Abstract
Quadriceps strength and activation deficits after anterior cruciate ligament (ACL) injury or surgery are typically evaluated at joint positions that are biomechanically advantageous to the quadriceps muscle. However, the effect of knee joint position and the associated changes in muscle length on strength and activation is currently unknown in this population. Here, we examined the effect of knee angle on quadriceps strength, activation, and electrically evoked torque in individuals with ACL reconstruction. Furthermore, we evaluated whether knee angle mediated the relationship between quadriceps weakness and functional performance after ACL reconstruction. Knee strength and activation were tested bilaterally at 90° and 45° of knee flexion in 11 subjects with ACL reconstruction using an interpolated triplet technique. The magnitude of electrically evoked torque at rest was used to quantify peripheral muscle contractile property changes, and the single-leg hop for distance test was used to evaluate functional performance. The results indicated that although quadriceps strength deficits were similar between knee angles, voluntary activation deficits were significantly higher in the reconstructed leg at 45° of knee flexion. On the contrary, the side-to-side evoked torque at rest ratio [i.e., (reconstructed/nonreconstructed) × 100] was significantly lower at 90° than at 45° of knee flexion. The association between quadriceps strength and functional performance was stronger at 45° of knee flexion. The results provide novel evidence that quadriceps activation is selectively affected at 45° of knee flexion and emphasize the importance of assessing quadriceps strength and activation at this position when feasible because it better captures activation deficits.
Keywords: muscle weakness, inhibition, twitch interpolation, central activation, rehabilitation
anterior cruciate ligament (ACL) injury is one of the most common serious sports injuries, occurring in about 100,000 individuals each year in the United States (20). A large proportion of individuals with ACL injury must undergo surgical reconstruction of their torn ACL to successfully return to sports. Quadriceps weakness is commonly observed after ACL injury and surgery, and persists even years after ACL reconstruction (52, 53). Regaining quadriceps strength after ACL reconstruction is important because a growing body of evidence indicates that quadriceps weakness is strongly associated with functional limitations and poor knee function after ACL reconstruction (21). Recent evidence also indicates that quadriceps weakness is associated with potentially dangerous biomechanics (11, 49), which has been theorized to contribute to early posttraumatic knee osteoarthritis (39, 52).
A clear understanding of the mechanisms that contribute to quadriceps weakness is critical for optimizing rehabilitation interventions after ACL injury and reconstruction. Existing evidence from people with acute ACL injury indicates that both quadriceps atrophy (i.e., reduction in muscle size) and activation failure (i.e., inability to drive the quadriceps muscle maximally during a contraction) contribute to quadriceps weakness (55). However, the factors contributing to chronic quadriceps weakness after ACL reconstruction remain elusive, particularly due to the limited research on this topic. Some scientists have implicated quadriceps activation failure as the primary cause of persistent quadriceps weakness (17, 24, 39), whereas others have attributed weakness to quadriceps atrophy (4, 29). The confluence of evidence suggests that both atrophy and activation failure contribute to quadriceps weakness immediately after surgery, but voluntary activation level recovers about 2 yr after surgery (24, 29, 38). A recent study that performed a detailed neuromuscular evaluation in individuals with chronic ACL reconstruction (2-15 yr after surgery) indicated that quadriceps weakness was primarily due to the peripheral changes and not to levels of voluntary activation or antagonistic muscle activity at 90° of knee flexion (29). Specifically, these authors reported that the magnitude of electrically evoked torque at rest, which is a surrogate measure of peripheral morphological/contractile status of the muscle (i.e., intrinsic muscle strength), was significantly lower in reconstructed legs and correlated strongly with quadriceps weakness after ACL reconstruction. However, they reported that voluntary activation levels were similar between sides and did not contribute to quadriceps weakness.
It is to be noted that quadriceps strength and activation in these studies are typically tested at longer muscle lengths (i.e., 90° of knee flexion) due to the inherent safety and biomechanical advantage of the quadriceps muscles at these positions (16, 23). However, subjects rarely load their quadriceps muscles in these knee positions during functional activities such as walking, running, or jumping. For example, the quadriceps muscles are maximally loaded between 20° to 50° of knee flexion during walking, stair climbing, and running (12, 45, 54). As a result, it is not clear whether strength and activation deficits are more profound at shorter muscle lengths, which are often used during activities of daily living. Recent evidence from individuals with ACL injury suggests that potential noncopers (i.e., people who cannot cope after an ACL injury without surgery) exhibit larger quadriceps strength deficit at knee angles ≤40° (13). There is also evidence from healthy individuals that quadriceps activation in general is lower at shorter muscle lengths compared with longer muscle lengths (7), perhaps due to reduced input from Ia afferents, or to increased strain on the ACL at extended knee positions, or both (7, 9, 16). Taken together, this evidence suggests that it is likely that individuals with ACL reconstruction would have greater deficits in strength and activation at shorter muscle lengths than at longer muscle lengths. However, this premise has not been experimentally verified. Therefore, the primary purpose of this study was to examine the effect of knee joint angle on quadriceps muscle strength and activation in individuals with ACL reconstruction. We hypothesized that the leg with ACL reconstruction would exhibit significantly greater deficits in knee extensor strength and activation at 45° than at 90° of knee flexion. We also evaluated whether or not the association between quadriceps weakness and functional performance (as measured by the single-leg hop for distance test) was significantly affected by the angle at which the strength testing was performed. We hypothesized that the relationship between knee strength and hop performance would be stronger at 45° than at 90° of knee flexion.
MATERIALS AND METHODS
Subjects.
Eleven active adults (Tegner Activity Score ≥4) with a unilateral ACL reconstruction volunteered to participate in this study. Subjects ranged in age from 19 to 47 yr. Subjects were excluded if they had surgery <6 mo previously, more than one ACL reconstruction, any additional significant knee injury or surgery, significant anterior knee pain, a history of contralateral knee injury or surgery, a recent history of lower quarter fracture or nerve injury, radiculopathy or spinal instability, uncontrolled diabetes or hypertension, or were deemed to be medically unstable. All subjects completed physical therapy regimens ranging from 3 to 6 mo after ACL reconstruction. Before participation, subjects signed a written informed consent document that was approved by the University of Michigan Human Subjects Institutional Review Board. Subjects were then briefly evaluated to rule out any conditions that would prevent them from participating in the study. After completing the physical evaluation, subjects completed the Knee Injury and Osteoarthritis Outcomes Survey (KOOS), the Lysholm Knee Questionnaire, and the Shelbourne and Trumper Anterior Knee Pain Questionnaire.
Testing procedures.
Subjects were instructed to refrain from participating in any strenuous activity for 24 h before testing. The testing procedure was initiated with a 5-min warmup on a static bicycle ergometer (Keiser, Fresno, CA). The warmup was followed by a functional performance evaluation using the single-leg hop for distance test and isometric knee strength and activation testing using an interpolated triplet technique (29, 33, 35).
Single-leg hop for distance test.
The single-leg hop for distance test was evaluated using a marker-based kinematic tracking approach (46). Before testing, retroreflective markers were placed over the hip, knee, and the ankle joint. The ankle marker positions in pixel coordinates were converted to actual distance coordinates using a calibration scale and were tracked via a high-definition motion-capture camera device using the Myovideo motion tracking software (Noraxon, Scottsdale, AZ). Subjects performed the test with their hands on their hips with instructions to hop as far as possible on the leg to be tested while landing on the same leg (34). To be considered a valid trial, the landing had to be on one leg, and under the subject's complete control. If the subject landed with an early touchdown of the contralateral leg, had a loss of balance, lifted the hands from their hips, or had additional hops after landing, the trial was repeated. Testing was performed first with the nonreconstructed leg, followed by the reconstructed leg. Three practice trials and five test trials were performed on each leg. The average of the five test trials was used to compute the side-to-side hop distance ratio (i.e., limb symmetry index) in which the average reconstructed leg hop distances were expressed as a percentage of average nonreconstructed leg hop distances (36).
Knee strength and activation testing.
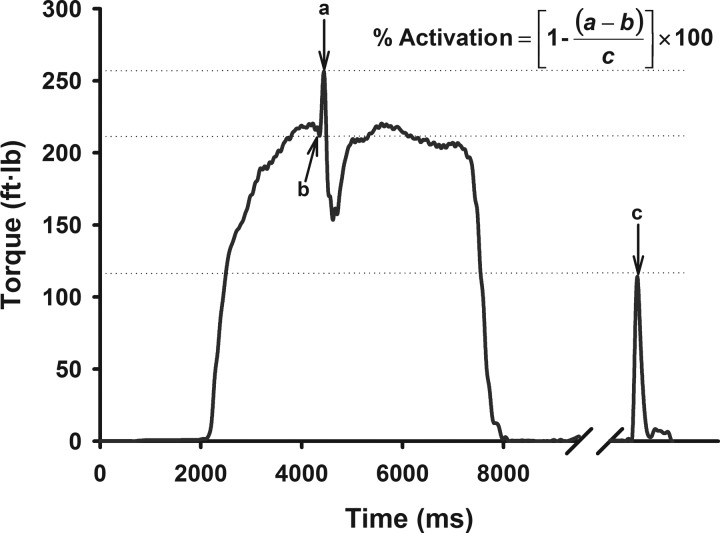
Knee extensor strength and voluntary quadriceps muscle activation were evaluated using the interpolated triplet technique (Fig. 1). In this technique, an electrical stimulus is delivered during a maximal voluntary isometric contraction (MVIC) with the goal of activating motor units that are inactive or firing submaximally (47). When incomplete activation is present, the superimposed electrical stimulus augments the force/torque generated by the muscle during the contraction [(a − b) in the equation in Fig. 1]. Conversely, when there is complete activation, there is no increase in force/torque when the electrical stimulus is introduced. The torque increment associated with the electrical stimulus during contraction (i.e., evoked torque during contraction) is then compared with the torque generated by an identical electrical stimulus delivered when the muscle is at rest (i.e., evoked torque at rest) to quantify voluntary activation. The magnitude of electrically evoked torque at rest (c in the equation in Fig. 1) can also provide information on the peripheral musculotendious adaptations (i.e., morphological and physiological changes) (18, 29, 44).
Fig. 1.
Schematic representation of the interpolated triplet technique, in which voluntary activation is quantified by comparing the torque increment associated with the electrical stimulus during contraction [i.e., evoked torque during contraction; (a − b) in the equation] to the torque generated by an identical electrical stimulus delivered when the muscle is at rest (i.e., evoked torque at rest; c in the equation).
Knee strength and activation testing was performed bilaterally on a Biodex Isokinetic Dynamometer (System 4 Pro; Biodex Medical Systems, Shirley, NY) at 45° and 90° of knee flexion. The nonreconstructed leg was tested first followed by the reconstructed leg, and the starting angle for testing was randomized such that approximately one-half of the subjects began testing at 90° of knee flexion and the other half began at 45° of knee flexion. Each subject was seated on the testing system's chair with hip at ∼90° of flexion, and tightly secured to it with thigh, hip, and torso straps. The chair was then adjusted to align the subject's lateral femoral epicondyle with the axis of rotation of the Biodex torque arm. The subject's distal shank was then firmly strapped to the Biodex torque arm pad. Before testing, the skin of the anterior thigh was cleansed with alcohol swabs and two self-adhesive electrodes (2.75 × 5.0 inches, Dura-Stick II; Chatanooga Group, Hixon, TN) were placed over the proximal and distal quadriceps muscles (28). To standardize test positions during testing, subjects were instructed to cross their hands across the chest and hold onto the chest straps during maximal contractions (42). The weight of the limb on the torque arm due to gravity was corrected before testing using a custom program written in LabView (v2011; National Instruments, Austin, TX).
Three submaximal practice trials at 50 to 75% of each subject's perceived maximum effort were performed to warm up and familiarize subjects with the testing procedures (4, 29), following which subjects performed a practice isometric knee extension contraction at their maximal effort to potentiate the quadriceps muscles (6, 29). Three mild electrical pulses were then delivered using a high-voltage, constant-current electrical stimulator (DS7AH; Digitimer, Hertfordshire, U.K.) to familiarize subjects with the electrical stimuli. Maximal current intensities were determined for each participant before testing by stimulating the quadriceps muscles at rest with pulse trains (three pulses, 100 Hz, 200-μs pulse duration, 400 V) in sequential steps of 100 mA (beginning at 100 mA) until the electrically evoked torque from the stimulated muscle contractions no longer increased, but decreased (29). The current was then reduced by 50 mA, and a final stimulus was provided. The current intensity that produced the maximal triplet-evoked torque at rest was used for testing. The magnitude of this evoked torque at rest was used to quantify peripheral musculotendinous adaptations after ACL reconstruction (29). The discomfort associated with the electrical stimulus delivered at rest was rated by subjects using an 11-point numeric rating scale (0 = none, 10 = worst imaginable).
Two minutes of rest was given before performing three test trials to evaluate maximal knee extensor strength and voluntary quadriceps muscle activation. During the test trials, the electrical stimulus at predetermined current intensity was superimposed on each subject's maximal effort to evaluate the level of voluntary drive to the quadriceps muscles (47, 51). The electrical stimulator was triggered at a predicted near-maximal volitional torque level using an automated torque-based triggered approach because this approach is known to improve stimulus timing precision in activation tests (27, 29). Loud verbal encouragement and visual feedback of a subject's torque curve were provided to facilitate maximal effort. Two minutes of rest was provided between each maximal contraction and the discomfort associated with the electrical stimulus superimposed during maximal contraction was rated by subjects using the same numeric rate scale mentioned above.
The torque signals from the Biodex dynamometer and the synchronization pulses from the electrical stimulator were analog-filtered at 100 Hz (SCXI-1143; National Instruments, Austin, TX) and sampled at 1,000 Hz using a desktop personal computer with an 18-bit high-accuracy M-series multifunction data acquisition module (USB 6281; National Instruments). The raw torque signals were converted to torque values (ft·lb) using equations obtained from a calibration cycle performed before testing. The trial that produced the highest voluntary torque during stimulation was used for further analysis. Voluntary activation was estimated using the following equation (35):
Statistical analyses.
All statistical analyses were performed using SPSS for Windows version 22.0 (SPSS, Chicago, IL). Descriptive statistics were computed for each variable to summarize demographic, torque, and activation data. A two-factor repeated-measures ANOVA with angle (45° and 90°) and leg (reconstructed and nonreconstructed) as repeated measures was performed to evaluate the effect of knee joint angle and surgery on muscle strength, voluntary activation, and evoked torque at rest. Significant interaction effects were followed by post hoc analyses using paired t-tests. Paired t-tests were also used to compare the side-to-side peak torque, evoked torque at rest, and voluntary activation ratios obtained at 45° and 90° of knee flexion. Pearson's product moment correlation coefficient was used to evaluate the association between side-to-side knee extensor torque ratio and side-to-side hop distance ratio. A significance level of α = 0.05 was established for all statistical analyses. Estimates of effect size were reported using partial η2.
RESULTS
Voluntary peak torque.
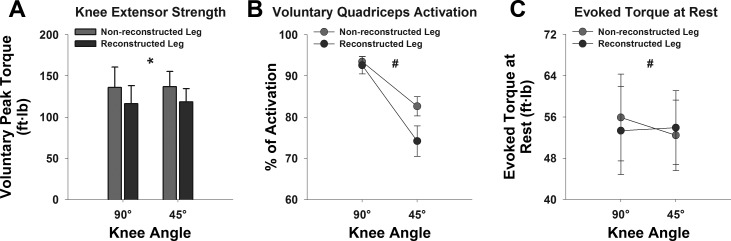
Subject demographics and clinical characteristics are provided in Tables 1 and 2. The two-factor repeated-measures ANOVA revealed a significant main effect of leg on peak knee extensor torque measurements [F(1,10) = 6.922, P = 0.025, partial η2 = 0.409, observed power = 0.660, Fig. 2A], indicating that subjects' reconstructed legs were significantly weaker compared with their nonreconstructed legs. However, there was neither a significant main effect of knee joint angle [F(1,10) = 0.025, partial η2 = 0.003, observed power = 0.052, P = 0.877] nor a leg-by-angle interaction effect [F(1,10) = 0.017, partial η2 = 0.002, observed power = 0.052, P = 0.898] on peak torque measurements.
Table 1.
Demographics and clinical characteristics of the ACL reconstructed subjects
| Subject | Age, yr | Sex | BMI | Graft Type | Meniscus Involvement | Injury to Surgery, mo | Surgery to Testing, mo | Tegner Activity Score* | Marx Activity Rating† |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | F | 20.2 | BPTB | No | 1.8 | 55.8 | 6 | 16 |
| 2 | 47 | M | 27.5 | Allograft | No | 1.3 | 22.0 | 6 | 11 |
| 3 | 30 | M | 27.1 | BPTB | No | 4.5 | 18.0 | 10 | 16 |
| 4 | 26 | F | 22.1 | STG | No | 6.2 | 14.8 | 6 | 10 |
| 5 | 19 | F | 28.3 | BPTB | No | 5.0 | 42.6 | 5 | 11 |
| 6 | 28 | M | 37.1 | BPTB | No | 1.8 | 145.8 | 6 | 12 |
| 7 | 25 | F | 34.5 | BPTB | No | 3.9 | 33.3 | 6 | 8 |
| 8 | 35 | F | 24.1 | BPTB | Yes | 8.2 | 210.4 | 4 | 10 |
| 9 | 21 | F | 20.8 | STG | Yes | 9.7 | 20.5 | 6 | 14 |
| 10 | 23 | M | 22.7 | BPTB | No | 1.6 | 10.0 | 7 | 9 |
| 11 | 25 | F | 21.7 | STG | No | 3.0 | 25.0 | 8 | 14 |
| Average | 27.2 | 26.0 | 4.3 | 54.4 | 6.4 | 11.9 |
BMI, body mass index; BPTB, bone-patellar tendon-bone graft; STG, semitendinosus-gracilis.
Out of 10.
Out of 16.
Table 2.
Self-reported ratings about their knee and associated problems
| Subjects | Lysholm Score | Knee Pain* | KOOS |
||||
|---|---|---|---|---|---|---|---|
| Pain | Symptoms | ADL | Sport | QOL | |||
| 1 | 93 | 70 | 86 | 100 | 94 | 100 | 81 |
| 2 | 89 | 79 | 81 | 75 | 84 | 55 | 69 |
| 3 | 95 | 95 | 97 | 100 | 100 | 100 | 88 |
| 4 | 100 | 95 | 100 | 93 | 99 | 100 | 94 |
| 5 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 6 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| 7 | 90 | 100 | 97 | 93 | 100 | 95 | 88 |
| 8 | 95 | 100 | 97 | 86 | 99 | 80 | 81 |
| 9 | 90 | 90 | 97 | 93 | 100 | 95 | 88 |
| 10 | 90 | 85 | 69 | 64 | 85 | 55 | 50 |
| 11 | 95 | 100 | 100 | 93 | 99 | 95 | 88 |
| Average | 94.3 | 92.2 | 93.1 | 90.6 | 96.3 | 88.6 | 84.3 |
Scores in all measures are out of 100.
Evaluated using the Shelbourne and Trumper anterior knee pain questionnaire. KOOS, Knee Injury and Osteoarthritis Outcome Survey; ADL, activities of daily living; QOL, quality of life.
Fig. 2.
Bar charts and line graphs representing peak voluntary knee extensor torque (ft·lb) (A), peak voluntary quadriceps muscle activation (B), and maximal electrically evoked torque at rest (C) in reconstructed and non-reconstructed legs at 90° and 45° of knee flexion in our sample. Error bars indicate standard error of the mean. *Significant main effect of leg at P < 0.05. #Significant interaction effect at P < 0.05.
Voluntary activation.
Repeated-measures ANOVA indicated a significant main effect of leg [F(1,10) = 6.805, partial η2 = 0.405, observed power = 0.653, P = 0.026] and of angle [F(1,10) = 25.238, partial η2 = 0.716, observed power = 0.994, P = 0.001] as well as a leg-by-angle interaction effect on voluntary activation measurements [F(1,10) = 6.766, partial η2 = 0.404, observed power = 0.651, P = 0.026, Fig. 2B]. The significant leg-by-angle interaction was due to greater between-leg differences (nonreconstructed leg − reconstructed leg) in voluntary activation at 45° than at 90° of knee flexion (8.4 ± 3.0% vs. 0.9 ± 1.4%, P = 0.03). Specifically, voluntary activation was similar between sides at 90° of knee flexion, but it was substantially lower on the reconstructed leg at 45° of knee flexion (Fig. 2B). These differences were not due to differences in current intensities used or subject discomfort levels, because they were similar between sides and knee angles (Table 3).
Table 3.
Current intensities used in voluntary activation testing and self-reported discomfort* associated with electrical stimulus at rest and during contraction
| Reconstructed Leg |
Nonreconstructed Leg |
|||||
|---|---|---|---|---|---|---|
| Angle | Mean ± SD | Median | Range | Mean ± SD | Median | Range |
| 45° | 268.2 ± 56.0 | 250 | 200–400 | 281.8 ± 56.0 | 300 | 200–400 |
| 90° | 281.8 ± 46.2 | 250 | 250–400 | 281.8 ± 51.3 | 300 | 200–400 |
| Discomfort | ||||||
| Rest | 6.5 ± 2.8 | 8.0 | 1.0–9.0 | 6.3 ± 2.9 | 7.0 | 1.0–9.0 |
| Contraction | 3.7 ± 2.3 | 3.0 | 1.0–8.0 | 3.8 ± 2.2 | 4.0 | 1.0–8.0 |
All values are in mA.
On a scale of 0 = none to 10 = worst imaginable.
Electrically evoked torque at rest.
There was no significant main effect of leg [F(1,10) = 0.187, partial η2 = 0.018, observed power = 0.068, P = 0.674] or of angle [F(1,10) = 0.621, partial η2 = 0.058, observed power = 0.110, P = 0.449] on evoked torque at rest. However, there was a significant leg-by-angle interaction effect on evoked torque at rest [F(1,10) = 8.881, partial η2 = 0.470, observed power = 0.766, P = 0.014, Fig. 2C]. The leg-by-angle interaction effect for evoked torque measurements at rest was due to the observation of greater between-leg differences (nonreconstructed leg − reconstructed leg) in evoked torque values at 90° than at 45° of knee flexion (2.5 ± 1.6 ft·lb vs. −1.5 ± 1.2 ft·lb, P = 0.01). Specifically, evoked torque at rest was lower in the reconstructed leg at 90° of knee flexion, but was higher in the reconstructed leg at 45° of knee flexion (Fig. 2C).
Side-to-side ratios.
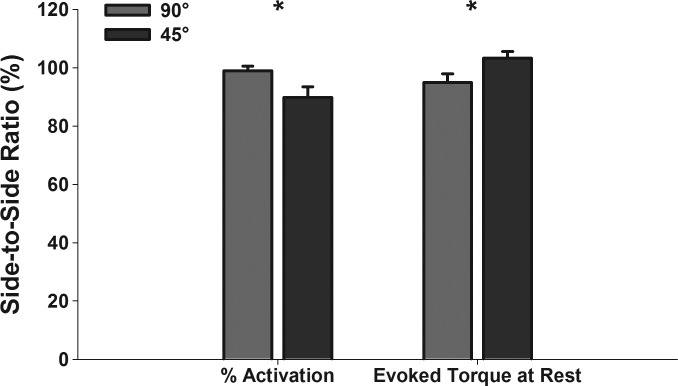
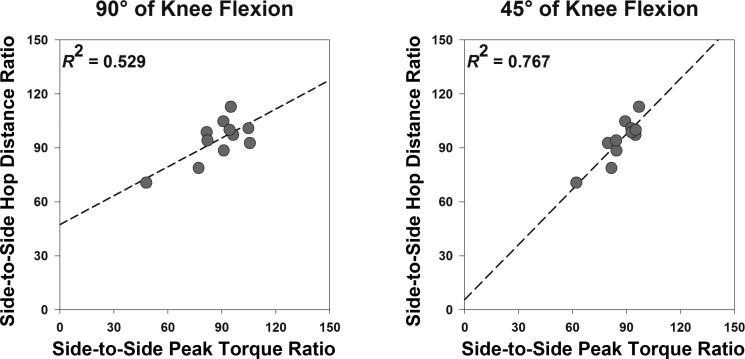
Paired t-tests revealed significant differences between side-to-side evoked torque at rest ratio and side-to-side voluntary activation ratio observed at 45° and 90° of knee flexion [t(10) = 2.968, P = 0.014 and t(10) = −2.520, P = 0.030, Fig. 3]. However, there were no differences between side-to-side voluntary peak torque ratios (i.e., quadriceps weakness) observed at 45° and 90° of knee flexion [86.8 ± 3.2% vs. 87.7 ± 4.8%; t(10) = −0.251, P = 0.807]. Pearson's correlation tests indicated significant associations between side-to-side peak torque ratios and side-to-side hop distance ratios at both angles (P < 0.05), but the association was stronger at 45° of knee flexion (r = 0.73 vs. r = 0.88, Fig. 4).
Fig. 3.
Bar graphs representing the mean side-to-side ratio (reconstructed/nonreconstructed leg × 100) of voluntary quadriceps muscle activation and electrically evoked torque at rest at 90° and 45° of knee flexion in our sample. Error bars indicate standard error of the mean. *Significant differences at P < 0.05.
Fig. 4.
Scatterplots demonstrating the relationship between side-to-side peak torque ratio and side-to-side hop distance ratio at 90° (A) and 45° (B) of knee flexion. Dashed lines indicate regression line. Note that the relationship between side-to-side peak torque ratio and side-to-side hop distance ratio was stronger at 45° of knee flexion, which was primarily due to the observation of lower variability between subjects in side-to-side peak torque ratios at 45° (SD = 10%) than at 90° (SD = 16%) of knee flexion.
DISCUSSION
In the current study, the effect of knee joint angle on quadriceps strength and activation was evaluated in individuals with ACL reconstruction. Contrary to our hypothesis, we found that the magnitude of strength deficits were similar between 45° and 90° of knee flexion. However, as hypothesized, the between-leg differences in voluntary activation varied depending on the knee joint angle used for testing. Specifically, voluntary activation of the reconstructed leg was lower than that of the nonreconstructed leg at 45°, but not at 90° of knee flexion. In general, the reduction in voluntary activation due to changes in knee joint angle was more pronounced on the reconstructed leg compared with the nonreconstructed leg. This finding was consistent in the sample studied; only one subject had an activation profile that deviated from this pattern. Another interesting finding was that the between-leg differences in evoked torque at rest, which is a measure of peripheral muscular changes (i.e., mechanical contractile status), was significantly greater at 90° than at 45° of knee flexion. Collectively, these findings suggest that the extent to which peripheral and central factors contributed to the observed muscle weakness varied depending on the angle at which testing was performed. Specifically, quadriceps weakness appeared to be primarily mediated by peripheral changes in the quadriceps muscle at 90° of knee flexion, whereas weakness appeared to be more related to the levels of voluntary activation at 45° of knee flexion. Finally, there was a significant association between quadriceps strength and single-leg hop performance, and this relationship was stronger at 45° than at 90° of knee flexion, indicating the functional relevance of strength measurements performed at more extended knee angles.
Quadriceps weakness is ubiquitous after ACL injury, and reconstruction and has been linked to the pathogenesis of osteoarthritis (39, 52). Despite substantial rehabilitation efforts, quadriceps weakness is known to persist for years after surgery (40). Similar to other studies, we found about 12–13% strength deficit on the reconstructed leg compared with the nonreconstructed leg (10, 52). However, contrary to our expectation, the magnitude of the observed quadriceps weakness was not significantly different between 90° and 45° of knee flexion. It is important to note, though, that the between-subjects variability in side-to-side peak torque ratios was lower at 45° than 90° of knee flexion (10% vs. 16%), indicating that the sensitivity/power to detect strength deficits would be greater at shorter muscle lengths than at longer muscle lengths. Moreover, some of the subjects who appeared to be stronger on their involved legs at 90° of knee flexion were indeed weaker on their involved legs at 45° of knee flexion. Furthermore, as mentioned earlier, the association between quadriceps weakness and single-leg hop performance was stronger at 45° than at 90° of knee flexion. These findings indicate that although either of these angles can be used to adequately capture strength deficits after ACL reconstruction, the measurement of quadriceps strength at shorter muscle lengths appears to be clinically more meaningful.
The contribution of voluntary muscle activation deficits to chronic quadriceps weakness is a highly debated topic and remains elusive (17, 25, 29, 39). Although some scientists have implicated quadriceps activation failure as the primary source of lingering quadriceps weakness after ACL reconstruction, others have attributed weakness to quadriceps atrophy that develops rapidly after the injury/surgery. Unfortunately, not many studies have evaluated the mechanisms of quadriceps muscle weakness in individuals with chronic ACL reconstruction. The results of a recent study that performed a detailed neuromuscular evaluation in individuals with chronic ACL reconstruction indicated that quadriceps weakness was primarily due to the peripheral changes and not due to levels of voluntary activation (29). However, their conclusions were based on testing at 90° of knee flexion, which may be an optimal position for evaluating strength deficits, but may not be ideal for quantifying activation deficits because the magnitude of these deficits vary as a function of knee angle and are maximal at shorter muscle lengths (7). As a result, it may not be possible to generalize the findings observed at 90° of knee flexion to other joint angles. This study fills this important gap and provides further insights into the mechanisms mediating chronic quadriceps weakness. Specifically, our findings provide novel and important evidence indicating that quadriceps activation failure is much more evident at shorter muscle lengths than at longer muscle lengths and contributes to strength deficits at 45° of knee flexion. This finding has important clinical and research implications. For example, rehabilitation programs after ACL injury and surgery should focus more on strategies that mitigate neural activation deficits (e.g., eccentric training, electrical stimulation, etc.) particularly at shorter muscle lengths. From a research point of view, evaluating quadriceps activation deficit at shorter muscle lengths (<45° of knee flexion) in addition to the conventional way of measuring it at longer muscle lengths (60° or 90° of knee flexion) is critical to adequately capture the effects of an injury or an intervention on quadriceps dysfunction.
The results of the study suggest that factors contributing to chronic quadriceps weakness may differ on the basis of the angle at which the testing is performed. Although evoked torque at rest was lower on the reconstructed legs, there were no differences in voluntary quadriceps muscle activation between legs at 90° of knee flexion. This suggests that chronic quadriceps weakness is related to peripheral changes in the quadriceps muscle and not to levels of voluntary activation at longer muscle lengths, which is consistent with the results of our previous study (29). However, voluntary quadriceps activation was profoundly affected on the reconstructed legs at 45° of knee flexion, which suggests that activation deficits may be the primary contributing factor for the observed quadriceps weakness at shorter muscle lengths.
The precise mechanisms for selective inhibition of the quadriceps muscles at shorter muscle lengths are unclear. However, the observed activation deficits at 45° of knee flexion suggest a reduction in Ia afferent input to the α-motor neuron pool of the quadriceps muscles, which may be mediated by a decrease in γ-efferent activity after ACL injury (7, 14). This is less of a problem at 90° of knee flexion because Ia afferents are already at heightened activity due to the elongated position of the muscles and require less input from γ-motor neurons (19, 26). Reduced activation at 45° of knee flexion could also be due to a maladaptive protective mechanism initiated early after the injury to minimize anterior shear forces induced by the quadriceps contractions at extended knee positions (16). Alternatively, selective inhibition of the affected quadriceps muscle at shorter muscle lengths may be indicative of neural excitability alterations at the cortical or spinal level (i.e., a central phenomenon) (32, 40, 41).
The peripheral muscular changes reflected by the evoked torque at rest at 90° of knee flexion may include chronic atrophy, changes in compliance of the series-elastic components of the muscle-tendon units, and alterations in the architectural structure and composition of the quadriceps muscle (2, 4, 55). However, given the profound atrophy that rapidly follows ACL injury and reconstruction, the reduction in side-to-side evoked torque at rest at 90° of knee flexion is most likely indicative of chronic quadriceps atrophy. It is important to note, however, that many of the subjects had greater evoked torque at rest in the reconstructed leg than in their nonreconstructed leg at 45° of knee flexion and the side-to-side evoked torque at rest ratios were significantly greater at 45° than at 90° of knee flexion. These findings suggest the possibility of angle-specific morphological adaptations similar to those observed after high-intensity resistance training (37) or a shift in torque-angle relation (i.e., shift of optimum length for peak tension) toward shorter muscle length, which would be advantageous to the quadriceps muscle to produce relatively greater torque (both electrically evoked and volitional) at extended knee positions. We note that although the precise mechanism for the observed differences in contractile property changes between knee angles is unclear, it does suggest that the alterations in contractile properties of the quadriceps muscle after an ACL injury/reconstruction appears to be more complex than previously thought.
The observed differences in contractile property changes between knee angles could also explain why knee extensor torque deficits were comparable between knee angles even though voluntary activation deficits were larger at 45° of knee flexion. The net volitional torque generated by a muscle is dependent on both central (voluntary activation) and peripheral (morphological/biomechanical) factors. Therefore, a reduction in force-generating capacity of the skeletal muscle would most likely happen only under the following circumstances: 1) if one or both factors were negatively affected (i.e., side-to-side ratios <100%) or 2) if the negatively affected factor was disproportionately larger compared with the other that was positively affected (i.e., side-to-side ratios >100%), a scenario that occurs when the direction of changes were different between the two factors. The results of our study show that the knee angle-mediated changes in peripheral contractile properties were in the opposite direction to voluntary activation changes, and the extent of those changes were relatively similar between the two factors (see Fig. 3). Thus any effect of reduced voluntary activation on quadriceps peak torque could have been nullified by the observed peripheral muscular changes, which could explain the observation of similar relative torque levels between knee angles despite lower activation at 45° of knee flexion. We recognize that the proposed explanation is simply speculation and that further research is required to fully understand the precise mechanisms underlying this observation.
The findings of this study have potential clinical implications. As stated above, we found that knee extensor weakness was similar between knee angles despite greater deficits in quadriceps voluntary activation at 45° of knee flexion. This suggest that either angle could be used for knee strength testing if a clinician or researcher is interested only in the magnitude of strength deficits after ACL injury or surgery. It is important to note that although the sensitivity of quadriceps strength testing appears to be slightly better at 45° of knee flexion, testing at 90° may still be preferable at times when excessive shear stress on the graft tissue is a concern (e.g., acutely after ACL reconstruction). On the contrary, if information about quadriceps activation failure is crucial, then testing at 45° of knee flexion is preferable because this angle appears to better capture activation deficits. In our opinion, if the time associated with strength and activation testing is not limited, testing at both shorter and longer muscle lengths would be ideal because it would provide a complete picture of a subject's neuromuscular profile. From a rehabilitation perspective, the presence of activation deficits at shorter muscle lengths even years after surgery suggests that great room exists to improve quadriceps strength at functional knee positions, and that clinicians should consider initiating aggressive treatment strategies early on to minimize chronic activation failure after ACL surgery. This is particularly important considering that quadriceps weakness and activation deficits have been theorized in the pathogenesis of posttraumatic osteoarthritis (39, 40, 52).
There are some limitations to this study. First and foremost, the small sample size of this study is a clear limitation; however, the observed results were robust and consistent among subjects irrespective of the chronicity of the ACL surgery, the presence or absence of concomitant meniscal injury, and the graft type. Second, the lack of a control group limited our ability to identify whether voluntary activation deficits observed on the nonreconstructed leg at 45° of knee flexion is normal or pathological. However, when comparing the results with those of previous publications (5, 7, 31), it is clear that the extent of activation failure observed on the nonreconstructed leg in our sample is comparable to those reported in a population of healthy, uninjured individuals, indicating that bilateral activation deficits that are typically observed early after injury and surgery recover over time. Third, the order in which the testing was performed could have affected the results. We tested the reconstructed leg after testing the nonreconstructed leg because this is a common practice in ACL research (13, 48, 52). It is possible that subjects could have developed some amount of central fatigue in the reconstructed leg because it was always preceded by maximal contractions of the nonconstructed leg. However, if that is the case, then the likelihood of this observation should in theory remain the same between 90° and 45° of knee flexion because we randomized the order of starting angle for testing. Hence, any differences observed between knee positions are less likely due to the order of testing. Finally, the number of maximal voluntary isometric contractions (MVICs) performed may not have been sufficient to capture the peak voluntary torque. The number of MVICs on each leg was limited to three per angle because we wanted to minimize fatigue and discomfort associated with electrical stimulus. Although it is typical that most studies restrict the number of MVICs to three trials, it is not clear whether provision of additional trials would have improved the level of voluntary activation on the reconstructed legs. In our opinion, this is unlikely because the peak torque analysis indicated that the between-trial variability was small and peak torque values reached a plateau between the second and third trials.
Although the techniques used for quantifying voluntary activation and peripheral contractile properties are well established in the literature, the choice of electrical stimulation duration and technique used for quantifying voluntary activation are also worth discussing. We chose to use a short-duration electrical stimulation (30 ms) as opposed to long-duration electrical stimulation (100 or 500 ms) for several reasons. Although some scientists favor the use of long-duration electric stimulation because the evoked force increments are larger and more readily detected (1, 22), many studies have shown that the number of electrical stimuli (i.e., the duration of electrical stimulation) has a negligible effect on the estimates of voluntary activation (3, 5, 8, 47). This is because a very strong linear association exists between torques obtained from the short- and long-duration pulse trains (Fig. 5A) and therefore, a shorter-duration train will have the same effect on the superimposed and resting torque. Because the superimposed torque is normalized to the resting torque during the % of activation calculation, any effects of stimulus duration on absolute torque is nullified when calculating voluntary muscle activation. It is to be noted that we compared only the responses to opposite side or between knee angles, and the evoked twitches were normalized to the resting twitches evoked with the same stimulus parameters. Therefore, our results would not have been any different if we had used a longer train. Moreover, short-duration electrical stimulation is more comfortable and tolerable to subjects compared with long-duration electrical stimulation. For example, a recent study by Grindstaff and Threlkeld (15) indicates that the discomfort associated with a doublet (two-pulse train) is 50% lower than a 10-pulse train of electrical stimuli. Furthermore, the use of long-duration electrical stimulation could potentially raise a safety concern for individuals with ACL reconstruction because prior studies have reported cases of patella fracture or ACL rupture with high-intensity electrical stimulation/exercise contractions (43, 50). Finally, the use of long-duration currents could potentially yield less valid results due to contamination of electrically evoked torque (both at rest and during contraction) by volitional and reflex responses. Because the length of the tetanic torque elicited by long-duration electric currents are much longer, there is more time for reflex and volitional responses to contaminate the torque signals during the rising phase of the evoked torque.
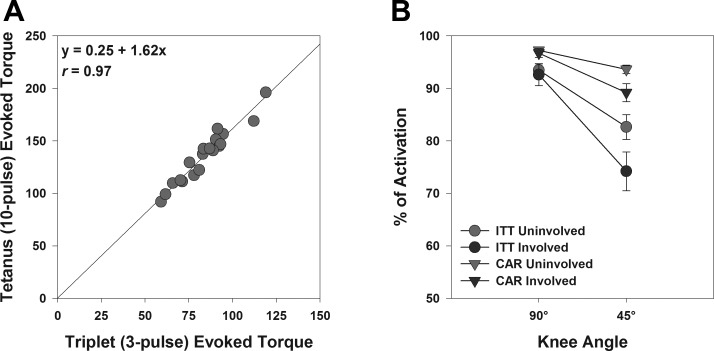
Fig. 5.
A: scatterplot demonstrating the relationship between electrically evoked torques obtained using short- and long-duration currents in 19 subjects. B: line chart representing peak voluntary quadriceps muscle activation estimates of reconstructed and nonreconstructed legs at 90° and 45° of knee flexion derived using two different quantification techniques (ITT, interpolated twitch technique; CAR, central activation ratio). Note that the CAR technique overestimates voluntary activation compared with ITT, but it yields similar trends to those observed in ITT.
In our study, we used an interpolated twitch technique (ITT) that was originally proposed by Merton (35), in which activation is quantified by scaling the superimposed torque increments during contraction to control responses evoked in relaxed muscles. Alternatively, some scientists have proposed the use of central activation ratio (CAR), in which activation is quantified by expressing MVIC torque as a percentage of total torque produced during the superimposed response (22). The advantage of CAR technique is that it is more tolerable because the electrical stimulus is provided only during maximal contraction and not during rest (Table 3). However, because of the lack of control twitches during rest, it becomes imperative to use long-duration electrical stimulation, which has potential problems as mentioned above. Furthermore, it is well known that the CAR technique overestimates activation failure compared with ITT (5, 15, 30, 47) because of the inherent false assumption that the combination of the superimposed train and the MVIC will evoke the muscle's true maximum force (30, 47). However, we have previously shown that that there is a strong correlation between CAR- and ITT-based activation estimates (30) and therefore, using CAR would not have changed our conclusions. Indeed, when we reanalyzed the data using CAR, we found similar results [significant effect of angle (P = 0.001), leg (P = 0.012), and angle × leg interaction (P = 0.011); Fig. 5B], suggesting that the choice of quantification technique did not affect our results.
In conclusion, the results of this study provide evidence for the first time that voluntary quadriceps activation in individuals with ACL reconstruction is selectively affected at 45° of knee flexion. This finding suggests that the observation of lack of voluntary activation deficits in many of the earlier studies might have been partially due to the use of longer muscle lengths (i.e., 60° to 90° of knee flexion) for testing quadriceps strength and activation. The results also suggest that the chronic strength deficits observed after ACL reconstruction were not angle-dependent; however, the factors explaining those strength deficits were angle-dependent. Finally, the association between quadriceps strength and single-leg hop performance was also stronger at 45° compared with 90° of knee flexion. These findings suggest that clinicians and researchers should consider testing quadriceps strength and activation at more than one knee angle (i.e., shorter and longer muscle lengths) to obtain a complete overview of an individual's neuromuscular profile and also to fully characterize the potential benefits of an intervention targeted to address strength and activation deficits after ACL injury or surgery.
GRANTS
This work was supported in part by University of Michigan Rehabilitation Robotics Interdisciplinary Faculty Initiative funds. Dr. Krishnan's effort is partly funded by the National Institutes of Health (R01-EB019834).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.K. conception and design of research; C.K. and P.T. performed experiments; C.K. and P.T. analyzed data; C.K. and P.T. interpreted results of experiments; C.K. prepared figures; C.K. and P.T. drafted manuscript; C.K. edited and revised manuscript; C.K. and P.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank two anonymous reviewers for their insightful comments and suggestions on earlier versions of the manuscript.
REFERENCES
- 1.Adams GR, Harris RT, Woodard D, Dudley GA. Mapping of electrical muscle stimulation using MRI. J Appl Physiol 74: 532–537, 1993. [DOI] [PubMed] [Google Scholar]
- 2.Adriani E, Mariani PP, Maresca G, Santori N. Healing of the patellar tendon after harvesting of its mid-third for anterior cruciate ligament reconstruction and evolution of the unclosed donor site defect. Knee Surg Sports Traumatol Arthrosc 3: 138–143, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Allen GM, McKenzie DK, Gandevia SC. Twitch interpolation of the elbow flexor muscles at high forces. Muscle Nerve 21: 318–328, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Arangio GA, Chen C, Kalady M, Reed JF 3rd. Thigh muscle size and strength after anterior cruciate ligament reconstruction and rehabilitation. J Orthop Sports Phys Ther 26: 238–243, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Bampouras TM, Reeves ND, Baltzopoulos V, Maganaris CN. Muscle activation assessment: effects of method, stimulus number, and joint angle. Muscle Nerve 34: 740–746, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Baudry S, Duchateau J. Postactivation potentiation in a human muscle: effect on the rate of torque development of tetanic and voluntary isometric contractions. J Appl Physiol 102: 1394–1401, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Becker R, Awiszus F. Physiological alterations of maximal voluntary quadriceps activation by changes of knee joint angle. Muscle Nerve 24: 667–672, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Behm DG, St-Pierre DM, Perez D. Muscle inactivation: assessment of interpolated twitch technique. J Appl Physiol 81: 2267–2273, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop 16: 1–12, 1992. [DOI] [PubMed] [Google Scholar]
- 10.Beynnon BD, Johnson RJ, Fleming BC, Kannus P, Kaplan M, Samani J, Renstrom P. Anterior cruciate ligament replacement: comparison of bone-patellar tendon-bone grafts with two-strand hamstring grafts. A prospective, randomized study. J Bone Joint Surg Am 84-A: 1503–1513, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Butler RJ, Minick KI, Ferber R, Underwood F. Gait mechanics after ACL reconstruction: implications for the early onset of knee osteoarthritis. Br J Sports Med 43: 366–370, 2009. [DOI] [PubMed] [Google Scholar]
- 12.Costigan PA, Deluzio KJ, Wyss UP. Knee and hip kinetics during normal stair climbing. Gait Posture 16: 31–37, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Eitzen I, Eitzen TJ, Holm I, Snyder-Mackler L, Risberg MA. Anterior cruciate ligament-deficient potential copers and noncopers reveal different isokinetic quadriceps strength profiles in the early stage after injury. Am J Sports Med 38: 586–593, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grande G, Cafarelli E. Ia Afferent input alters the recruitment thresholds and firing rates of single human motor units. Exp Brain Res 150: 449–457, 2003. [DOI] [PubMed] [Google Scholar]
- 15.Grindstaff TL, Threlkeld AJ. Optimal stimulation parameters to detect deficits in quadriceps voluntary activation. J Strength Cond Res 28: 381–389, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Gross MT, Tyson AD, Burns CB. Effect of knee angle and ligament insufficiency on anterior tibial translation during quadriceps muscle contraction: a preliminary report. J Orthop Sports Phys Ther 17: 133–143, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Hart JM, Pietrosimone B, Hertel J, Ingersoll CD. Quadriceps activation following knee injuries: a systematic review. J Athl Train 45: 87–97, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horstman AM, de Ruiter CJ, van Duijnhoven NT, Hopman MT, de Haan A. Changes in muscle contractile characteristics and jump height following 24 days of unilateral lower limb suspension. Eur J Appl Physiol 112: 135–144, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houk JC, Rymer WZ, Crago PE. Dependence of dynamic response of spindle receptors on muscle length and velocity. J Neurophysiol 46: 143–166, 1981. [DOI] [PubMed] [Google Scholar]
- 20.Huston LJ, Greenfield ML, Wojtys EM. Anterior cruciate ligament injuries in the female athlete. Potential risk factors. Clin Orthop Relat Res Mar: 50–63, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Keays SL, Bullock-Saxton JE, Newcombe P, Keays AC. The relationship between knee strength and functional stability before and after anterior cruciate ligament reconstruction. J Orthop Res 21: 231–237, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Kent-Braun JA, Le Blanc R. Quantitation of central activation failure during maximal voluntary contractions in humans. Muscle Nerve 19: 861–869, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Knapik JJ, Wright JE, Mawdsley RH, Braun J. Isometric, isotonic, and isokinetic torque variations in four muscle groups through a range of joint motion. Phys Ther 63: 938–947, 1983. [DOI] [PubMed] [Google Scholar]
- 24.Konishi Y, Oda T, Tsukazaki S, Kinugasa R, Fukubayashi T. Relationship between quadriceps femoris muscle volume and muscle torque at least 18 months after anterior cruciate ligament reconstruction. Scand J Med Sci Sports 22: 791–796, 2012. [DOI] [PubMed] [Google Scholar]
- 25.Krishnan C. Quadriceps inhibition may not be a predecessor of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev 38: 38;author reply 39,2010. [DOI] [PubMed] [Google Scholar]
- 26.Krishnan C, Allen EJ, Williams GN. Effect of knee position on quadriceps muscle force steadiness and activation strategies. Muscle Nerve 43: 563–573, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krishnan C, Allen EJ, Williams GN. Torque-based triggering improves stimulus timing precision in activation tests. Muscle Nerve 40: 130–133, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan C, Williams GN. Evoked tetanic torque and activation level explain strength differences by side. Eur J Appl Physiol 106: 769–774, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krishnan C, Williams GN. Factors explaining chronic knee extensor strength deficits after ACL reconstruction. J Orthop Res 29: 633–640, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krishnan C, Williams GN. Quantification method affects estimates of voluntary quadriceps activation. Muscle Nerve 41: 868–874, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubo K, Tsunoda N, Kanehisa H, Fukunaga T. Activation of agonist and antagonist muscles at different joint angles during maximal isometric efforts. Eur J Appl Physiol 91: 349–352, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Lepley AS, Ericksen HM, Sohn DH, Pietrosimone BG. Contributions of neural excitability and voluntary activation to quadriceps muscle strength following anterior cruciate ligament reconstruction. Knee 21: 736–742, 2014. [DOI] [PubMed] [Google Scholar]
- 33.Madhavan S, Krishnan C, Jayaraman A, Rymer WZ, Stinear JW. Corticospinal tract integrity correlates with knee extensor weakness in chronic stroke survivors. Clin Neurophysiol 122: 1588–1594, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manske R, Reiman M. Functional performance testing for power and return to sports. Sports Health 5: 244–250, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Munro AG, Herrington LC. Between-session reliability of four hop tests and the agility T-test. J Strength Cond Res 25: 1470–1477, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Noorkoiv M, Nosaka K, Blazevich AJ. Neuromuscular adaptations associated with knee joint angle-specific force change. Med Sci Sports Exerc 46: 1525–1537, 2014. [DOI] [PubMed] [Google Scholar]
- 38.Otzel DM, Chow JW, Tillman MD. Long-term deficits in quadriceps strength and activation following anterior cruciate ligament reconstruction. Phys Ther Sport 16: 22–28, 2015. [DOI] [PubMed] [Google Scholar]
- 39.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev 37: 147–153, 2009. [DOI] [PubMed] [Google Scholar]
- 40.Palmieri-Smith RM, Thomas AC, Wojtys EM. Maximizing quadriceps strength after ACL reconstruction. Clin Sports Med 27: 405–424, vii–ix, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil 22: 1–6, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Pincivero DM, Salfetnikov Y, Campy RM, Coelho AJ. Angle- and gender-specific quadriceps femoris muscle recruitment and knee extensor torque. J Biomech 37: 1689–1697, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Piva SR, Childs JD, Klucinec BM, Irrgang JJ, Almeida GJ, Fitzgerald GK. Patella fracture during rehabilitation after bone-patellar tendon-bone anterior cruciate ligament reconstruction: 2 case reports. J Orthop Sports Phys Ther 39: 278–286, 2009. [DOI] [PubMed] [Google Scholar]
- 44.Prasartwuth O, Allen TJ, Butler JE, Gandevia SC, Taylor JL. Length-dependent changes in voluntary activation, maximum voluntary torque and twitch responses after eccentric damage in humans. J Physiol 571: 243–252, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riley PO, Dicharry J, Franz J, Della Croce U, Wilder RP, Kerrigan DC. A kinematics and kinetic comparison of overground and treadmill running. Med Sci Sports Exerc 40: 1093–1100, 2008. [DOI] [PubMed] [Google Scholar]
- 46.Roos PE, Button K, Rimmer PA, van Deursen RW. Fear of re-injury impacts rehabilitation of ACL injured patients. J Bone Joint Surg Br 94: 56–56, 2012.22219248 [Google Scholar]
- 47.Shield A, Zhou S. Assessing voluntary muscle activation with the twitch interpolation technique. Sports Med 34: 253–267, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Snyder-Mackler L, De Luca PF, Williams PR, Eastlack ME, Bartolozzi AR 3rd. Reflex inhibition of the quadriceps femoris muscle after injury or reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am 76: 555–560, 1994. [DOI] [PubMed] [Google Scholar]
- 49.Sturnieks DL, Besier TF, Hamer PW, Ackland TR, Mills PM, Stachowiak GW, Podsiadlo P, Lloyd DG. Knee strength and knee adduction moments following arthroscopic partial meniscectomy. Med Sci Sports Exerc 40: 991–997, 2008. [DOI] [PubMed] [Google Scholar]
- 50.Tay GH, Warrier SK, Marquis G. Indirect patella fractures following ACL reconstruction: a review. Acta Orthop 77: 494–500, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Taylor JL. Point: the interpolated twitch does/does not provide a valid measure of the voluntary activation of muscle. J Appl Physiol 107: 354–355, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Tourville TW, Jarrell KM, Naud S, Slauterbeck JR, Johnson RJ, Beynnon BD. Relationship between isokinetic strength and tibiofemoral joint space width changes after anterior cruciate ligament reconstruction. Am J Sports Med 42: 302–311, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urbach D, Nebelung W, Becker R, Awiszus F. Effects of reconstruction of the anterior cruciate ligament on voluntary activation of quadriceps femoris a prospective twitch interpolation study. J Bone Joint Surg Br 83: 1104–1110, 2001. [DOI] [PubMed] [Google Scholar]
- 54.Webster KE, Wittwer JE, O'Brien J, Feller JA. Gait patterns after anterior cruciate ligament reconstruction are related to graft type. Am J Sports Med 33: 247–254, 2005. [DOI] [PubMed] [Google Scholar]
- 55.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med 33: 402–407, 2005. [DOI] [PubMed] [Google Scholar]