropubic prostatectomies. J Urol. 2004;172:2227–2231. doi: 10.1097/01.ju.0000145222.94455.73. [DOI] [PubMed] [Google Scholar]
- 4.Ayyathurai R, Manoharan M, Nieder AM, Kava B, Soloway MS. Factors affecting erectile function after radical retropubic prostatectomy: results from 1620 consecutive patients. BJU Int. 2008;101:833–836. doi: 10.1111/j.1464-410X.2007.07409.x. [DOI] [PubMed] [Google Scholar]
- 5.Latini DM, Penson DF, Lubeck DP, Wallace KL, Henning JM, Lue TF. Longitudinal differences in disease specific quality of life in men with erectile dysfunction: results from the Exploratory Comprehensive Evaluation of Erectile Dysfunction study. J Urol. 2003;169:1437–1442. doi: 10.1097/01.ju.0000049203.33463.9e. [DOI] [PubMed] [Google Scholar]
- 6.Chevret M, Jaudinot E, Sullivan K, Marrel A, De Gendre AS. Quality of sexual life and satisfaction in female partners of men with ED: psychometric validation of the Index of Sexual Life (ISL) questionnaire. J Sex Marital Ther. 2004;30:141–515. doi: 10.1080/00926230490262339. [DOI] [PubMed] [Google Scholar]
- 7.Mulhall J, Land S, Parker M, Waters WB, Flanigan RC. The use of an erectogenic pharmacotherapy regimen following radical prostatectomy improves recovery of spontaneous erectile function. J Sex Med. 2005;2:540–542. doi: 10.1111/j.1743-6109.2005.00081_1.x. [DOI] [PubMed] [Google Scholar]
- 8.Mazzola C, Mulhall JP. Penile rehabilitation after prostate cancer treatment: outcomes and practical algorithm. Urol Clin North Am. 2011;38:105–118. doi: 10.1016/j.ucl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Montague DK. Penile prosthesis implantation for end-stage erectile dysfunction after radical prostatectomy. Rev Urol. 2005;(suppl 1):S51–57. [PMC free article] [PubMed] [Google Scholar]
- 10.Raina R, Pahlajani G, Agarwal A, Zippe CD. Early penile rehabilitation following radical prostatectomy: Cleveland clinic experience. Int J Impot Res. 2007;20:121–126. doi: 10.1038/sj.ijir.3901573. [DOI] [PubMed] [Google Scholar]
- 11.Chung E, Brock G. Sexual rehabilitation and cancer survivorship: a state of art review of current literature and management strategies in male sexual dysfunction among prostate cancer survivors. J Sex Med. 2013;(suppl 1):102–111. doi: 10.1111/j.1743-6109.2012.03005.x. [DOI] [PubMed] [Google Scholar]
- 12.Althof SE. When an erection alone is not enough: biopsychosocial obstacles to lovemaking. Int J Impot Res. 2002;(suppl 1):S99–S104. doi: 10.1038/sj.ijir.3900799. [DOI] [PubMed] [Google Scholar]
- 13.Hackett GI. What do patients expect from erectile dysfunction therapy? Eur Urol. 2002;(suppl 1):4–11. [Google Scholar]
- 14.Klotz T, Mathers M, Klotz R, Sommer F. Why do patients with erectile dysfunction abandon effective therapy with sildenafil (Viagra)? Int J Impot Res. 2005;17:2–4. doi: 10.1038/sj.ijir.3901252. [DOI] [PubMed] [Google Scholar]
- 15.Irwin MB, Kata EJ. High attrition rate with intracavernous injection of prostaglandin E1 for impotency. Urology. 1994;43:84–87. doi: 10.1016/s0090-4295(94)80272-6. [DOI] [PubMed] [Google Scholar]
- 16.Ströberg P, Hedelin H, Bergström A. Is sex only for the healthy and wealthy? J Sex Med. 2007;4:176–182. doi: 10.1111/j.1743-6109.2006.00233.x. [DOI] [PubMed] [Google Scholar]
- 17.Benson CR, Serefoglu EC, Hellstrom WJ. Sexual dysfunction following radical prostatectomy. J Androl. 2012;33:1143–1154. doi: 10.2164/jandrol.112.016790. [DOI] [PubMed] [Google Scholar]
- 18.Frey AU, Sønksen J, Fode M. Neglected side effects after radical prostatectomy: a systematic review. J Sex Med. 2014;11:374–385. doi: 10.1111/jsm.12403. [DOI] [PubMed] [Google Scholar]
- 19.Messaoudi R, Menard J, Ripert T, Parquet H, Staerman F. Erectile dysfunction and sexual health after radical prostatectomy: impact of sexual motivation. Int J Impot Res. 2011;3:81–86. doi: 10.1038/ijir.2011.8. [DOI] [PubMed] [Google Scholar]
- 20.Rosen RC, Cappelleri JC, Smith MD, Lipsky J, Peña BM. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. Int J Impot Res. 1999;11:319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 21.Mulhall JP, Goldstein I, Bushmakin AG, Cappelleri JC, Hvidsten K. Validation of the erection hardness score. J Sex Med. 2007;4:1626–1634. doi: 10.1111/j.1743-6109.2007.00600.x. [DOI] [PubMed] [Google Scholar]
- 22.Parahoo K, McDonough S, McCaughan E, Noyes J, Semple C, Halstead EJ, et al. Psychosocial interventions for men with prostate cancer. Cochrane Database Syst Rev. 2013 Dec 24;12:CD008529. doi: 10.1002/14651858.CD008529.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borchers H, Brehmer B, Kirschner-Hermanns R, Reineke T, Tietze L, Jakse G. Erectile function after non-nerve-sparing radical prostatectomy: fact or fiction? Urol Int. 2006;76:213–216. doi: 10.1159/000091621. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan R, Katz D, Nelson CJ, Mulhall JP. Erectile function recovery in patients after non-nerve sparing radical prostatectomy. Andrology. 2014;2:951–954. doi: 10.1111/andr.282. [DOI] [PubMed] [Google Scholar]
- 25.Briganti A, Di Trapani E, Abdollah F, et al. Choosing the best candidates for penile rehabilitation after bilateral nerve-sparing radical prostatectomy. J Sex Med. 2012;9:608–617. doi: 10.1111/j.1743-6109.2011.02580.x. [DOI] [PubMed] [Google Scholar]
- 26.Montorsi F, Brock G, Stolzenburg JU, et al. Effects of tadalafil treatment on erectile function recovery following bilateral nerve-sparing radical prostatectomy: a randomised placebo-controlled study (REACTT) Eur Urol. 2014;65:587–596. doi: 10.1016/j.eururo.2013.09.051. [DOI] [PubMed] [Google Scholar]
- 27.Montorsi F, Brock G, Lee J, Shapiro J, Van Poppel H, Graefen M, Stief C. Effect of nightly versus on-demand vardenafil on recovery of erectile function in men following bilateral nerve-sparing radical prostatectomy. Eur Urol. 2008;54:924–931. doi: 10.1016/j.eururo.2008.06.083. [DOI] [PubMed] [Google Scholar]
- 28.Gandaglia G, Gallina A, Suardi N, et al. Preoperative erectile function is the only predictor of the use of a high number of phosphodiesterase type-5 inhibitors after bilateral nerve-sparing radical prostatectomy. Int J Impot Res. 2014;26:201–204. doi: 10.1038/ijir.2014.10. [DOI] [PubMed] [Google Scholar]
- 29.Fode M, Ohl DA, Ralph D, Sønksen J. Fode M, Ohl DA, Ralph D, Sønksen J, editors. Penile rehabilitation after radical prostatectomy: what the evidence really says. BJU Int. 2013;112:998–1008. doi: 10.1111/bju.12228. [DOI] [PubMed] [Google Scholar]
- 30.Kimura M, Bañez LL, Schroeck FR, et al. Factors predicting early and late phase decline of sexual health-related quality of life following radical prostatectomy. J Sex Med. 2011;8:2935–2943. doi: 10.1111/j.1743-6109.2011.02387.x. [DOI] [PubMed] [Google Scholar]
- 31.Burns SM, Mahalik JR. Understanding how masculine gender scripts may contribute to men's adjustment following treatment for prostate cancer. Am J Mens Health. 2007;1:250–261. doi: 10.1177/1557988306293380. [DOI] [PubMed] [Google Scholar]
- 32.Canada AL, Neese LE, Sui D, Schover LR. Pilot intervention to enhance sexual rehabilitation for couples after treatment for localized prostate carcinoma. Cancer. 2005;104:2689–2700. doi: 10.1002/cncr.21537. [DOI] [PubMed] [Google Scholar]
- 33.Mulhall JP. The role and structure of a postradical prostatectomy penile rehabilitation program. Curr Urol Rep. 2009;10:219–225. doi: 10.1007/s11934-009-0037-4. [DOI] [PubMed] [Google Scholar]
- 34.Tutolo M, Briganti A, Suardi N, et al. Optimizing postoperative sexual function after radical prostatectomy. Ther Adv Urol. 2012;4:347–365. doi: 10.1177/1756287212450063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Levine LA. Diagnosis and treatment of erectile dysfunction. Am J Med. 2000;109(suppl 9A):3S–12S. doi: 10.1016/s0002-9343(00)00655-0. [DOI] [PubMed] [Google Scholar]