Abstract
Pulmonary surfactant reduces surface tension in the lung and prevents alveolar collapse. Following a deep inspiration (DI), respiratory elastance first drops then gradually increases due to surface film and tissue viscoelasticity. In acute lung injury (ALI), this increase is faster and governed by alveolar collapse due to increased surface tension. We hypothesized that the rate of increase in elastance reflects the deficiency of surfactant in the lung. To test this, mice were ventilated before (baseline) and after saline lavage obtained by injecting 0.8 ml and withdrawing 0.7 ml fluid (severe ALI) or injecting 0.1 ml (mild ALI). After two DIs, elastance was tracked for 10 min followed by a full lavage to assess surfactant proteins B (SP-B) and C (SP-C) content. Following 2 DIs, the increases in elastance during 10 min ventilation (ΔH) were 3.60 ± 0.61, 5.35 ± 1.04, and 8.33 ± 0.84 cmH2O/ml in baseline mice and mice with mild and severe ALI, respectively (P < 0.0001). SP-B and SP-C in the lavage fluid dropped by 32.4% and 24.9% in the mild and 50.4% and 39.6% in the severe ALI, respectively. Furthermore, ΔH showed a strong negative correlation with both SP-B (r2 = 0.801) and SP-C (r2 = 0.810) content. The ΔH was, however, much smaller when the lavage fluid also contained exogeneous SP-B and SP-C. Thus ΔH can be interpreted as an organ level measure of surface film functionality in lavage-induced ALI in mice. This method could prove useful in clinical situations such as diagnosing surfactant problems, monitoring recovery from lung injury or the effectiveness of surfactant therapy.
Keywords: elastance, surfactant protein B, surfactant protein C, acute lung injury
the surface tension at the air-liquid interface of the lung contributes to lung recoil (43). Pulmonary surfactant, a complex mixture of lipids and proteins (1, 14, 32), greatly reduces the surface tension and hence prevents alveolar and small airway collapse. As a consequence, lung compliance remains high even at lower lung volumes stabilizing the lung at end expiration (5, 7, 8, 11, 43).
Acute lung injury/acute respiratory distress syndrome (ALI/ARDS) as well as respiratory distress syndrome (RDS) in preterm babies are invariably associated with surfactant deficiency, dysfunction, or inactivation of surfactant (5, 9, 17, 21, 22). Loss of surfactant function causes the lung to partially collapse with nonuniform lung aeration, reduced surface area for gas exchange, and increased risk of lung injury (57). Thus patients suffering from surfactant deficiency often require mechanical ventilation supplemented with oxygen and/or exogenous surfactant (11, 15, 18, 20, 45, 53, 57).
The first surfactant replacement therapy for human neonatal RDS was reported in 1980 (15), and the procedure has since become a standard therapy (45, 53). Mechanisms of surfactant delivery and spreading are summarized by Grotberg and Gaver (19) and Halpern et al. (23). Several clinical trials evaluated the effectiveness of surfactant replacement therapy in adult ALI/ARDS (18, 49). A single study showed that the administration of bovine surfactant in repeated large doses improved patient survival (18), whereas a large multicenter clinical study provided no evidence of benefits of using recombinant surfactant likely due to the lack of lowering surface tension in the lung (50).
Evaluation of the effectiveness of surfactant therapy in these clinical studies is based on several parameters such as mortality, the ratio of oxygen partial pressure to fraction of inspired oxygen (PaO2/FiO2), APACH score, chest X-ray, or CT imaging (49, 56). However, these parameters are not specific to surfactant function. The only direct method is to carry out an invasive partial lavage procedure followed by biophysical or biochemical analysis of the lavage fluid samples (27, 54, 55). However, even if the surface tension of the lavage sample is determined in vitro, its actual value may not be relevant to the surface tension in vivo because the lavage itself generates some injury (55). The primary goal of this study is to introduce a novel in vivo functionality assay of the surface film in the lung.
After a deep inspiration (DI), respiratory elastance in the normal lung drops immediately due to stretching of the alveolar septal walls and possibly opening collapsed airways and alveoli. The drop in elastance is then followed by a slow increase which depends on the viscoelasticity of the surface film and the connective tissue (2, 3). However, in ALI/ARDS, the increase in respiratory elastance is faster and governed by alveolar collapse and increased surface tension (2, 3). We hypothesized that the rate of increase in respiratory elastance after a DI directly reflects the functionality of the surface film in the lung. To test this hypothesis, we recorded the time course of respiratory elastance after two DIs in normal mice and in two mouse lavage models of ALI/ARDS. The changes in elastance were then correlated with surfactant protein B (SP-B) and C (SP-C) content of the surface film both of which are responsible for the low surface tension and absorption-respreading properties of surfactant at the air-liquid interface (10, 27).
MATERIALS AND METHODS
Animal preparation.
All procedures were approved by the Animal Care and Use Committee of Boston University. Male C57BL/6J mice (n = 16, body wt 27.9 ± 1.6 g, Charles River Laboratories, Boston, MA) at age 10 wk were used. The animals were anesthetized by intraperitoneal injection of pentobarbital sodium (70 mg/kg), tracheostomized and cannulated with an 18-gauge steel needle in the supine position, and placed on a heated pad to maintain constant body temperature (37°C) throughout the experiment. Extra doses of pentobarbital sodium (20 mg/kg) were administered every 20 min to keep the animal deeply anesthetized. The tracheal cannula was connected to a computer-controlled small animal ventilator (flexiVent, SCIREQ, Montreal, Quebec, Canada).
Controlled ventilation.
The animals were mechanically ventilated with room air using a controlled ventilation mode (55) at a frequency (f) of 240 breaths/min and a positive end-expiratory pressure (PEEP) of 3 cmH20. During regular mechanical ventilation, animals are ventilated by prescribing the piston displacement volume to be 8 ml/kg. However, the delivered tidal volume (VT) by the ventilator depends on the mechanical impedance of the mouse (55). Thus, in controlled ventilation mode, the volume displacement of the piston was manually adjusted on a regular basis using the delivered VT reported by the flexiVent to make sure that the delivered VT was 8 ml/kg.
Ventilation protocol.
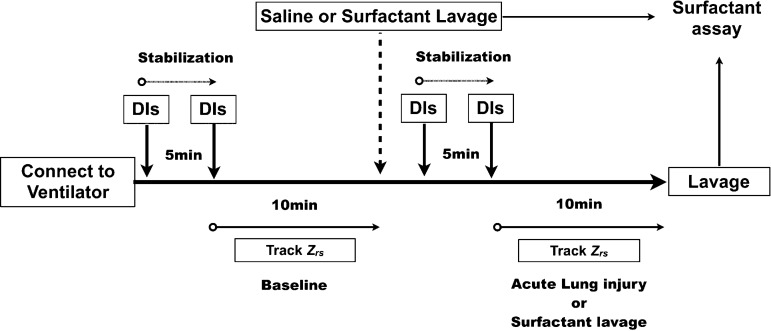
Figure 1 shows the time course of the experiment. After 5 min of stabilization, baseline measurements of respiratory input impedance (Zrs) were obtained. Next, two DIs, defined as an increase in tracheal pressure to 30 cmH20 kept for 3 s, were applied to standardize lung volume history. The Zrs was then measured for 10 min. After the baseline ventilation protocol, we divided the animals into two groups. In the first group, acute lung injury was induced by instilling 0.8 ml of warm saline (37°C) via the tracheal cannula and slowly retrieving 0.7 ml (severe ALI group, n = 4). The retrieved lavage sample represented the baseline surface film composition of the normal lung. Immediately after the first lavage, the mice were given two DIs to recruit the lung. The animals were then ventilated using the same ventilation setup as described above. After another 5 min stabilization, two DIs were administered and Zrs was tracked for 10 min. At the end, a second bronchioalveolar lavage was performed. This second lavage sample represented the composition of the surface film in the severe ALI group. In the second group, we simply injected 0.1 ml of warm saline via the tracheal cannula (mild ALI group, n = 4). These animals were also given two DIs to recruit the lung, and ventilated using the same protocol as the severe ALI group. At the end, bronchioalveolar lavage was performed to sample the surface film composition. The severe ALI procedure leaves 0.1 ml saline in the lung after the first lavage similarly to the mild ALI procedure, but it significantly dilutes the surfactant.
Fig. 1.
Time course of the protocol. Once the animal was connected to the ventilator, two deep inspirations (DIs) were immediately applied followed by a 5-min stabilization period. A second set of two DIs was then applied and respiratory impedance (Zrs) was measured during a 10-min ventilation period. Next, following a saline lavage or saline containing surfactant, the animals were connected to the ventilator and given the same stabilization and Zrs measurements as before the lavage. At the end of the protocol, another lavage sample was obtained.
To assess whether the changes in Zrs following the DIs were due to the decreased lung surfactant as a result of the lavage procedure, we carried out additional experiments using a commercial surfactant, which contains SP-B and SP-C. In this case, the protocol (Fig. 1) was repeated but the lavage fluid also contained calfactant at a manufacturer recommended dose of 3.0 ml/kg (Infasurf, Ony, Amherst, NY) which contained 35 mg/ml of phospholipids and 0.7 mg proteins including 0.26 mg/ml of SP-B and 8.1 μg protein·μmol−1·l−1 phospholipid of SP-C dissolved in 0.1 ml saline. Mice were given 0.8 ml solution orotracheally followed by slowly retrieving 0.7 ml (0.8-0.7 surf group, n = 4) or simply injected 0.1 ml (0.1 surf group, n = 4) of solution. The mice were given two DIs and then ventilated using the same ventilation setup. After stabilization, additional two DIs were administered and Zrs was tracked for 10 min. At the end, bronchioalveolar lavage was performed. After adding protease inhibitors, the lavage samples were stored at −20°C for further analysis.
Impedance measurement.
Dynamic respiratory mechanics were measured in the closed-chest condition using the forced oscillation technique combined with the optimal ventilator waveform (OVW) (40). The OVW is a composite waveform consisting of 5 sine waves (2, 5, 11, 19, and 31 Hz) with amplitudes and phase angles selected so that the waveform in the time domain looks similar to a tidal breath while allowing a smooth estimation of the impedance (29, 54, 55). During measurement, the peak-to-peak OVW amplitude was matched to the VT delivered by the ventilator in the controlled ventilation mode to minimize the interruption of lung stretch by tidal ventilation. Two cycles of the OVW were delivered while airway pressure and flow were recorded by the ventilator. The Zrs was then estimated as the ratio of the cross-power spectrum of pressure and flow and the power spectrum of flow ensemble averaged over 8 overlapping windows.
Model description and parameter estimation.
To extract mechanical parameters of the respiratory system, Zrs spectra were fitted to the constant-phase model (24) as follows. The model is composed of an airway resistance (Raw) and an airway inertance (Iaw) connected in series with a viscoelastic constant phase tissue impedance (24):
| (1) |
where j is the imaginary unit, ω is the circular frequency, ωn = ω/ω0 with ω0 = 1 rad/s, α = 2/π arctan(G/H), and G and H are the coefficients of tissue damping and elastance, respectively. For the total respiratory system, the chest wall also contributes to the resistance and model provides an estimate of the total Newtonian resistance (R). However, the contribution of the chest wall in the mouse is small and R is a good surrogate of Raw (29). Using an optimization procedure, G, H, Raw, and Iaw were estimated by minimizing the root mean square error between Eq. 1 and the measured Zrs spectra.
Total protein and western blot analysis.
The total protein in the lavage samples was determined using the BCA protein assay reagent kit (Pierce, Rockford, IL). Equal amounts of total protein or equal volume of samples were separated by SDS polyacrylamide gel electrophoresis using precasted 4-20% gradient gels (BioRad Laboratories, Hercules, CA), then transferred to polyvinylidene fluoride membrane (Millipore, Bedford, MA). Western blot analysis was performed with a polyclonal anti-rabbit SP-B antibody (1:3000, Chemicon, Temecula, CA) and polyclonal anti-rabbit SP-C antibody (1:1000, Santa Cruz, Dallas, TX, and Abcam, Cambridge, MA). The primary and secondary antibody incubation as well as the bovine serum albumin blocking step were done for 1 h. The immune complexes were detected with a chemiluminescence kit (SuperSignal West Pico, Pierce Biotechnology, Rockford, IL) and quantified by computerized optical densitometry.
Statistical analysis.
All data are presented as means ± standard deviation (SD). Different groups were tested with 1- or 2-way ANOVA, and paired t-test using Prism 6.0e (GraphPad Software, San Diego, CA). Before the ANOVA, the statistical package automatically tests for both equal variance and normality. A significant difference was defined as P < 0.05.
RESULTS
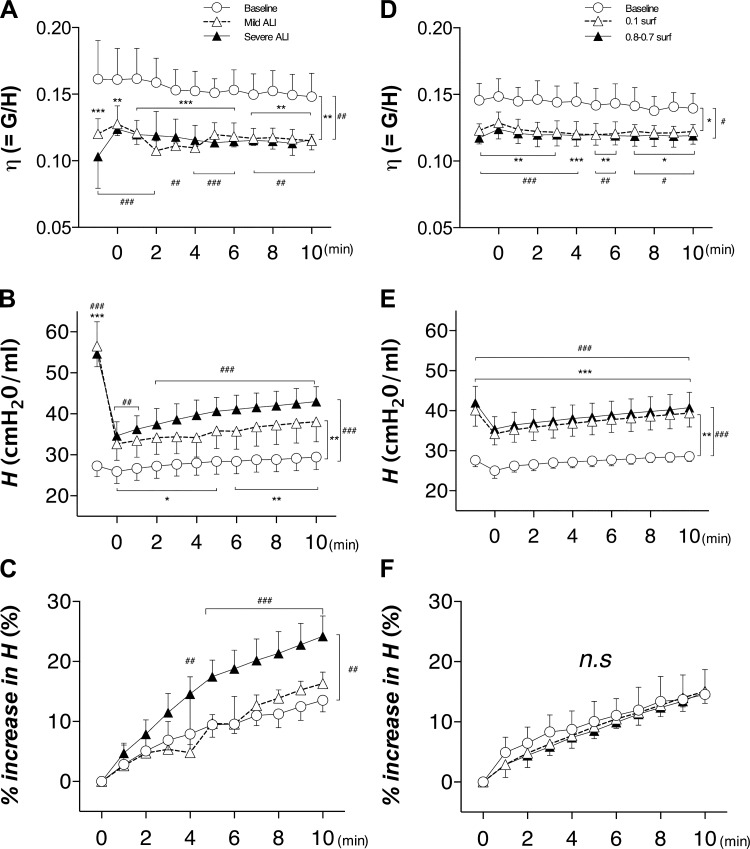
The time courses of the mechanical parameters from the constant phase model are shown in Fig. 2. Hysteresivity, defined as η = G/H, depended on the treatment group (P = 0.002) and time (P = 0.011) with significantly lower values in the mild and severe ALI groups compared with the baseline group throughout the 10-min period (Fig. 2A). In the surfactant lavage groups (Fig. 2D), η also depended on the treatment (P = 0.011) and time (P < 0.0001) and there was an interaction between time and treatment (P = 0.0013). The parameter H (Fig. 2, B and E) decreased immediately after DIs, and during the 10-min observation window, it increased steadily in all groups. The H significantly depended on both time (P < 0.0001) and treatment (P = 0.0013) with interactions (P < 0.0001) including the baseline and the mild and severe ALI groups (Fig. 2B). The H in the surfactant lavage group (Fig. 2E) also depended on time (P < 0.0001) and treatment (P = 0.0006) with a significant interaction (P < 0.0001). The % increase in H in the ALI groups (Fig. 2C) showed the strongest dependence on both time (P < 0.0001) and treatment (P = 0.0038), and there was an interaction between time and treatment (P < 0.0001), whereas % increase in H in the surfactant lavage group (Fig. 2F) depended only on time (P < 0.0001). Furthermore, the severe ALI group (Fig. 2C) showed an increase compared with both the baseline group (24.2 ± 3.4 vs. 14.3 ± 3.2%, respectively, P = 0.0023) and the mild ALI group (16.3 ± 1.9%, P = 0.01) at the end of the 10-min ventilation period. In contrast, there were no differences between baseline, 0.1 surf, and 0.8-0.7 surf groups (Fig. 2F) at any time point. These data provide evidence that lavaging the lung with surfactant does not result in a rapid increase in H following DIs.
Fig. 2.
Mechanical parameters obtained from the single compartment model of Zrs. The graphs compare the time course of hysteresivity (η = G/H; A), tissue elastance (H; B), and % increase in H (C) during 10 min of ventilation after DIs in acute lung injury (ALI) groups, whereas D, E, and F are η, H, and % increase in H, respectively, obtained in surfactant-lavaged animals. Vertical brackets denote overall group differences and horizontal brackets are post hoc differences compared with the baseline group at the same time points. Significant differences between baseline and the mild ALI or 0.1-surf groups: *P < 0.05, **P < 0.01, ***P < 0.001. Significant differences between baseline and the severe ALI or 0.8-0.7 surf groups: #P < 0.05, ##P < 0.01, ###P < 0.001.
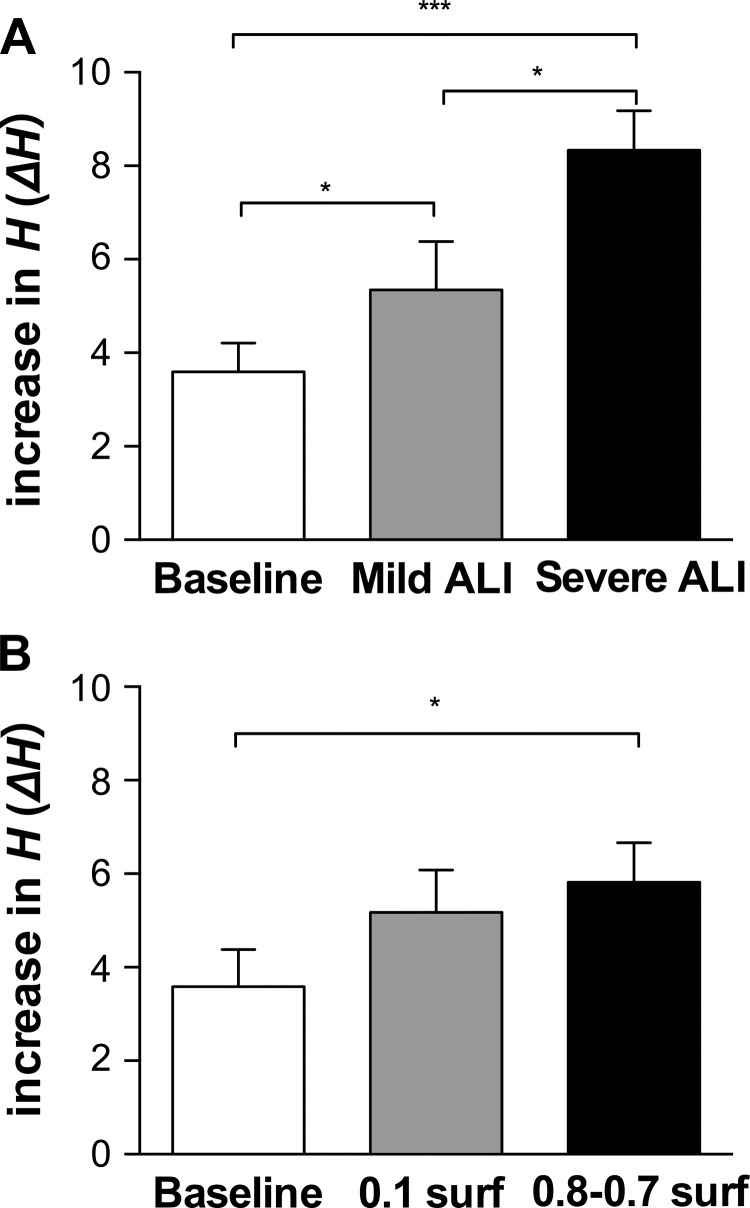
Figure 3 shows the increase in H (ΔH) from time 0 right after the DIs to the end point of 10-min ventilation (time 10). The ΔH was elevated both in the mild and severe ALI groups (5.35 ± 1.04 and 8.33 ± 0.84 cmH2O/ml, respectively) compared with baseline (3.60 ± 0.61 cmH2O/ml), and all group comparisons were significant (Fig. 3A). On the other hand, ΔH in both surfactant lavaged groups was only slightly higher (0.1 surf and 0.8-0.7 surf were 5.18 ± 0.90 and 5.82 ± 0.85 cmH2O/ml, respectively) than in baseline (3.59 ± 0.79 cmH2O/ml), and there was a significant difference only between the baseline group and the 0.8-0.7 surf group (P = 0.0118, Fig. 3B).
Fig. 3.
The increase in H (ΔH) after the two DIs from time 0 to the end of 10-min ventilation (time 10) in ALI groups (A) and in surfactant-lavaged groups (B). *P < 0.05, ***P < 0.001.
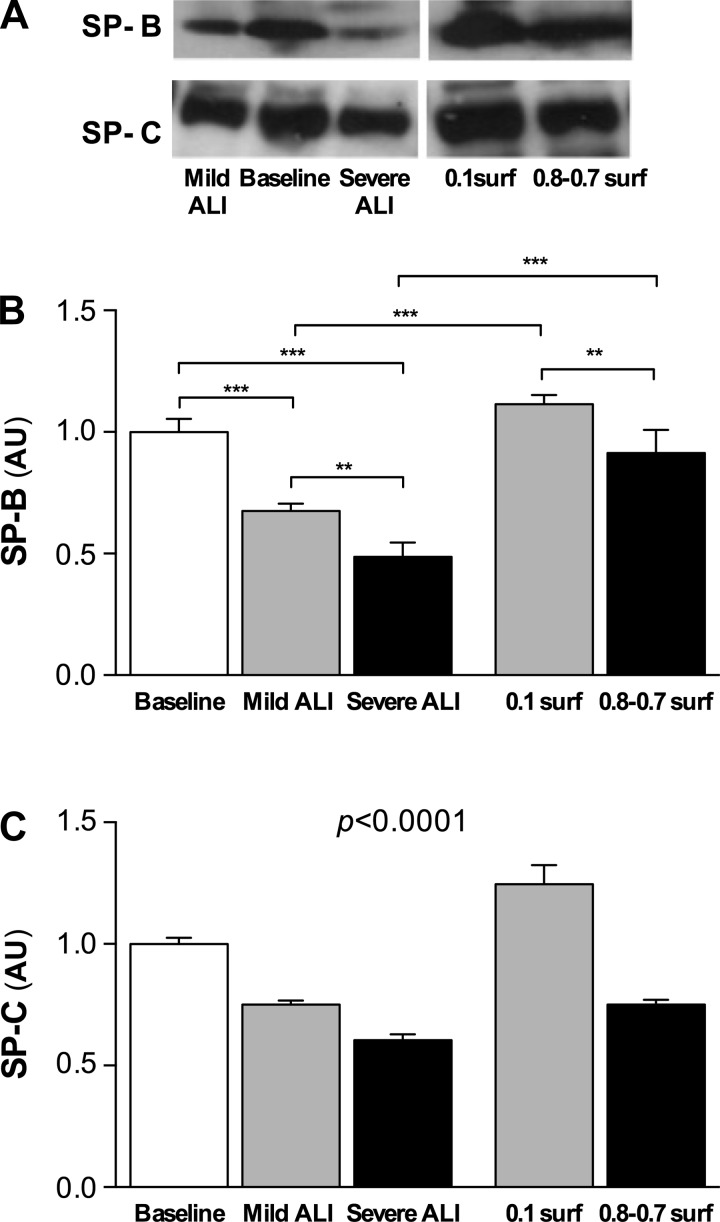
Representative Western blots for SP-B and SP-C are shown in Fig. 4A and the corresponding statistics of the protein levels normalized to the mean of the baseline group are summarized in Fig. 4, B and C. The SP-B expression (Fig. 4B) decreased significantly in both the mild (0.68 ± 0.03) and the severe ALI (0.49 ± 0.06) groups compared with baseline (P < 0.001, respectively), and SP-B was also lower in the severe ALI group than in the mild ALI group (P < 0.01). The surfactant groups had more SP-B than the corresponding lavage groups and the 0.1 surfactant group also had more SP-B than the 0.8-0.7 surfactant group. SP-C concentrations exhibited a very similar pattern (Fig. 4C): SP-C was lower in both the mild and severe ALI groups compared with baseline (0.75 ± 0.02 and 0.60 ± 0.02, P < 0.001, respectively). For SP-C, every group was different from every other group (P < 0.0001). Furthermore, there was a high correlation between SP-B and SP-C (r2 = 0.945, P < 0.0001, not shown).
Fig. 4.
A: example images of Western blots for SP-B and SP-C. Surfactant protein-B (SP-B) (B) and surfactant protein-C (SP-C) (C) levels in the lavage samples after 10 min ventilation normalized with the mean of the expression in the baseline group (n = 4 in each group). For SP-C, every group was different from every other group (P < 0.0001). **P < 0.01, ***P < 0.001.
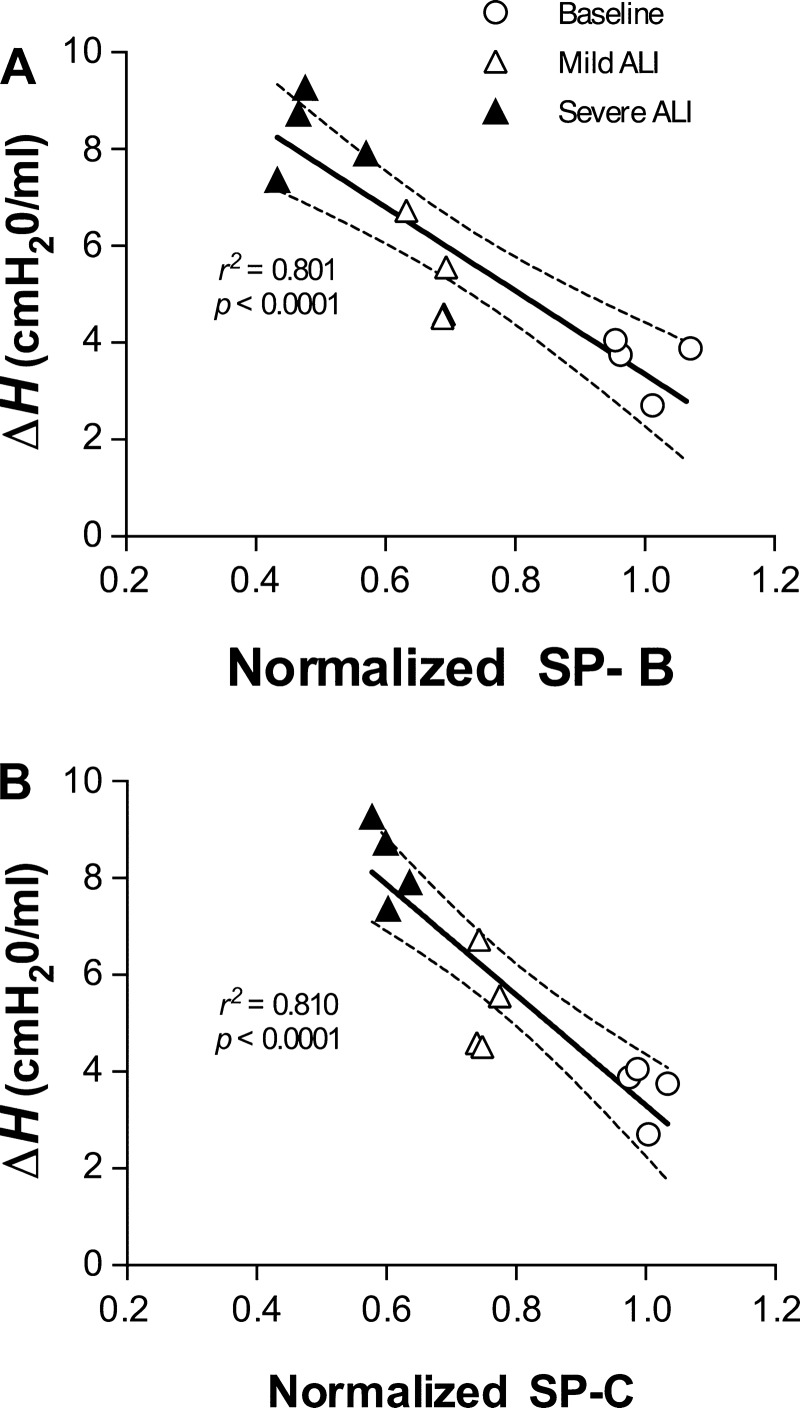
For the baseline and lavage groups, the relationship between the normalized SP-B and SP-C levels and ΔH are shown in Fig. 5. Both the SP-B and SP-C levels correlated strongly with ΔH with r2 values of 0.801 (P < 0.0001) and 0.810 (P < 0.0001), respectively.
Fig. 5.
Individual values of the changes in H (ΔH) are plotted against the corresponding normalized SP-B (A) and SP-C (B) concentration. Note the strong correlations between ΔH and normalized SP-B (r2 = 0.801, P < 0.0001) and SP-C (r2 = 0.810, P < 0.0001). The black line shows the linear regression, and the dotted lines are the 95% confidence intervals.
DISCUSSION
The purpose of this study was to determine whether the time-dependent mechanical properties of the respiratory system, specifically the elastance, following DIs are related to SP-B and SP-C levels in the surface film of the lung. The main findings of this study are as follows. 1) The changes in H after the DIs measured over a time period of 10 min were larger in mice with lavage-induced mild and severe ALI than in healthy animals. 2) Both SP-B and SP-C concentrations in the lavage samples significantly decreased in the ALI groups compared with baseline. Furthermore, both protein levels were lower in the severe than in the mild ALI group, and they exhibited a similar pattern. 3) The change in H with time (ΔH) showed a strong negative correlation with SP-B and SP-C levels, suggesting that the DI-induced increase in elastance at the whole organ level reflects the lack of proper SP-B and SP-C concentrations in the surface film. 4) When the lavage fluid also contained exogeneous SP-B and SP-C, the mechanical consequences of lavaging the lung were significantly attenuated.
It is well known that respiratory elastance after DIs or sighs immediately drops (36) due to several mechanisms including the stretching of the surface film and recruiting surfactant, the opening of airways and alveoli, as well as stretching the septal walls. Allen et al. (2) measured H after DIs before and after saline lavaging of mice and reported that the rise in H increased and the time constant τ of the recovery of H after a DI was much shorter in lavaged mice, suggesting that changes in H reflect ongoing derecruitment. In our study, τ in the severe ALI group was also smaller than in the baseline group (4.27 ± 0.75 vs. 7.08 ± 1.63 min, P < 0.05), whereas τ in the mild ALI group was intermediate (5.84 ± 1.41 min) between severe ALI and baseline groups, but the differences did not reach statistical significance. On the other hand, H after 10 min ventilation (Htime10) in the mild ALI group (38.0 ± 4.9 cmH20/min) was significantly higher than in the baseline group (29.4 ± 3.1 cmH20/min). Furthermore, when we examined ΔH following the DIs, we found that it was also different between the mild and severe ALI groups (5.35 ± 1.04 and 8.33 ± 0.84 cmH20/min, P < 0.01), suggesting that ΔH is a more sensitive index of derecruitment than H or τ.
Surfactant needs to be able to maintain a low surface tension with a rigid air-liquid interface at end-expiration while it should also enable a rapid expansion of the interface during inspiration. Surfactant proteins play a key role in both (10). While these processes likely contribute to the time course of H following the DIs, several other phenomena should also be considered. At the smallest scale, the increase of H in the baseline case may indeed reflect surface film adsorption and respreading governed by SP-B and SP-C (10). However, tissue viscoelasticity generates a similar increase in H following the DIs due to stress adaptation. The relative contributions of surface film adsorption and respreading and tissue viscoelasticity are still debated (52). The response of the ALI lungs to DIs is likely dominated by surface film effects since the lavage is not likely to alter tissue viscoelasticity during the short time scale of the experiments. The elimination or dilution of surfactant leads to slow adsorption rates of surfactant during stretching the alveolar surface, which leads to large surface stresses (16) and hence increased H. However, the time course of gradual derecruitment should contribute more to the increase in H than any change in the physicochemical properties of the surface film because the biggest effect an alveolus can have on H is when it collapses. Nevertheless, the two mechanisms are not independent since slow adsorption and increased surface tension promote collapse. While sorting out these mechanisms in vivo is difficult, our data together with the surfactant lavage results imply that surfactant proteins play a key role in maintaining surface stability and avoiding collapse.
Hysteresivity (η) is thought to be a material property of the lung tissue (13). However, it was found that airway heterogeneity of the lung can also increase η (39). Increases in η have been shown to occur with changes in the intrinsic mechanical properties of the lung tissue (13, 47), or with increased regional heterogeneity throughout the lung (30, 38, 39), both of which could generate deterioration of gas exchange and even damage to the tissue. Several animal studies reported increased η such as elastase-treated or TNFα-overexpressing mouse models of emphysema (28, 37) as well as decreased η in acute lung injury (54). In this study, η was also significantly lower in the ALI groups than in baseline (Fig. 2A). H in the ALI groups was significantly increased (Fig. 2B), while G did not change (not shown). As a result, η became significantly lower in the ALI group (Fig. 2A). This may reflect a decrease in alveolar surface film hysteresis in the presence of edema or could be a result of partitioning of total resistance to airway and tissue components (29). To test this, we measured the pressure-volume curve superimposed on PEEP in several mice (not shown) and we found that the area enclosed by these curves followed the same trend as η. These results suggest that changes in η as well as pressure-volume hysteresis in our experiments likely reflect alterations in surface film properties (44).
Our data were collected using the OVW approach which mimics normal tidal breathing during dynamic measurements because the peak-to-peak oscillatory amplitude of the OVW volume is matched to the VT of ventilation (40). The amplitude of the forced oscillations is expected to affect the estimates of the mechanical parameters (42). For example, Ito et al. (29) reported that the airway-tissue partitioning was affected by the VT. The smaller tissue resistance fraction at the larger VT is a result of the tissues being softer. Furthermore, in small animals such as mice, elastance can increase due to the fact that respiratory impedance, which is the load impedance to the ventilator, is high. The reason is that the actual delivered VT to a mouse is significantly smaller than the prescribed value (55), especially in animal models with high impedance such as in ARDS. Thus a smaller delivered VT allows more derecruitment which in turn results in high H and G (29, 55). In our study, we controlled the delivered VT by adjusting it based on the load dependence of the ventilator (55). Thus the VT was likely larger than the VT delivered in other studies which might affect the recovery curve of H. Indeed, H rapidly increased during the first minute after the DI in injured animals or at low PEEP levels in other studies (2, 37), whereas our data showed a more steady increase over the 10 min ventilation even in the severe ALI group. Furthermore, it is likely that due to progressive derecruitment, the open units of the lung received progressively larger regional VTs by the end of the 10 min ventilation period. Since η was found to decrease in mice when VT was increased from 4 to 8 ml/kg (29), this is another possible mechanism explaining the decrease in η (Fig. 2, A and D). Thus, due to the load-dependence of the ventilator, the contribution of VT to estimates of the mechanical parameters should be carefully considered when partitioning of airway and tissue properties is attempted during substantial changes in the mechanical properties of the lung.
The surfactant proteins SP-B and SP-C are hydrophobic proteins and their primary function is to promote the reduction of surface tension at the air-liquid interface together with the lipid components of surfactant (33, 58). The pathogenesis of surfactant dysfunction in ALI/ARDS is still not fully understood. Animal injury models and in vitro models of surfactant dysfunction suggest that changes in surfactant composition directly alter surface tension and contribute to lung injury (20, 25, 31, 46). Furthermore, preterm infants born with RDS have few surfactant-producing type II alveolar epithelial cells because of mature type II cells develop only late in gestation (12). In the present study, SP-B and SP-C concentration in the lavage samples (Fig. 4) decreased in both ALI groups. A previous study showed that a smaller delivered VT decreased SP-B secretion in mouse lavage model of ALI (55). Several reports also revealed that the amount and composition of surfactant are largely determined by the dynamic stretching pattern of the lung parenchyma (4, 59). One might argue that in our protocol, the DI itself triggers surfactant secretion. However, because of the delayed secretion following a big stretch (48), this is not expected to affect our data. Therefore, any intervention that reduces the SP-B concentration in the lung also deteriorates the surface tension-reducing functionality of the surface film.
Respiratory elastance is a measure of the stiffness of the respiratory system including chest wall and lung (34). Lung elastance includes components from the connective tissues and the alveolar surface film (51). When one or two consecutive DIs are delivered to the lung, most airways and alveoli open which significantly reduces H. Following the DIs, H starts to increase back to its pre-DI level and the rate of increase depends on tissue and surface film viscoelasticity. However, in ALI, changes in H following the DIs come predominantly from changes in surface tension (2, 3) and our results demonstrate that the severity of ALI increases the rate of increase in H. Furthermore, the strong inverse relation between the ΔH and the amount of SP-B (Fig. 5A) and SP-C (Fig. 5B) quantitatively links organ level mechanics with the levels of SP-B and SP-C in ALI. We also analyzed the correlation between SP-B and the ratio of H at 10 and 0 min time points (H10min/H0min) or just the value of H at 10 min (H10min). These indexes also gave good correlations (r2 = 0.6842, P = 0.009, and r2 = 0.7889, P < 0.0001, respectively). However, ΔH showed the strongest relationship with SP-B (Fig. 5A, r2 = 0.801) and SP-C (Fig. 5B, r2 = 0.810). Therefore, ΔH following DIs is a more sensitive index of derecruitment than the other indexes of H and provides a simple index that can test the overall functionality of the surface film.
When the lavage fluid contained exogeneous SP-B and SP-C, the % increase in H following the DIs was exactly the same as in baseline (Fig. 2F). This is in good agreement with the results in Fig. 4B that neither the 0.1 surf nor the 0.8-0.7 surf treatment produce different SP-B levels from baseline (Fig. 4B). Nevertheless, the results for η were nearly identical during saline and surfactant lavage (Fig. 2, A vs. D) suggesting that η is most likely determined by the 0.1 ml fluid that remains in the lung after both saline and surfactant lavages whereas the increase in H is more sensitive to the surfactant protein levels.
A limitation of this study is that there is no single animal model that can reproduce all the characteristics of ALI/ARDS in humans (41). The saline lavage model of ALI generates mild inflammation and tissue damage compared with other models (41), such as chemically or bacterially induced ALI, unless followed by a second hit (e.g., high VT mechanical ventilation). However, this model is one of the most commonly used, especially as a surfactant depletion model of ALI (6, 35). Another limitation of using ΔH following DIs as a surrogate of surface film functionality is that when applied to ALI with unknown etiology, we will not know what biophysical changes in the surface film make surface tension high in a given lung. Protein exudates from the blood, e-cadherin from compromised cell-cell interactions, and/or lack or altered composition of surfactant can all increase surface tension (26). On the other hand, a significant advantage of this assay is that it separates surface film effects from the effects of reopenings because the assay starts with an open lung after the DIs. Additionally, ΔH is an organ level measure of how fast surfactant produces closure deep in the lung. The method we propose is simple, noninvasive, and can provide immediate results as opposed to the traditional biochemical analysis which needs several hours or often a day. Additionally, the method provides a true assessment of the functionality of the surface film in the presence of injury.
In summary, we measured respiratory impedance after DIs in normal mice and in mice with lavage-induced ALI. We found that tracking the elastance for 10 min provides information about the functionality of the surface film in vivo. This method has the potential to reduce the time and cost of assessment of surface film functionality and help doctors diagnose and fine-tune therapy. Since it requires little data analysis and can be implemented on any ventilator, it is also suitable for guiding mechanical ventilation. Nevertheless, the effectiveness of the method should be evaluated in other models of ALI and in larger species with lungs more similar to those of human patients.
GRANTS
This study was supported by National Institutes of Health Grant HL-111745.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: A.T. and B.S. conception and design of research; A.T. and E.B.-S. performed experiments; A.T., E.B.-S., and A.M. analyzed data; A.T. prepared figures; A.T. and B.S. drafted manuscript; E.B.-S., A.M., and B.S. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank an anonymous reviewer for suggesting the surfactant lavage study.
REFERENCES
- 1.Abrams ME. Isolation and quantitative estimation of pulmonary surface-active lipoprotein. J Appl Physiol 21: 718–720, 1966. [DOI] [PubMed] [Google Scholar]
- 2.Allen G, Lundblad L, Parsons P, Bates JH. Transient mechanical benefits of a deep inflation in the injured mouse lung. J Appl Physiol 93: 1709–1715, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Allen GB, Pavone LA, DiRocco JD, Bates JH, Nieman GF. Pulmonary impedance and alveolar instability during injurious ventilation in rats. J Appl Physiol 99: 723–730, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 296: L574–L581, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avery ME, Mead J. Surface properties in relation to atelectasis and hyaline membrane disease. AMA J Dis Child 97: 517–523, 1959. [DOI] [PubMed] [Google Scholar]
- 6.Boehme S, Bentley AH, Hartmann EK, Chang S, Erdoes G, Prinzing A, Hagmann M, Baumgardner JE, Ullrich R, Markstaller K, David M. Influence of inspiration to expiration ratio on cyclic recruitment and derecruitment of atelectasis in a saline lavage model of acute respiratory distress syndrome. Crit Care Med 43: e65–e74, 2015. [DOI] [PubMed] [Google Scholar]
- 7.Clements JA. Surface tension of lung extracts. Proc Soc Exp Biol Med 95: 170–172, 1957. [DOI] [PubMed] [Google Scholar]
- 8.Clements JA. Surface phenomena in relation to pulmonary function. Physiologist 5: 11–28, 1962. [PubMed] [Google Scholar]
- 9.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, Pathology Cooperative Group, Albright EA, Askin FB, Baker P, Chou PM, Cool CM, Coventry SC, Cutz E, Davis MM, Dishop MK, Galambos C, Patterson K, Travis WD, Wert SE, White FV; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med 176: 1120–1128, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding J, Takamoto DY, von Nahmen A, Lipp MM, Lee KY, Waring AJ, Zasadzinski JA. Effects of lung surfactant proteins, SP-B and SP-C, and palmitic acid on monolayer stability. Biophys J 80: 2262–2272, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enhorning G. Surfactant can be supplemented before the neonate needs it. J Perinat Med 15: 479–483, 1987. [DOI] [PubMed] [Google Scholar]
- 12.Flecknoe SJ, Wallace MJ, Cock ML, Harding R, Hooper SB. Changes in alveolar epithelial cell proportions during fetal and postnatal development in sheep. Am J Physiol Lung Cell Mol Physiol 285: L664–L670, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Fredberg JJ, Stamenovic D. On the imperfect elasticity of lung tissue. J Appl Physiol 67: 2408–2419, 1989. [DOI] [PubMed] [Google Scholar]
- 14.Frosolono MF, Charms BL, Pawlowski R, Silvka S. Isolation, characterization, and surface chemistry of a surface-active fraction from dog lung. J Lipid Res 11: 439–457, 1970. [PubMed] [Google Scholar]
- 15.Fujiwara T, Maeta H, Chida S, Morita T, Watanabe Y, Abe T. Artificial surfactant therapy in hyaline-membrane disease. Lancet 1: 55–59, 1980. [DOI] [PubMed] [Google Scholar]
- 16.Ghadiali SN, Gaver DP 3rd. An investigation of pulmonary surfactant physicochemical behavior under airway reopening conditions. J Appl Physiol 88: 493–506, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Gregory TJ, Longmore WJ, Moxley MA, Whitsett JA, Reed CR, Fowler AA 3rd, Hudson LD, Maunder RJ, Crim C, Hyers TM. Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome. J Clin Invest 88: 1976–1781, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory TJ, Steinberg KP, Spragg R, Gadek JE, Hyers TM, Longmore WJ, Moxley MA, Cai GZ, Hite RD, Smith RM, Hudson LD, Crim C, Newton P, Mitchell BR, Gold AJ. Bovine surfactant therapy for patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 155: 1309–1315, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Grotberg JB, Gaver DP 3rd. A synopsis of surfactant spreading research. J Colloid Interface Sci 178: 377–378, 1996. [Google Scholar]
- 20.Günther A, Ruppert C, Schmidt R, Markart P, Grimminger F, Walmrath D, Seeger W. Surfactant alteration and replacement in acute respiratory distress syndrome. Respir Res 2: 353–364, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Günther A, Siebert C, Schmidt R, Ziegler S, Grimminger F, Yabut M, Temmesfeld B, Walmrath D, Morr H, Seeger W. Surfactant alterations in severe pneumonia, acute respiratory distress syndrome, and cardiogenic lung edema. Am J Respir Crit Care Med 153: 176–184, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Hallman M, Spragg R, Harrell JH, Moser KM, Gluck L. Evidence of lung surfactant abnormality in respiratory failure. Study of bronchoalveolar lavage phospholipids, surface activity, phospholipase activity, and plasma myoinositol. J Clin Invest 70: 673–683, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halpern D, Fujioka H, Takayama S, Grotberg JB. Liquid and surfactant delivery into pulmonary airways. Respir Physiol Neurobiol 163: 222–231, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Holm BA, Wang Z, Notter RH. Multiple mechanisms of lung surfactant inhibition. Pediatr Res 46: 85–93, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Ingenito EP, Mark L, Davison B. Effects of acute lung injury on dynamic tissue properties. J Appl Physiol 77: 2689–2697, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Ingenito EP, Mora R, Cullivan M, Marzan Y, Haley K, Mark L, Sonna LA. Decreased surfactant protein-B expression and surfactant dysfunction in a murine model of acute lung injury. Am J Respir Cell Mol Biol 25: 35–44, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effect of elastase treatment. J Appl Physiol 97: 204–212, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Ito S, Lutchen KR, Suki B. Effects of heterogeneities on the partitioning of airway and tissue properties in normal mice. J Appl Physiol 102: 859–869, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Kaczka DW, Ingenito EP, Israel E, Lutchen KR. Airway and lung tissue mechanics in asthma. Effects of albuterol. Am J Respir Crit Care Med 159: 169–178, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Kim DK, Fukuda T, Thompson BT, Cockrill B, Hales C, Bonventre JV. Bronchoalveolar lavage fluid phospholipase A2 activities are increased in human adult respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol 269: L109–L118, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Klaus MH, Clements JA, Havel RJ. Composition of surface-active material isolated from beef lung. Proc Natl Acad Sci USA 47: 1858–1859, 1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, Tashiro K, Yamamoto K, Nitta S, Ohmura S, Suzuki Y. Effects of surfactant proteins SP-B and SP-C on dynamic and static mechanics of immature lungs. J Appl Physiol 83: 1849–1856, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Lai Y, Chou H. Respiratory mechanics and maximal expiratory flow in the anesthetized mouse. J Appl Physiol 88: 939–943, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JF, Tabor B, Ikegami M, Jobe AH, Joseph M, Absolom D. Lung function and surfactant distribution in saline-lavaged sheep given instilled vs. nebulized surfactant. J Appl Physiol 74: 1256–1264, 1993. [DOI] [PubMed] [Google Scholar]
- 36.Loring SH, Ingram RH, Drazen JM. Effects of lung inflation on airway and tissue responses to aerosol histamine. J Appl Physiol 51: 806–811, 1981. [DOI] [PubMed] [Google Scholar]
- 37.Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med 171: 1363–1370, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lutchen KR, Greenstein JL, Suki B. How inhomogeneities and airway walls affect frequency dependence and separation of airway and tissue properties. J Appl Physiol 80: 1696–1707, 1996. [DOI] [PubMed] [Google Scholar]
- 39.Lutchen KR, Hantos Z, Peták F, Adamicza A, Suki B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J Appl Physiol 80: 1841–1849, 1996. [DOI] [PubMed] [Google Scholar]
- 40.Lutchen KR, Yang K, Kaczka DW, Suki B. Optimal ventilation waveforms for estimating low-frequency respiratory impedance. J Appl Physiol 75: 478–488, 1993. [DOI] [PubMed] [Google Scholar]
- 41.Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 295: L379–L399, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moriya HT, Moraes JC, Bates JH. Nonlinear and frequency-dependent mechanical behavior of the mouse respiratory system. Ann Biomed Eng 31: 318–326, 2003. [DOI] [PubMed] [Google Scholar]
- 43.Von Nergaar K. Neue Auffassungen über einen Grundbegriff der Atemmechanic. Die Retraktionskraft der Lunge, abhängig von der Oberflächenspannung in den Alveolen. Z Gesamte Exp Med 66: 373–394, 1929. [Google Scholar]
- 44.Notter RH, Wang Z, Egan EA, Holm BA. Component-specific surface and physiological activity in bovine-derived lung surfactants. Chem Phys Lipids 114: 21–34, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Polin RA, Carlo WA; Committee on Fetus and Newborn; American Academy of Pediatrics. Surfactant replacement therapy for preterm and term neonates with respiratory distress. Pediatrics 133: 156–163, 2014. [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-Capote K, Manzanares D, Haines T, Possmayer F. Reactive oxygen species inactivation of surfactant involves structural and functional alterations to surfactant proteins SP-B and SP-C. Biophys J 90: 2808–2821, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakai H, Ingenito EP, Mora R, Abbay S, Cavalcante FS, Lutchen KR, Suki B. Hysteresivity of the lung and tissue strip in the normal rat: effects of heterogeneities. J Appl Physiol 91: 737–747, 2001. [DOI] [PubMed] [Google Scholar]
- 48.Spieth PM, Carvalho AR, Pelosi P, Hoehn C, Meissner C, Kasper M, Hübler M, von Neindorff M, Dassow C, Barrenschee M, Uhlig S, Koch T, de Abreu MG. Variable tidal volumes improve lung protective ventilation strategies in experimental lung injury. Am J Respir Crit Care Med 179: 684–693, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, Witte MC, Richards GA, Rippin G, Rathgeb F, Häfner D, Taut FJ, Seeger W. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med 351: 884–892, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Spragg RG, Taut FJ, Lewis JF, Schenk P, Ruppert C, Dean N, Krell K, Karabinis A, Günther A. Recombinant surfactant protein C-based surfactant for patients with severe direct lung injury. Am J Respir Crit Care Med 183: 1055–1061, 2011. [DOI] [PubMed] [Google Scholar]
- 51.Stamenović D, Barnas GM. Effect of surface forces on oscillatory behavior of lungs. J Appl Physiol 79: 1578–1585, 1995. [DOI] [PubMed] [Google Scholar]
- 52.Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol 1: 1317–1351, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, Saugstad OD, Simeoni U, Speer CP, Vento M, Halliday HL; European Association of Perinatal Medicine. European Consensus Guidelines on the management of neonatal respiratory distress syndrome in preterm infants—2013 update. Neonatology 103: 353–368, 2013. [DOI] [PubMed] [Google Scholar]
- 54.Thammanomai A, Hamakawa H, Bartolák-Suki E, Suki B. Combined effects of ventilation mode and positive end-expiratory pressure on mechanics, gas exchange and the epithelium in mice with acute lung injury. PLoS One 8: e53934, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thammanomai A, Majumdar A, Bartolák-Suki E, Suki B. Effects of reduced tidal volume ventilation on pulmonary function in mice before and after acute lung injury. J Appl Physiol 103: 1551–1559, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Walsh BK, Daigle B, DiBlasi RM, Restrepo RD; American Association for Respiratory Care. AARC Clinical Practice Guideline Surfactant replacement therapy: 2013. Respir Care 58: 367–375, 2013. [DOI] [PubMed] [Google Scholar]
- 57.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 342: 1334–1349, 2000. [DOI] [PubMed] [Google Scholar]
- 58.Weaver TE, Conkright JJ. Function of surfactant proteins B and C. Annu Rev Physiol 63: 555–578, 2001. [DOI] [PubMed] [Google Scholar]
- 59.Wirtz HR, Dobbs LG. Calcium mobilization and exocytosis after one mechanical stretch of lung epithelial cells. Science 250: 1266–1269, 1990. [DOI] [PubMed] [Google Scholar]