Abstract
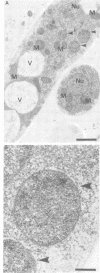
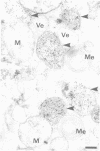
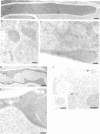
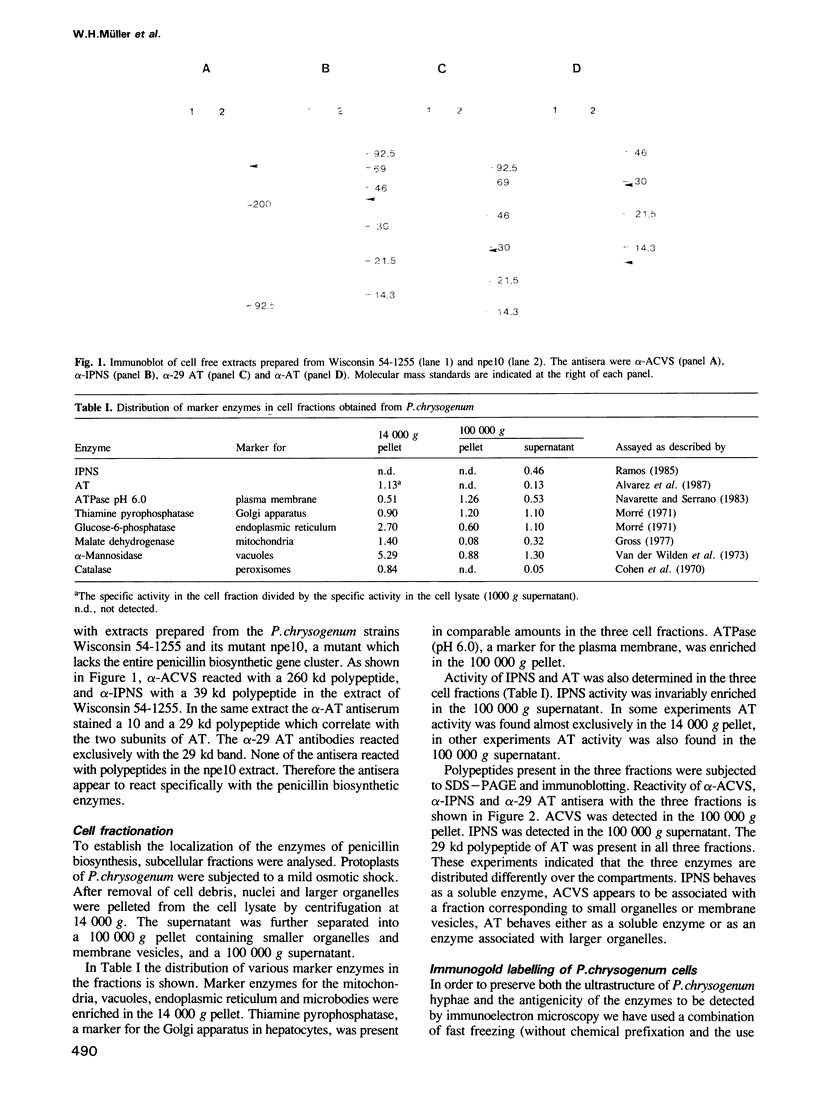
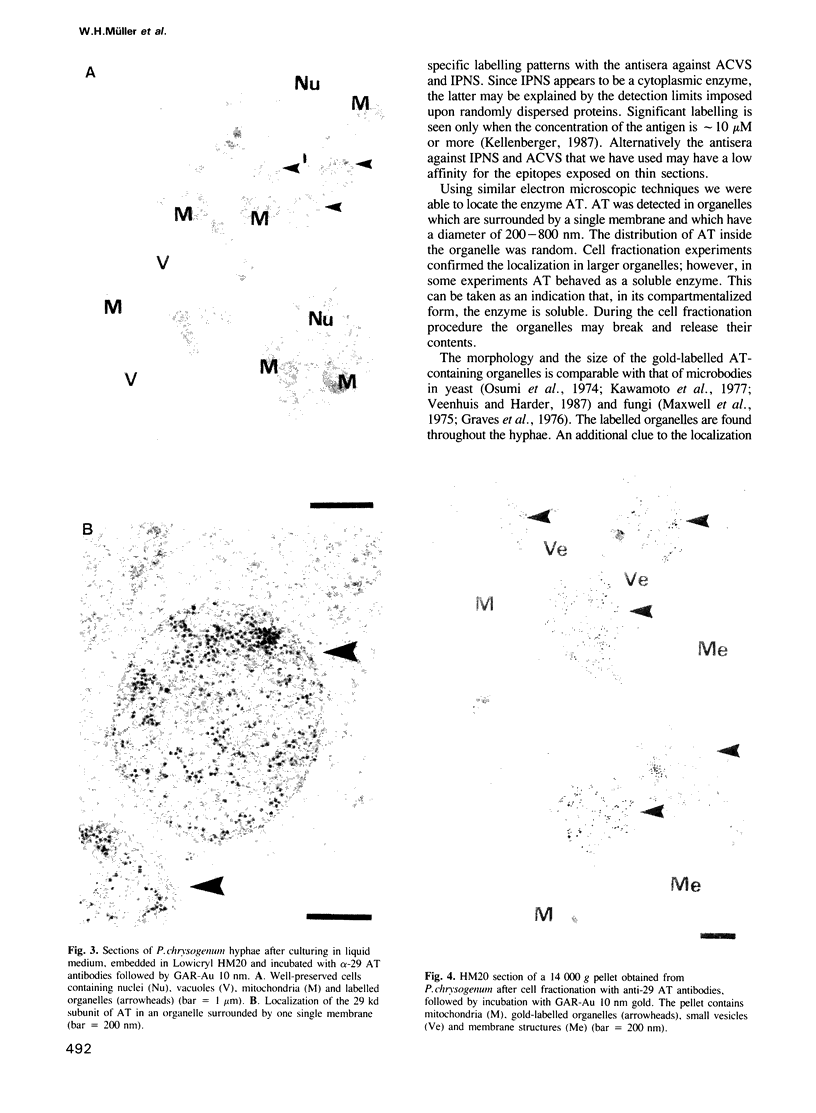
The localization of the enzymes involved in penicillin biosynthesis in Penicillium chrysogenum hyphae has been studied by immunological detection methods in combination with electron microscopy and cell fractionation. The results suggest a complicated pathway involving different intracellular locations. The enzyme delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase was found to be associated with membranes or small organelles. The next enzyme isopenicillin N-synthetase appeared to be a cytosolic enzyme. The enzyme which is involved in the last step of penicillin biosynthesis, acyltransferase, was located in organelles with a diameter of 200-800 nm. These organelles, most probably, are microbodies. A positive correlation was found between the capacity for penicillin production and the number of organelles per cell when comparing different P. chrysogenum strains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez E., Cantoral J. M., Barredo J. L., Díez B., Martín J. F. Purification to homogeneity and characterization of acyl coenzyme A:6-aminopenicillanic acid acyltransferase of Penicillium chrysogenum. Antimicrob Agents Chemother. 1987 Nov;31(11):1675–1682. doi: 10.1128/aac.31.11.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J. L., van Solingen P., Díez B., Alvarez E., Cantoral J. M., Kattevilder A., Smaal E. B., Groenen M. A., Veenstra A. E., Martín J. F. Cloning and characterization of the acyl-coenzyme A: 6-aminopenicillanic-acid-acyltransferase gene of Penicillium chrysogenum. Gene. 1989 Nov 30;83(2):291–300. doi: 10.1016/0378-1119(89)90115-7. [DOI] [PubMed] [Google Scholar]
- Brunner R., Rohr M. Phenacyl:coenzyme A ligase. Methods Enzymol. 1975;43:476–481. doi: 10.1016/0076-6879(75)43107-x. [DOI] [PubMed] [Google Scholar]
- Carr L. G., Skatrud P. L., Scheetz M. E., 2nd, Queener S. W., Ingolia T. D. Cloning and expression of the isopenicillin N synthetase gene from Penicillium chrysogenum. Gene. 1986;48(2-3):257–266. doi: 10.1016/0378-1119(86)90084-3. [DOI] [PubMed] [Google Scholar]
- Cohen G., Dembiec D., Marcus J. Measurement of catalase activity in tissue extracts. Anal Biochem. 1970 Mar;34:30–38. doi: 10.1016/0003-2697(70)90083-7. [DOI] [PubMed] [Google Scholar]
- Fawcett P., Abraham E. P. Delta-(alpha-aminoadipyl)cysteinylvaline synthetase. Methods Enzymol. 1975;43:471–473. doi: 10.1016/0076-6879(75)43105-6. [DOI] [PubMed] [Google Scholar]
- Fernández-Cañn J. M., Reglero A., Martínez-Blanco H., Luengo J. M. Uptake of phenylacetic acid by Penicillium chrysogenum Wis 54-1255: a critical regulatory point in benzylpenicillin biosynthesis. J Antibiot (Tokyo) 1989 Sep;42(9):1398–1409. doi: 10.7164/antibiotics.42.1398. [DOI] [PubMed] [Google Scholar]
- Gould S. J., Keller G. A., Hosken N., Wilkinson J., Subramani S. A conserved tripeptide sorts proteins to peroxisomes. J Cell Biol. 1989 May;108(5):1657–1664. doi: 10.1083/jcb.108.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Aist J. R. Hyphal tip cell ultrastructure of the fungus Fusarium: improved preservation by freeze-substitution. J Ultrastruct Res. 1979 Mar;66(3):224–234. doi: 10.1016/s0022-5320(79)90120-5. [DOI] [PubMed] [Google Scholar]
- Kawamoto S., Tanaka A., Yamamura M., Teranishi Y., Fukui S. Microbody of n-alkane-grown yeast. Enzyme localization in the isolated microbody. Arch Microbiol. 1977 Feb 4;112(1):1–8. doi: 10.1007/BF00446647. [DOI] [PubMed] [Google Scholar]
- Kellenberger E., Dürrenberger M., Villiger W., Carlemalm E., Wurtz M. The efficiency of immunolabel on Lowicryl sections compared to theoretical predictions. J Histochem Cytochem. 1987 Sep;35(9):959–969. doi: 10.1177/35.9.3302020. [DOI] [PubMed] [Google Scholar]
- Kuryłowicz W., Kurzatkowski W., Kurzatkowski J. Biosynthesis of benzylpenicillin by Penicillium chrysogenum and its Golgi apparatus. Arch Immunol Ther Exp (Warsz) 1987;35(5):699–724. [PubMed] [Google Scholar]
- Navarrete R., Serrano R. Solubilization of yeast plasma membranes and mitochondria by different types of non-denaturing detergents. Biochim Biophys Acta. 1983 Mar 9;728(3):403–408. doi: 10.1016/0005-2736(83)90512-6. [DOI] [PubMed] [Google Scholar]
- Nüesch J., Heim J., Treichler H. J. The biosynthesis of sulfur-containing beta-lactam antibiotics. Annu Rev Microbiol. 1987;41:51–75. doi: 10.1146/annurev.mi.41.100187.000411. [DOI] [PubMed] [Google Scholar]
- Osumi M., Miwa N., Teranishi Y., Tanaka A., Fukui S. Ultrastructure of Candida yeasts grown on n-alkanes. Appearance of microbodies and its relationship to high catalase activity. Arch Microbiol. 1974;99(3):181–201. doi: 10.1007/BF00696234. [DOI] [PubMed] [Google Scholar]
- Queener S. W. Molecular biology of penicillin and cephalosporin biosynthesis. Antimicrob Agents Chemother. 1990 Jun;34(6):943–948. doi: 10.1128/aac.34.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos F. R., López-Nieto M. J., Martín J. F. Isopenicillin N synthetase of Penicillium chrysogenum, an enzyme that converts delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1985 Mar;27(3):380–387. doi: 10.1128/aac.27.3.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada Y., Baldwin J. E., Singh P. D., Solomon N. A., Demain A. L. Cell-free cyclization of delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine to isopenicillin N. Antimicrob Agents Chemother. 1980 Sep;18(3):465–470. doi: 10.1128/aac.18.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. J., Bull J. H., Edwards J., Turner G. Amplification of the isopenicillin N synthetase gene in a strain of Penicillium chrysogenum producing high levels of penicillin. Mol Gen Genet. 1989 Apr;216(2-3):492–497. doi: 10.1007/BF00334395. [DOI] [PubMed] [Google Scholar]
- Smith D. J., Burnham M. K., Edwards J., Earl A. J., Turner G. Cloning and heterologous expression of the penicillin biosynthetic gene cluster from penicillum chrysogenum. Biotechnology (N Y) 1990 Jan;8(1):39–41. doi: 10.1038/nbt0190-39. [DOI] [PubMed] [Google Scholar]
- Weibull C., Christiansson A., Carlemalm E. Extraction of membrane lipids during fixation, dehydration and embedding of Acholeplasma laidlawii-cells for electron microscopy. J Microsc. 1983 Feb;129(Pt 2):201–207. doi: 10.1111/j.1365-2818.1983.tb04174.x. [DOI] [PubMed] [Google Scholar]
- Whiteman P. A., Abraham E. P., Baldwin J. E., Fleming M. D., Schofield C. J., Sutherland J. D., Willis A. C. Acyl coenzyme A: 6-aminopenicillanic acid acyltransferase from Penicillium chrysogenum and Aspergillus nidulans. FEBS Lett. 1990 Mar 26;262(2):342–344. doi: 10.1016/0014-5793(90)80224-7. [DOI] [PubMed] [Google Scholar]
- van Bergen en Henegouwen P. M., Leunissen J. L. Controlled growth of colloidal gold particles and implications for labelling efficiency. Histochemistry. 1986;85(1):81–87. doi: 10.1007/BF00508657. [DOI] [PubMed] [Google Scholar]
- van Liempt H., von Döhren H., Kleinkauf H. delta-(L-alpha-aminoadipyl)-L-cysteinyl-D-valine synthetase from Aspergillus nidulans. The first enzyme in penicillin biosynthesis is a multifunctional peptide synthetase. J Biol Chem. 1989 Mar 5;264(7):3680–3684. [PubMed] [Google Scholar]