Abstract
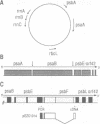
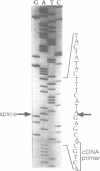
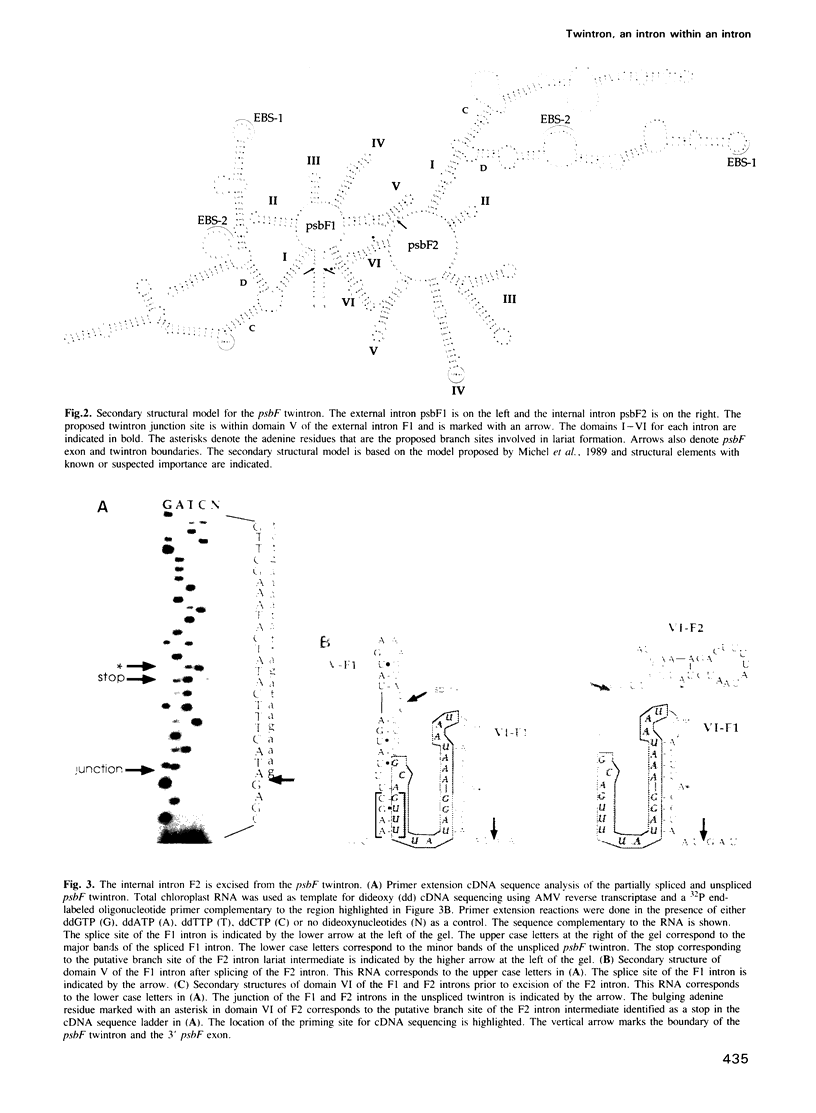
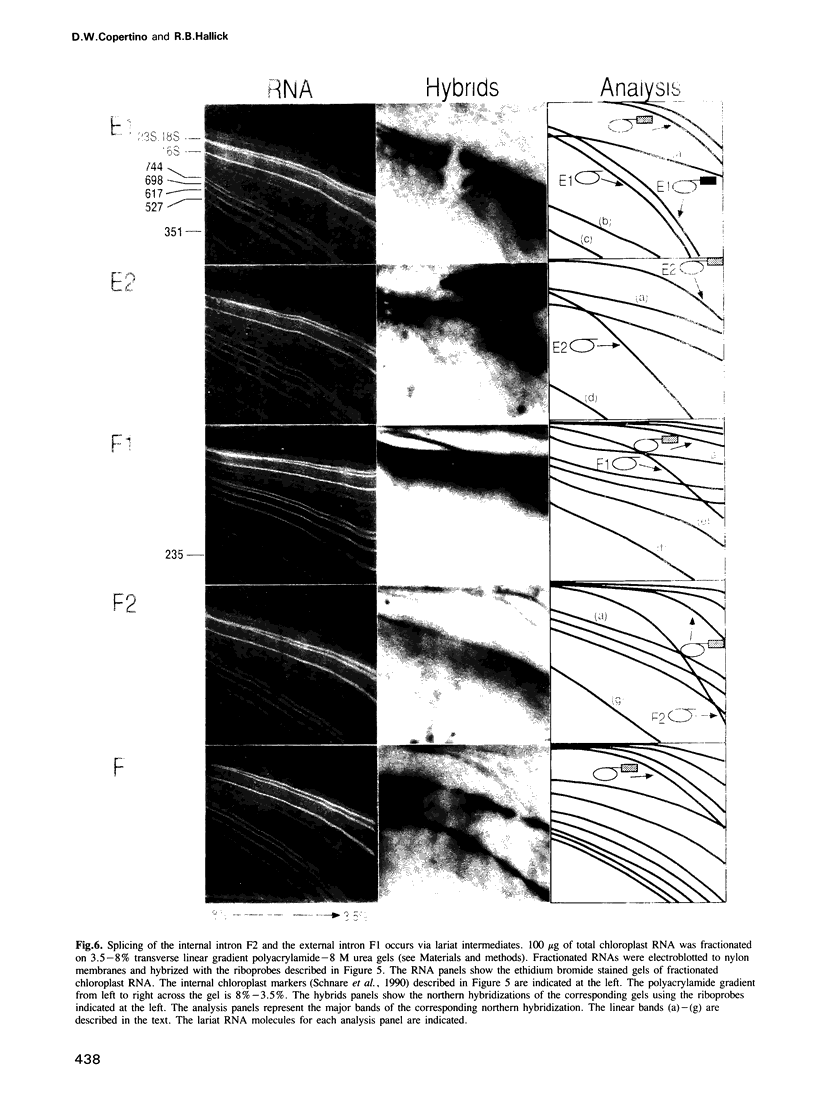
The psbF gene of chloroplast DNAs encodes the beta-subunit of cytochrome b-559 of the photosystem II reaction center. The psbF locus of Euglena gracilis chloroplast DNA has an unusual 1042 nt group II intron that appears to be formed from the insertion of one group II intron into structural domain V of a second group II intron. Using both direct primer extension cDNA sequencing and cDNA cloning and sequencing, we have determined that a 618 nt internal intron is first excised from the 1042 nt intron of psbF pre-mRNA, resulting in a partially spliced pre-mRNA containing a 424 nt group II intron with a spliced domain V. The 424 nt intron is then removed to yield the mature psbF mRNA. Therefore, the 1042 nt intron of psbF is a group II intron within another group II intron. We use the term 'twintron' to define this new type of genetic element. Intermediates in the splicing pathway were detected by northern hybridization. Splicing of both the internal and external introns occurs via lariat intermediates. Twintron splicing was found to proceed by a sequential pathway, the internal intron being removed prior to the excision of the external intron. A possible mechanism for twintron formation by intron transposition is discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augustin S., Müller M. W., Schweyen R. J. Reverse self-splicing of group II intron RNAs in vitro. Nature. 1990 Jan 25;343(6256):383–386. doi: 10.1038/343383a0. [DOI] [PubMed] [Google Scholar]
- Bass B. L., Cech T. R. Ribozyme inhibitors: deoxyguanosine and dideoxyguanosine are competitive inhibitors of self-splicing of the Tetrahymena ribosomal ribonucleic acid precursor. Biochemistry. 1986 Aug 12;25(16):4473–4477. doi: 10.1021/bi00364a001. [DOI] [PubMed] [Google Scholar]
- Boynton J. E., Gillham N. W., Harris E. H., Hosler J. P., Johnson A. M., Jones A. R., Randolph-Anderson B. L., Robertson D., Klein T. M., Shark K. B. Chloroplast transformation in Chlamydomonas with high velocity microprojectiles. Science. 1988 Jun 10;240(4858):1534–1538. doi: 10.1126/science.2897716. [DOI] [PubMed] [Google Scholar]
- Carrillo N., Seyer P., Tyagi A., Herrmann R. G. Cytochrome b-559 genes from Oenothera hookeri and Nicotiana tabacum show a remarkably high degree of conservation as compared to spinach. The enigma of cytochrome b-559: highly conserved genes and proteins but no known function. Curr Genet. 1986;10(8):619–624. doi: 10.1007/BF00418129. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Conserved sequences and structures of group I introns: building an active site for RNA catalysis--a review. Gene. 1988 Dec 20;73(2):259–271. doi: 10.1016/0378-1119(88)90492-1. [DOI] [PubMed] [Google Scholar]
- Cech T. R. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell. 1986 Jan 31;44(2):207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- Chakhmakhcheva O. G., Andreeva A. V., Buryakova A. A., Reverdatto S. V., Efimov V. A. Nucleotide sequence of the barley chloroplast psbE, psbF genes and flanking regions. Nucleic Acids Res. 1989 Apr 11;17(7):2858–2858. doi: 10.1093/nar/17.7.2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher D. A., Hallick R. B. Complex RNA maturation pathway for a chloroplast ribosomal protein operon with an internal tRNA cistron. Plant Cell. 1990 Jul;2(7):659–671. doi: 10.1105/tpc.2.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopher D. A., Hallick R. B. Euglena gracilis chloroplast ribosomal protein operon: a new chloroplast gene for ribosomal protein L5 and description of a novel organelle intron category designated group III. Nucleic Acids Res. 1989 Oct 11;17(19):7591–7608. doi: 10.1093/nar/17.19.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman J. C., Christopher D. A., Little M. C., Hallick R. B., Price C. A. Organization of the psbE, psbF, orf38, and orf42 gene loci on the Euglena gracilis chloroplast genome. Curr Genet. 1988 Feb;13(2):173–180. doi: 10.1007/BF00365652. [DOI] [PubMed] [Google Scholar]
- Cushman J. C., Hallick R. B., Price C. A. The two genes for the P700 chlorophyll a apoproteins on the Euglena gracilis chloroplast genome contain multiple introns. Curr Genet. 1988 Feb;13(2):159–171. doi: 10.1007/BF00365651. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Hallick R. B. The Euglena gracilis chloroplast ribulose-1,5-bisphosphate carboxylase gene. I. Complete DNA sequence and analysis of the nine intervening sequences. J Biol Chem. 1985 Dec 25;260(30):16156–16161. [PubMed] [Google Scholar]
- Grabowski P. J., Padgett R. A., Sharp P. A. Messenger RNA splicing in vitro: an excised intervening sequence and a potential intermediate. Cell. 1984 Jun;37(2):415–427. doi: 10.1016/0092-8674(84)90372-6. [DOI] [PubMed] [Google Scholar]
- Haley J., Bogorad L. Alternative promoters are used for genes within maize chloroplast polycistronic transcription units. Plant Cell. 1990 Apr;2(4):323–333. doi: 10.1105/tpc.2.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi M., Koike H., Inoue Y. N-terminal sequencing of low-molecular-mass components in cyanobacterial photosystem II core complex. Two components correspond to unidentified open reading frames of plant chloroplast DNA. FEBS Lett. 1989 Aug 14;253(1-2):178–182. doi: 10.1016/0014-5793(89)80954-8. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Michel F. Multiple exon-binding sites in class II self-splicing introns. Cell. 1987 Jul 3;50(1):17–29. doi: 10.1016/0092-8674(87)90658-1. [DOI] [PubMed] [Google Scholar]
- Jarrell K. A., Dietrich R. C., Perlman P. S. Group II intron domain 5 facilitates a trans-splicing reaction. Mol Cell Biol. 1988 Jun;8(6):2361–2366. doi: 10.1128/mcb.8.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. A., Anziano P. Q., Shark K., Sanford J. C., Butow R. A. Mitochondrial transformation in yeast by bombardment with microprojectiles. Science. 1988 Jun 10;240(4858):1538–1541. doi: 10.1126/science.2836954. [DOI] [PubMed] [Google Scholar]
- Koller B., Clarke J., Delius H. The structure of precursor mRNAs and of excised intron RNAs in chloroplasts of Euglena gracilis. EMBO J. 1985 Oct;4(10):2445–2450. doi: 10.1002/j.1460-2075.1985.tb03954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolosov V. L., Klezovich O. N., Abdulaev N. G., Zolosharev A. S. Fotosistema II rzhi. Nukleotidnaia posledovatelnost' genov psbE, psbF, psbL i OPC40 khloroplastnoi DNK. Bioorg Khim. 1989 Sep;15(9):1284–1286. [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Michel F., Dujon B. Conservation of RNA secondary structures in two intron families including mitochondrial-, chloroplast- and nuclear-encoded members. EMBO J. 1983;2(1):33–38. doi: 10.1002/j.1460-2075.1983.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel F., Jacquier A., Dujon B. Comparison of fungal mitochondrial introns reveals extensive homologies in RNA secondary structure. Biochimie. 1982 Oct;64(10):867–881. doi: 10.1016/s0300-9084(82)80349-0. [DOI] [PubMed] [Google Scholar]
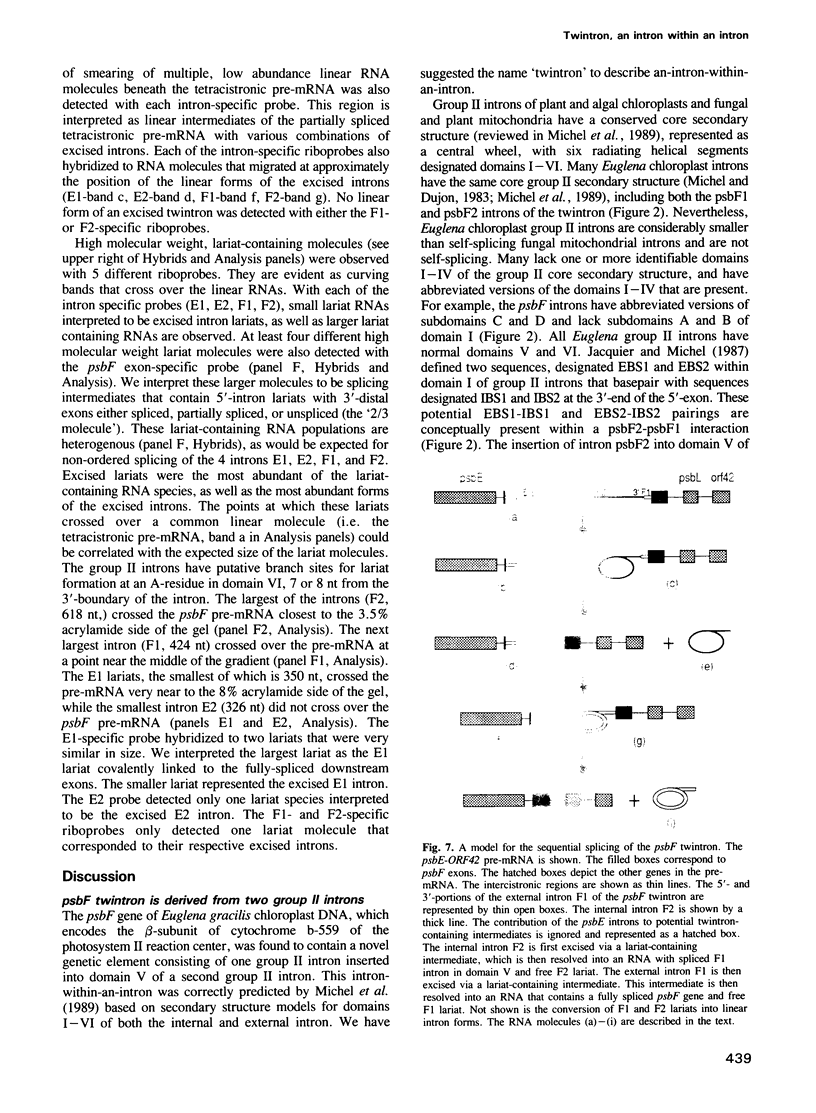
- Michel F., Umesono K., Ozeki H. Comparative and functional anatomy of group II catalytic introns--a review. Gene. 1989 Oct 15;82(1):5–30. doi: 10.1016/0378-1119(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Nanba O., Satoh K. Isolation of a photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci U S A. 1987 Jan;84(1):109–112. doi: 10.1073/pnas.84.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakrasi H. B., Williams J. G., Arntzen C. J. Targeted mutagenesis of the psbE and psbF genes blocks photosynthetic electron transport: evidence for a functional role of cytochrome b559 in photosystem II. EMBO J. 1988 Feb;7(2):325–332. doi: 10.1002/j.1460-2075.1988.tb02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peebles C. L., Perlman P. S., Mecklenburg K. L., Petrillo M. L., Tabor J. H., Jarrell K. A., Cheng H. L. A self-splicing RNA excises an intron lariat. Cell. 1986 Jan 31;44(2):213–223. doi: 10.1016/0092-8674(86)90755-5. [DOI] [PubMed] [Google Scholar]
- Schnare M. N., Gray M. W. Sixteen discrete RNA components in the cytoplasmic ribosome of Euglena gracilis. J Mol Biol. 1990 Sep 5;215(1):73–83. doi: 10.1016/S0022-2836(05)80096-8. [DOI] [PubMed] [Google Scholar]
- Shinozaki K., Ohme M., Tanaka M., Wakasugi T., Hayashida N., Matsubayashi T., Zaita N., Chunwongse J., Obokata J., Yamaguchi-Shinozaki K. The complete nucleotide sequence of the tobacco chloroplast genome: its gene organization and expression. EMBO J. 1986 Sep;5(9):2043–2049. doi: 10.1002/j.1460-2075.1986.tb04464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. W., Patton J. G., Nadal-Ginard B. Alternative splicing in the control of gene expression. Annu Rev Genet. 1989;23:527–577. doi: 10.1146/annurev.ge.23.120189.002523. [DOI] [PubMed] [Google Scholar]
- Tae G. S., Black M. T., Cramer W. A., Vallon O., Bogorad L. Thylakoid membrane protein topography: transmembrane orientation of the chloroplast cytochrome b-559 psbE gene product. Biochemistry. 1988 Dec 27;27(26):9075–9080. doi: 10.1021/bi00426a002. [DOI] [PubMed] [Google Scholar]
- Tae G. S., Cramer W. A. Lumen-side topography of the alpha-subunit of the chloroplast cytochrome b-559. FEBS Lett. 1989 Dec 18;259(1):161–164. doi: 10.1016/0014-5793(89)81518-2. [DOI] [PubMed] [Google Scholar]
- van der Veen R., Arnberg A. C., van der Horst G., Bonen L., Tabak H. F., Grivell L. A. Excised group II introns in yeast mitochondria are lariats and can be formed by self-splicing in vitro. Cell. 1986 Jan 31;44(2):225–234. doi: 10.1016/0092-8674(86)90756-7. [DOI] [PubMed] [Google Scholar]