Abstract
We tested the hypothesis that aging would be associated with slowed vasodilator kinetics in contracting muscle in part due to a reduced nitric oxide (NO) bioavailability. Young (n = 10; 24 ± 2 yr) and older (n = 10; 67 ± 2 yr) adults performed rhythmic forearm exercise (4 min each) at 10, 20, and 30% of max during saline infusion (control) and NO synthase (NOS) inhibition. Brachial artery diameter and velocities were measured using Doppler ultrasound. Forearm vascular conductance (FVC) was calculated for each duty cycle (1 s contraction/2 s relaxation) from forearm blood flow (FBF; ml/min) and blood pressure (mmHg) and fit with a monoexponential model. The main parameters derived from the model were the amplitude of the FBF and FVC response and the number of duty cycles for FBF and FVC to change 63% of the steady-state amplitude (τFBF and τFVC). Under control conditions 1) the amplitude of the FVC response at 30% maximal voluntary contraction (MVC) was lower in older compared with young adults (319 ± 33 vs. 462 ± 52 ml·min−1·100 mmHg−1; P < 0.05) and 2) τFVC was slower in older (10 ± 1, 13 ± 1, and 15 ± 1 duty cycles) compared with young (6 ± 1, 9 ± 1, and 11 ± 1 duty cycles) adults at all intensities (P < 0.05). In young adults, NOS inhibition blunted the amplitude of the FVC response at 30% MVC and prolonged the τFVC at all intensities (10 ± 2, 12 ± 1, and 16 ± 2 duty cycles; P < 0.05), whereas it did not change in older adults. Our data indicate that the blood flow and vasodilator kinetics in exercising muscle are altered with aging possibly due to blunted NO signaling.
Keywords: aging, exercise, blood flow, vasodilation, nitric oxide, kinetics
skeletal muscle blood flow increases rapidly at the onset of exercise in an effort to meet the muscle's increased metabolic needs. The mechanisms responsible for increasing blood flow at the onset of exercise as well as maintaining it over time involves a complex interaction between mechanical factors, the sympathetic nervous system, and local metabolic and endothelium-derived substances that influence vascular tone (40). To date, the majority of studies that have addressed the mechanisms involved in hyperemic and vasodilator responses during muscle contractions have focused primarily on limb (arm or leg) hemodynamics once steady-state exercise has been achieved. Under steady-state conditions endothelium-derived nitric oxide (NO) has been shown to make a modest contribution to the exercise hyperemic response during mild- to moderate-intensity exercise in humans (19, 22, 42), although others have found that inhibition of NO synthase (NOS) has no effect on skeletal muscle blood flow and vasodilation during dynamic exercise (21, 24, 31, 37).
It is important to note that the mechanisms influencing vascular tone at the onset of exercise may be different from those involved in the control during steady-state exercise (10). Along these lines we recently demonstrated that NOS inhibition substantially reduces (∼30-50%) the rapid hyperemic and vasodilator response to a single muscle contraction over a range of intensities (6). Despite these findings and others related to the mechanisms involved in the rapid vasodilator response following a single muscle contraction (3, 4, 6, 11, 28), there have only been a few studies related to the time course (kinetics) and mechanisms of hyperemia and vasodilation between the onset of exercise (initial contraction) and steady-state conditions in humans. Shoemaker and colleagues (43) found that neither acetylcholine nor NO is essential to observe the normal time course of the blood flow response during light-intensity forearm contractions. Conversely, we demonstrated that NOS inhibition attenuates the onset of vasodilation (expressed as the slope of the exercise response between rest and steady-state exercise during dynamic forearm exercise) and prolongs the time to reach steady-state values in young men (5). Similarly, combined inhibition of NO and prostanoids (via cyclooxygenase inhibition) substantially attenuates blood flow and vasodilation throughout the time course (10–240 s) of intense one-legged knee-extensor exercise (9).
Aging is associated with a variety of changes within the cardiovascular system that can compromise muscle blood flow or alter its regulation during dynamic exercise. Despite convincing evidence that there is a deficit in steady-state blood flow during submaximal exercise with aging (26, 28, 29, 33–36), little is known regarding the time course (i.e., kinetics) of exercise hyperemia and vasodilation in older adults. In resistance vessels of aged rats there is an altered ability to match oxygen delivery to consumption during the rest-to-contractions transition (2). This temporal mismatching is thought to be due to an impaired vasodilation. Along these lines, recent data suggest that the time course of endothelium-dependent vasodilation in isolated arterioles is significantly slower in skeletal muscle of old rats (1). Moreover, human aging is also associated with a reduction in NO bioavailability and decreased endothelium-dependent (NO-mediated) vasodilation (7, 14, 44, 45). Whether the time course of hyperemia and vasodilation in response to rhythmic muscle contractions is altered with aging and whether reductions in NO signaling contribute to the potential age-related differences remains unclear. Therefore we aimed to quantify and characterize the hyperemic and vasodilator kinetics during exercise in young and older humans. We hypothesized that compared with young adults, the speed of skeletal muscle vasodilation at the onset of rhythmic contractions will be slower in otherwise healthy aging humans. Additionally, we sought to test the hypothesis that a slower hyperemic and vasodilator kinetic profile in older adults is attributed in part to a reduced NO bioavailability.
METHODS
Subjects
A total of 10 young and 10 older healthy subjects volunteered to participate in protocol 1 of the study, which was part of a larger protocol with some of the data previously published (6). An additional seven young healthy subjects participated in protocol 2. Subjects completed written informed consent and underwent a standard screening and were healthy, nonobese [body mass index (BMI) ≤ 30 kg/m2], nonsmokers, not taking any vasoactive medications, and were sedentary to moderately active. Studies were performed after an overnight fast and with subjects refraining from exercise and caffeine for at least 24 h. Young female subjects were studied during the early follicular phase of the menstrual cycle or the placebo phase of oral contraceptives (32). All older female subjects were postmenopausal and were not taking any form of hormone replacement therapy. All study protocols were approved by the Institutional Review Board and were performed according to the Declaration of Helsinki.
Arterial Catheterization
In protocol 1, a 20-gauge, 5-cm (model RA-04020, Arrow International, Reading, PA) catheter was placed in the brachial artery of the experimental arm under aseptic conditions after local anesthesia (2% lidocaine) for administration of study drugs. The catheter was connected to a three-port connector in series, as previously described in detail (15). One port was linked to a pressure transducer positioned at heart level (model PX600F, Edwards Lifescience, Irvine, CA) to allow measurement of arterial pressure and was continuously flushed (3 ml/h) with saline with a stop-cock system to enable arterial blood sampling. The remaining two ports allowed arterial drug administration.
Heart Rate and Systemic Blood Pressure
Heart rate (HR) was recorded via continuous 3-lead ECG. In protocol 1, beat-to-beat systemic blood pressure was recorded via a brachial arterial catheter connected to a pressure transducer (Cardiocap/5, Datex-Ohmeda, Louisville, CO). In protocol 2, beat-to-beat systemic blood pressure was assessed with a finger plethysmograph (Nexfin; Edwards Lifesciences) on the nonexercising hand.
Forearm Blood Flow
Brachial artery mean blood velocity and brachial artery diameter were determined with a 12-MHz linear-array Doppler probe (model M12L, Vivid 7, General Electric, Milwaukee, WI). Brachial artery blood velocity was measured throughout each condition with a probe insonation angle previously calibrated to 60° and the lumen of the artery positioned parallel to the angle correction cursor. Brachial artery diameter measurements were obtained at end diastole at rest (immediately prior to the start of contractions), between contractions during steady-state exercise (last 15 s of exercise), and at the end of each recovery period. Forearm blood flow (FBF) was calculated as the product of mean blood velocity (cm/s) and brachial artery cross-sectional area (cm2) and expressed as milliliters per minute.
Forearm Exercise
Rhythmic forearm exercise was performed with a handgrip device by the nondominant arm at 10%, 20%, and 30% of each subject's maximal voluntary contraction (MVC). The weight was lifted 4–5 cm over a pulley at a duty cycle of 1-s contraction and 2-s relaxation (20 contractions/min) using a metronome to ensure correct timing. The average weight used in protocol 1 for the young subjects was 4.1 ± 0.5, 8.2 ± 1.0, and 12.4 ± 1.5 kg for 10, 20, and 30% MVC, respectively. The average weight used for the older subjects was 3.4 ± 0.3, 6.8 ± 0.7, and 10.2 ± 1.1 kg for 10, 20, and 30% MVC, respectively (P = 0.22–0.27 compared with young subjects). The average weight used for forearm exercise in protocol 2 was 5.3 ± 0.5, 10.5 ± 0.9, and 15.6 ± 1.3 kg for 10, 20, and 30% MVC, respectively. Workload intensity was randomized within each drug condition (protocol 1) and within each set of trials (protocol 2).
Pharmacological Infusions
NG-monomethyl-l-arginine (l-NMMA; NOS inhibitor; Bachem, Switzerland) was infused at a loading dose of 5 mg/min for 5 min and then at a maintenance dose of 1 mg/min for the remainder of the study. To test the efficacy of the NO synthase inhibition with l-NMMA, acetylcholine (ACh; a nonspecific muscarinic agonist) was infused intra-arterially at 4.0 μg·dl forearm volume−1·min−1 for 4 min before and after l-NMMA administration. Intra-arterial infusion of sodium nitroprusside (NTP) was used to determine whether l-NMMA administration has any nonspecific effects on forearm vasodilation. NTP was infused at 1.0 μg·dl forearm volume−1·min−1 for 4 min before and after l-NMMA administration.
Experimental Protocol
Each subject completed a total of six exercise trials (on a single study day) which consisted of rhythmic forearm contractions during saline (control) and l-NMMA administration. Exercise intensity (10, 20, and 30% MVC) was randomized within each condition. Each trial consisted of 2 min of rest, followed by 4 min of rhythmic forearm exercise and 3 min of recovery. Brachial artery velocity and hemodynamics were measured throughout the rest, exercise, and recovery periods. Additionally, intra-arterial infusions of ACh and NTP were performed after each set of exercise trials under each condition (saline and l-NMMA). Brachial artery velocity and hemodynamics were measured during the rest (2 min) and infusion (4 min) periods. Due to the long half-life of l-NMMA, NOS inhibition trials were always performed last. A rest period of 15–20 min was allowed between each exercise and infusion trial. As previously mentioned these rhythmic exercise trials were part of a larger protocol with some of the data published (6). The previously published results consisted of blood flow and vasodilator responses to single muscle contractions. The rhythmic forearm exercise trials in the current study were always performed following the single muscle contraction trials (within each drug condition) and following a 20-min rest period.
Protocol 2 was performed to determine the reproducibility of the hyperemic and vasodilator kinetic response to rhythmic exercise across multiple trials and varying intensities. The exact same design was used in protocol 2, with the exception that study drugs were not administered and only young adults were studied. That is each subject performed two sets of three separate rhythmic forearm exercise trials at 10, 20, and 30% MVC.
Data Analysis and Statistics
Data were collected at 250 Hz, stored on a computer, and analyzed off-line with signal-processing software (WinDaq, DATAQ Instruments, Akron, OH). Mean arterial pressure (MAP) was determined from the brachial artery pressure waveform, and HR was determined from the electrocardiogram. Baseline FBF and MAP represent an average of the last 30 s of the resting time period prior to exercise. Forearm vascular conductance (FVC) was calculated as (FBF/MAP) × 100 and expressed as milliliters per minute per 100 mmHg. During exercise FBF and FVC were calculated and averaged over each duty cycle (1-s contraction and 2-s relaxation). A minimum of 90% of complete duty cycles (all velocity envelopes clearly captured within the duty cycle) were obtained in all subjects for each exercise trial for protocol 1 and in six of the seven subjects in protocol 2. On average 95–99% of the duty cycles across exercise trials were used in the model described below. Additionally, FBF and FVC were averaged every 5 s during the recovery period. The on-transient kinetics for FBF and FVC during each exercise intensity were analyzed with a nonlinear least-squares curve-fitting procedure using the following monoexponential model
with the components of the model defined as follows: Y(t) represents the dependent variable (either FBF or FVC) at any time (t), Y(baseline) is the resting value immediately prior to the onset of exercise, Amp is the amplitude of the response, TD is the time delay, and τ represents the number of duty cycles required to achieve 63% of the steady-state amplitude.
Additionally, the FBF and FVC responses following exercise were analyzed to examine the impact of aging and NOS inhibition during off-transient kinetics. To quantify the magnitude of the recovery response total FBF and FVC were defined as the area under the curve after respective baseline values were subtracted (20). To further gain insight on the off-kinetics, we also quantified the time it took for end-exercise steady-state FBF and FVC to decay 50%.
All values are expressed as means ± SE. Analysis of variance (ANOVA) was used to analyze baseline differences between age groups. To determine the effect of age (group) and NOS inhibition (condition) on the on- and off-transient hyperemic and vasodilator kinetics, differences in the FBF and FVC amplitude, τFBF and τFVC, 50% decay rate, and total recovery FBF and FVC within each exercise intensity (10, 20, and 30% MVC) were determined via two-way repeated-measures ANOVA. Additional two-way repeated-measures ANOVA were performed to examine FBF and FVC responses to ACh and NTP within each condition (saline vs. l-NMMA) and between age groups. When a group × condition interaction was found, Tukey's post hoc analysis determined where statistical differences occurred. Statistical difference was set a priori at P < 0.05. Reproducibility was assessed via coefficients of variation and repeated-measures ANOVA (protocol 2).
RESULTS
Both young and older subject characteristics from protocol 1 are summarized in Table 1. Height, weight, BMI, forearm volume, and MVC were similar between age groups (P > 0.05 for all). Older subjects demonstrated greater cholesterol values (total, high-, and low-density lipoprotein) than their younger counterparts (P < 0.05 for all). Seven subjects (6M/1F) completed protocol 2. The subjects were 29 ± 2 yr of age and 179 ± 2 cm in height and weighed 82 ± 6 kg (BMI: 25.3 ± 1.3 kg/m2).
Table 1.
Subject characteristics
| Variable | Young | Older |
|---|---|---|
| Age, years | 24 ± 2 | 67 ± 2* |
| Men/Women | 5/5 | 5/5 |
| Height, cm | 174 ± 3 | 170 ± 3 |
| Weight, kg | 72 ± 5 | 72 ± 6 |
| BMI, kg/m2 | 23.5 ± 1.1 | 24.7 ± 1.1 |
| FAV, ml | 879 ± 71 | 841 ± 96 |
| MVC, kg | 41 ± 5 | 34 ± 4 |
| Total cholesterol, mmol/l | 3.8 ± 0.2 | 4.9 ± 0.2* |
| LDL, mmol/l | 2.1 ± 0.2 | 2.9 ± 0.2* |
| HDL, mmol/l | 1.2 ± 0.1 | 1.7 ± 0.2* |
| Triglycerides, mmol/l | 1.0 ± 0.2 | 1.1 ± 0.1 |
Values are means ± SE.
BMI, body mass index; FAV, forearm volume; MVC, maximal voluntary contraction; LDL, low-density lipoprotein, HDL, high-density lipoprotein.
P < 0.05 vs. young.
Protocol 1
Hemodynamic responses to forearm exercise in young and older adults.
Baseline (resting), exercise (steady state), and recovery (last 30 s) hemodynamics at each intensity and under each condition are shown in Table 2. HR, FBF, and FVC at rest were similar between age groups across all trails. MAP was consistently higher across all conditions in the older adults (P < 0.05). Despite a significant increase in steady-state FBF and FVC at all intensities in both age groups under control conditions, the steady-state hyperemic and vasodilator responses were attenuated at 30% MVC in older compared with young adults (P < 0.05). No age-related differences in end-recovery FBF and FVC were observed (P > 0.05, Table 2). Infusion of l-NMMA reduced baseline FBF and FVC and increased MAP in both young and older adults. In young adults NOS inhibition reduced the FVC at steady-state exercise at all intensities (P < 0.05). Conversely, steady-state exercise FBF and FVC across intensities were unchanged with l-NMMA in the older adults (P = 0.07–0.41). End-recovery FBF and FVC were reduced with l-NMMA at 10%, 20%, and 30% in both young and older adults (P < 0.05; Table 2).
Table 2.
Systemic and forearm hemodynamics at rest and during exercise and recovery under each drug condition
| 10% MVC |
20% MVC |
30% MVC |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline (Rest) | Exercise (Steady-state) | Recovery | Baseline (Rest) | Exercise (Steady-state) | Recovery | Baseline (Rest) | Exercise (Steady-state) | Recovery | |
| Young (n = 10) | |||||||||
| Control (saline) | |||||||||
| FBF, ml/min | 73 ± 10 | 276 ± 44† | 84 ± 17 | 76 ± 11 | 441 ± 56† | 207 ± 49† | 77 ± 12 | 600 ± 67† | 352 ± 54† |
| FVC, ml·min−1·100 mmHg−1 | 75 ± 9 | 283 ± 45† | 85 ± 17 | 78 ± 10 | 444 ± 53† | 213 ± 49† | 77 ± 11 | 578 ± 58† | 352 ± 50† |
| Heat rate, beats/min | 61 ± 2 | 62 ± 2 | 60 ± 2 | 60 ± 2 | 68 ± 2† | 61 ± 2 | 61 ± 3 | 79 ± 2† | 65 ± 3 |
| MAP, mmHg | 96 ± 2 | 96 ± 2 | 97 ± 2 | 96 ± 2 | 99 ± 2 | 97 ± 2 | 97 ± 2 | 103 ± 3† | 99 ± 2 |
| l-NMMA | |||||||||
| FBF, ml/min | 55 ± 9‡ | 216 ± 33†‡ | 62 ± 13‡ | 51 ± 7‡ | 391 ± 46† | 106 ± 25‡ | 49 ± 6‡ | 528 ± 59†‡ | 214 ± 38†‡ |
| FVC, ml·min−1·100 mmHg−1 | 54 ± 8‡ | 212 ± 31†‡ | 60 ± 13‡ | 49 ± 7‡ | 364 ± 37†‡ | 99 ± 22‡ | 48 ± 6‡ | 486 ± 49†‡ | 210 ± 36†‡ |
| Heat rate, beats/min | 59 ± 3 | 61 ± 3 | 61 ± 3 | 61 ± 2 | 69 ± 2† | 61 ± 2 | 58 ± 2 | 79 ± 3† | 66 ± 2 |
| MAP, mmHg | 100 ± 2‡ | 101 ± 2‡ | 102 ± 2‡ | 101 ± 2‡ | 106 ± 2†‡ | 104 ± 3‡ | 100 ± 2‡ | 108 ± 3†‡ | 101 ± 2 |
| Older (n = 10) | |||||||||
| Control (no drug) | |||||||||
| FBF, ml/min | 77 ± 7 | 254 ± 16† | 86 ± 6 | 76 ± 8 | 359 ± 32† | 166 ± 20† | 74 ± 6 | 449 ± 38*† | 267 ± 45† |
| FVC, ml·min−1·100 mmHg−1 | 72 ± 8 | 238 ± 18† | 80 ± 6 | 69 ± 8 | 322 ± 26*† | 153 ± 20† | 67 ± 6 | 375 ± 34*† | 245 ± 43† |
| Heat rate, beats/min | 61 ± 3 | 65 ± 3† | 61 ± 2 | 59 ± 3 | 70 ± 3† | 63 ± 3 | 60 ± 2 | 79 ± 3† | 66 ± 2 |
| MAP, mmHg | 108 ± 2* | 107 ± 1* | 107 ± 2* | 107 ± 2* | 113 ± 2*† | 110 ± 1* | 108 ± 1* | 120 ± 3*† | 110 ± 2* |
| l-NMMA | |||||||||
| FBF, ml/min | 60 ± 6‡ | 230 ± 24† | 65 ± 9‡ | 57 ± 7‡ | 352 ± 32† | 99 ± 14‡ | 56 ± 7‡ | 436 ± 36*† | 191 ± 32†‡ |
| FVC, ml·min−1·100 mmHg−1 | 52 ± 6‡ | 205 ± 21† | 57 ± 8‡ | 49 ± 6‡ | 297 ± 29*† | 85 ± 12‡ | 49 ± 6‡ | 353 ± 33*† | 170 ± 29†‡ |
| Heat rate, beats/min | 61 ± 4 | 63 ± 3 | 60 ± 3 | 61 ± 3 | 71 ± 3† | 64 ± 3 | 61 ± 2 | 79 ± 3† | 64 ± 3 |
| MAP, mmHg | 113 ± 2*‡ | 113 ± 1*‡ | 114 ± 1*‡ | 113 ± 2*‡ | 119 ± 2*†‡ | 116 ± 1*‡ | 112 ± 2*‡ | 123 ± 3*†‡ | 114 ± 2*‡ |
Values are means ± SE.
P < 0.05 vs. young;
P < 0.05 vs. baseline (rest);
P < 0.05 vs. control.
On-kinetics.
The mean fit for the on-transient vasodilator kinetics across exercise intensities in young and older adults under control (saline) and NOS inhibition (l-NMMA) conditions are illustrated in Fig. 1. As expected, there was a significant main effect of exercise intensity on the amplitude of the FBF and FVC responses in young and older adults under control conditions (P < 0.001). That is, there was a progressive rise in the amplitude of the FBF and FVC responses with increasing exercise intensity (Fig. 2, A and B) in both age groups. However, the amplitude of the FBF and FVC responses at 30% MVC under control conditions was lower in older compared with young subjects (P < 0.05 for both, Fig. 2, A and B). Additionally, there was a main effect of exercise intensity on τFBF and τFVC in both age groups (P < 0.001). Within each exercise intensity, older adults demonstrated a prolonged τFBF and τFVC compared with young adults (P < 0.05, Fig. 3, A and B). There was a significant main effect of NOS inhibition on the amplitude at all three exercise intensities; however, no group × condition interaction was observed except at the 30% MVC exercise level (P < 0.05; Fig. 2, A and B). Conversely, NOS inhibition prolonged the τFBF and τFVC at all exercise intensities in young adults (P < 0.05) while no change was observed in the older group (Fig. 3, A and B).
Fig. 1.
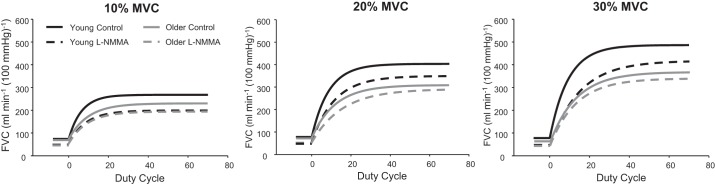
Mean fit for the on-transient vasodilator kinetics (forearm vascular conductance; FVC) in young and older adults at 10%, 20%, and 30% maximal voluntary contraction (MVC). l-NMMA, NG-monomethyl-l-arginine.
Fig. 2.
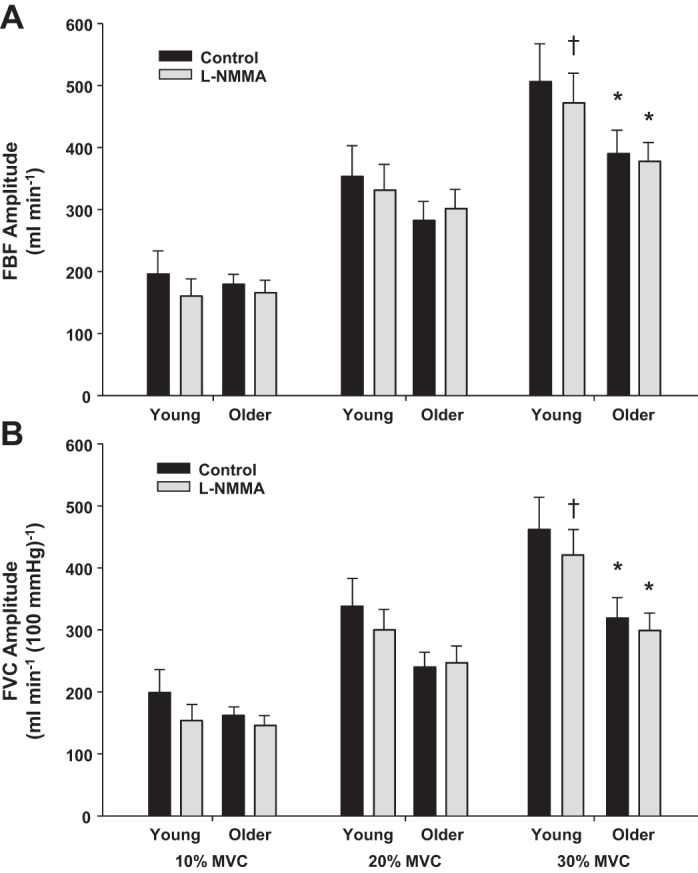
Amplitude of the forearm blood flow (FBF; A) and FVC (B) responses during dynamic forearm exercise at 10%, 20%, and 30% MVC in young and older adults under control (saline) and NO synthase (NOS) inhibition (l-NMMA) conditions. Under control conditions the amplitude of the FBF and FVC responses was attenuated in older adults at 30% MVC. NOS inhibition (l-NMMA) also blunted the amplitude of the hyperemic and vasodilator responses at 30% MVC in young adults. *P < 0.05 vs. young, †P < 0.05 vs. control.
Fig. 3.
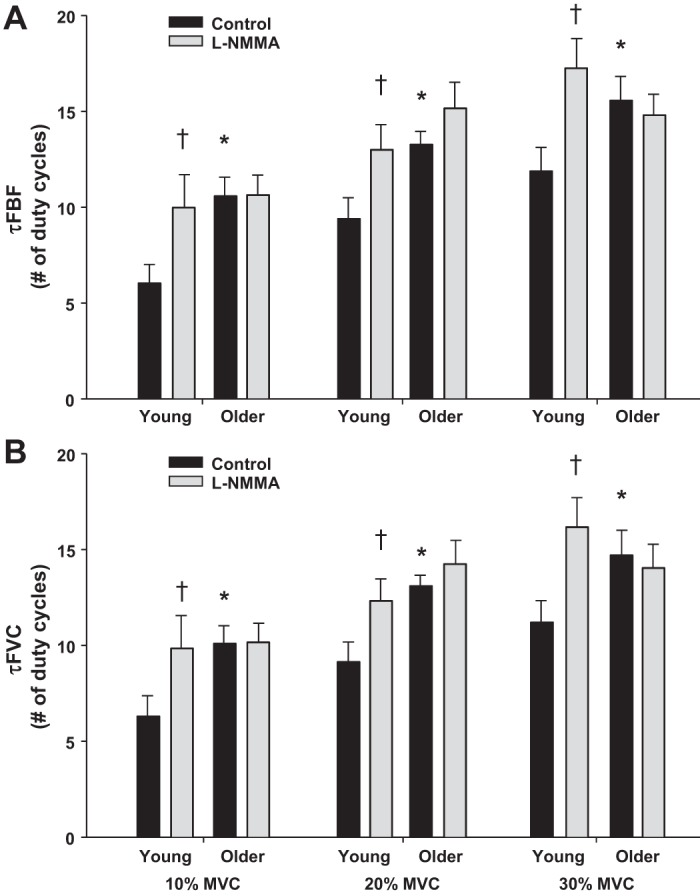
Number of duty cycles for FBF (A) and FVC (B) to reach 63% of the steady-state amplitude (τFBF and τFVC) during dynamic forearm exercise at 10%, 20% and 30% MVC in young and older adults under control (saline) and NOS inhibition (l-NMMA) conditions. The hyperemic and vasodilator responses were slower in older adults at all exercise intensities. In young adults, NO synthase inhibition (l-NMMA) prolonged the hyperemic and vasodilator responses (τFBF and τFVC) at all exercise intensities. *P < 0.05 vs. young, †P < 0.05 vs. control.
Recovery data.
Total postexercise FBF and FVC at each exercise intensity in young and older adults are shown in Fig. 4. During saline and l-NMMA infusions total postexercise FBF and FVC progressively increased with higher exercise intensities in both age groups (P < 0.001). There was a main effect of age on total postexercise FBF and FVC at 30% MVC (P < 0.05, Fig. 4, A and B). Additionally, there was a main effect of l-NMMA on the total postexercise FBF and FVC at all exercise intensities (P < 0.01, Fig. 4, A and B). However, there was no age × condition interaction observed within any of the exercise intensities.
Fig. 4.
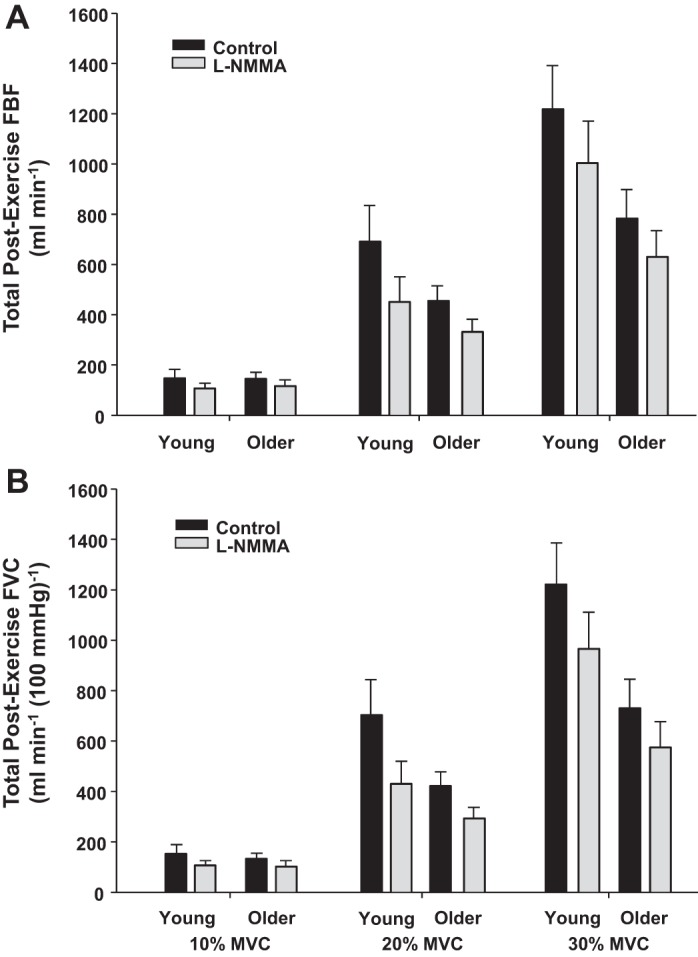
Total postexercise FBF and FVC during recovery at 10%. 20%, and 30% MVC in young and older adults under control (saline) and NOS inhibition (l-NMMA) conditions. A main effect of age was found at 30% MVC for total postexercise FBF and FVC (P < 0.05). There was also a main effect of l-NMMA on the total postexercise FBF and FVC at all exercise intensities (P < 0.01).
The recovery FBF and FVC response (off-kinetics) was also analyzed by the time it took for end-exercise steady-state FBF and FVC to decay 50%. No significant age effect was found at 10, 20, and 30% MVC. However, a significant main effect of condition was found at all exercise intensities. That is, l-NMMA significantly sped up the FBF and FVC decay rate at 10–30% MVC in young and older adults (P < 0.01 for all).
Blood flow and vasodilator responses to exogenous acetylcholine and sodium nitroprusside.
The hyperemic and vasodilator responses (change in FBF and FVC from baseline, respectively) to intra-arterial infusion of ACh were substantially lower in older compared with young adults (FBF = 186 ± 41 vs. 327 ± 39 ml/min; FVC = 168 ± 37 vs. 332 ± 41 ml·min−1·100 mmHg−1, P < 0.05 for both). Likewise, the hyperemic and vasodilator responses to intra-arterial infusion of NTP were also lower in the older compared with young adults (FBF = 149 ± 16 vs. 233 ± 23 ml/min; FVC = 146 ± 16 vs. 258 ± 30 ml·min−1·100 mmHg−1, P < 0.01 for both). Compared with control (saline) conditions, infusion of l-NMMA markedly reduced the FBF (209 ± 25 ml/min) and FVC (200 ± 25 ml·min−1·100 mmHg−1) responses to ACh in young adults (P < 0.001 for both), thus confirming effective NOS inhibition. However, l-NMMA did not alter the FBF (175 ± 38 ml/min; P = 0.49) and FVC (151 ± 31 ml·min−1·100 mmHg−1; P = 0.26) responses to ACh in the older adults. Consequently there were no age-related difference in the FBF (P = 0.48) and FVC (P = 0.23) responses to ACh during NOS inhibition. Moreover, NOS inhibition did not change the FBF and FVC responses to NTP in either age group.
Protocol 2
The reproducibility of the on-transient blood flow and vasodilator kinetic parameters during forearm exercise is presented in Table 3. We were unable to get adequate blood velocity tracings to fit the model in one subject during the 30% MVC exercise trials. Therefore the reproducibility of the kinetic parameters was assessed in 7 subjects at 10 and 20% MVC and six subjects at 30% MVC. The amplitude of the FBF and FVC responses, as well as τFBF and τFVC, did not differ between trials within each exercise intensity (10, 20, and 30% MVC). Furthermore each parameter of the on-transient blood flow and vasodilator kinetic response to forearm exercise was fairly reproducible.
Table 3.
Reproducibility of the hyperemic and vasodilator kinetic response to rhythmic exercise across multiple trials and varying intensities
| Trial 1 | Trial 2 | P Value | CV, % | |
|---|---|---|---|---|
| 10% MVC | ||||
| FBF amplitude, ml/min | 140 ± 17 | 157 ± 23 | 0.20 | 11.8 ± 1.8 |
| τFBF, no. of duty cycles | 5.1 ± 0.8 | 5.5 ± 1.0 | 0.39 | 8.7 ± 1.9 |
| FVC amplitude, ml·min−1·100 mmHg−1 | 165 ± 18 | 181 ± 23 | 0.18 | 9.4 ± 1.7 |
| τFVC, no. of duty cycles | 5.3 ± 0.9 | 5.7 ± 1.0 | 0.17 | 5.4 ± 1.8 |
| 20% MVC | ||||
| FBF amplitude, ml/min | 310 ± 47 | 319 ± 53 | 0.53 | 5.3 ± 1.9 |
| τFBF, no. of duty of cycles | 7.3 ± 0.9 | 7.9 ± 1.1 | 0.20 | 8.8 ± 2.5 |
| FVC amplitude, ml·min−1·100 mmHg−1 | 333 ± 47 | 324 ± 45 | 0.43 | 6.1 ± 1.2 |
| τFVC, no. of duty cycles | 6.6 ± 0.9 | 6.8 ± 1.0 | 0.59 | 9.0 ± 1.3 |
| 30% MVC | ||||
| FBF amplitude, ml/min | 509 ± 53 | 507 ± 61 | 0.90 | 5.3 ± 2.0 |
| τFBF, no. of duty cycles | 9.1 ± 1.2 | 8.7 ± 1.1 | 0.52 | 9.0 ± 2.4 |
| FVC amplitude, ml·min−1·100 mmHg−1 | 505 ± 41 | 515 ± 33 | 0.39 | 3.0 ± 0.7 |
| τFVC, no. of duty cycles | 8.6 ± 1.0 | 8.4 ± 1.0 | 0.72 | 9.6 ± 1.6 |
Values are means ± SE.
CV, coefficient of variation (presented as mean intra-individual CV).
DISCUSSION
We have previously demonstrated that the hyperemic and vasodilator response to a single muscle contraction is attenuated in older adults (4, 6), which is likely due to a diminished contribution of NO-mediated mechanisms (6). The present data are the first to demonstrate that the speed (i.e., kinetics) of skeletal muscle blood flow and vasodilation during repeated rhythmic contractions are slower in otherwise healthy aging humans (Fig. 1). Moreover, our present findings suggest that NO appears to play a role in the onset and kinetics of hyperemia and vasodilation during mild to heavy rhythmic forearm exercise in young adults, but a reduction in NO bioavailability and/or signaling likely contributes to the impaired kinetics in older adults. These conclusions are supported by 1) the slower τFBF and τFVC across all exercise intensities (Fig. 3, A and B) in older compared with young adults; and 2) NOS inhibition prolonged the τFBF and τFVC at all exercise intensities in young adults, but failed to alter the hyperemic and vasodilator kinetics in older adults, thus eliminating the age-related differences in the timing of the response observed under control conditions. Additionally, our reproducibility studies in young adults (protocol 2) suggest that the changes in the hyperemic and vasodilator kinetics during the NOS inhibition trials (protocol 1) are not likely explained by a bout order effect.
Age-Related Differences in Exercise Hyperemic and Vasodilator Kinetics
To our knowledge this is the first study to examine the impact of human aging on the hyperemic and vasodilator kinetics during exercise. Numerous other studies have examined the effect of aging on steady-state blood flow during or immediately following submaximal forearm and leg exercise, with several demonstrating an attenuated hyperemia (26, 28, 29, 33–36), whereas others reported no age-related differences in FBF between young and older adults (17, 25, 30). Our current data indicate that the absolute steady-state FBF and FVC responses of older adults are preserved at mild to moderate but reduced at heavier relative intensity forearm exercise (Table 2). Of particular interest to the present study, age-related differences in the modeled on-kinetic parameters were also observed. First, the amplitude of the hyperemic and vasodilator responses (which accounts for baseline FBF and FVC) were blunted at 30% MVC in older compared with young adults. Additionally, older adults also demonstrated prolonged τFBF and τFVC values, which indicate a longer duration to reach steady-state blood flow and vasodilation. This finding of a prolonged time course for steady-state vasodilation is in agreement with previous reports of impairments in the dynamics of vasodilation in skeletal muscle resistance vessels of older rats exposed to acetylcholine (1). Taken together, our current findings suggest that in addition to the age-related effects on steady state muscle blood flow and vasodilation, aging also appears to prolong the time needed to reach steady-state conditions during rhythmic exercise. When considered with the previously reported attenuated rapid hyperemic and vasodilator responses to a single muscle contraction in older adults (4, 6, 28), the age-related impairments in blood flow kinetics likely start within the first couple seconds of exercise.
Ultimately the rise in muscle blood flow and oxygen delivery during exercise occurs to match the metabolic activity of the contracting tissue. The slowed blood flow and vasodilator kinetic response in the older adults of the current study could be taken to mean that aging is associated with a mismatch between oxygen consumption and metabolic need over the course of a given bout of exercise. However, previous findings have demonstrated that the lower muscle blood flow during submaximal steady-state exercise observed in older adults is offset by a higher oxygen extraction within the contracting muscle (29, 34) although this finding has not been consistently reported (33, 35). If older adults do indeed extract oxygen at higher levels for a given workload to help compensate for the reduced muscle blood flow, then it is possible that the need for oxygen within the contracting muscle is being met over the time course of exercise despite the slower blood flow and vasodilator kinetics observed in the current study. In this context, duManoir and colleagues (18) concluded from studies involving near-infrared spectroscopy that microvascular blood flow kinetics are slowed with aging, but older adults rely on a greater oxygen extraction during the transition from rest to exercise.
Role of NO in the Onset of Hyperemia and Vasodilation During Exercise
Previous studies examining the effect of NOS inhibition on skeletal muscle blood flow during steady-state conditions in young adults suggest that exercise hyperemia is reduced on the order of 10–30% (19, 22, 42). Indeed we observed a ∼10–15% reduction in steady state FBF and FVC in the young adults during heavier intensity forearm exercise (30% MVC) in the current study (Table 2). However, the role of NO in the time course of exercise hyperemia (from rest to steady state) and onset of vasodilation in young adults has been studied to a much lesser extent, with previous studies reporting somewhat conflicting results. We have previously demonstrated that NOS inhibition can significantly blunt the immediate hyperemic and vasodilator response following a single forearm contraction (6). Moreover, when NOS inhibition is performed along with inhibition of K+-mediated vascular hyperpolarization and prostaglandin synthesis the rapid vasodilator response to a single muscle contraction is nearly abolished (11). Taken together these results suggest that NO contributes to the increase in blood flow as early as the first contraction during exercise. However, initial studies related to the time course of hyperemia and vasodilation in humans during repeated contractions indicated that NO was not obligatory to the onset of the hyperemic response during mild-intensity (∼10% MVC) forearm exercise (43). It should be noted that the NOS inhibition trials (in the previous report) were made in conjunction with muscarinic receptor blockade (atropine). Therefore, it is unclear whether the blood flow responses to exercise during NOS inhibition were affected by previous administration of atropine. In this context we previously found that the onset and timing of vasodilation during rhythmic moderate-intensity forearm exercise is reduced during single inhibition trials of NOS in young males (5). More recent evidence has also highlighted the importance of NO, as well as prostaglandins, for the regulation of blood flow at the onset and over the first 2 min of dynamic leg exercise (9). The results obtained from the modeled data sets used to quantify and characterize the hyperemic and vasodilator kinetics in the current study clearly suggest a role for NO in the timing of the exercise response across a range of contraction intensities and are in agreement with the aforementioned studies despite different analytical approaches (5, 9).
The impaired (relative to young adults) and lack of change in the on-kinetic parameters with NOS inhibition observed in the older adults of the current study (Fig. 1) likely suggest a reduced role of NO in the onset of hyperemia and vasodilation, which might be due to less bioavailable NO in older adults. Although the exact mechanism for a reduced NO bioavailability with aging is unknown, an enhanced scavenging of NO via reactive oxygen species could possibly contribute to some extent. Along these lines, acute administration of antioxidants improves endothelium-dependent vasodilation under resting conditions in older adults (44, 51). Moreover and of particular interest to the current findings, intra-arterial infusions of ascorbic acid can increase muscle blood flow during dynamic forearm exercise in older adults (28), which is mediated primarily via an increase in the bioavailability of NO derived from the NOS pathway (12). However, the effect of ascorbic acid on muscle blood flow in the aforementioned studies was examined only after steady-state exercise was achieved. Therefore, it is unclear if and to what extent scavenging of NO via reactive oxygen species contributes to the age-related changes in hyperemic and vasodilator kinetics. To this point, administration of ascorbic acid failed to improve the rapid hyperemic and vasodilator response to single muscle contractions in older adults (28), thus suggesting scavenging of NO via reactive oxygen species may not have a major role in the age-related impairments in vasodilation at the very onset of exercise. A more comprehensive series of studies is needed to determine if the slower vasodilator kinetics with aging, presumably via altered NO signaling, are due to oxidative stress-related mechanisms.
The prolonged and attenuated blood flow and vasodilator kinetics with aging may also be a result of greater sympathetic restraint of the vasculature in exercising muscles of the older adults. In young adults, sympathetic vasoconstrictor responses are blunted in the vascular beds of contracting limbs (i.e., functional sympatholysis) (50), which has been reported to occur early in exercise (13, 49). Conversely, functional sympatholysis during steady-state exercise has been shown to be diminished with aging (16, 27, 33). The precise local factor(s) responsible and/or mechanism of functional sympatholysis are a topic of debate (39). However, there is some evidence from experimental animals and humans that suggest a role of NO in the inhibition of sympathetic vasoconstriction in contracting skeletal muscle (8, 46, 47). If NO indeed is a mediator of functional sympatholysis, then a reduced bioavailable NO in older adults may not only attenuate direct vasodilation within the contracting skeletal muscle, but also limit the ability to blunt sympathetic vasoconstriction.
Although the results from the l-NMMA trials in the present study suggest a reduction in bioavailable NO contributes to the impaired blood flow and vasodilator kinetics in older adults, other factors are likely involved in the reported age-related differences. Along these lines, the older adults also demonstrated a blunted vasodilator response to intra-arterial infusions of NTP. This would indicate that attenuated vascular smooth muscle responsiveness to NO may explain, at least to some extent, the age-related alterations in vasodilator kinetics during rhythmic forearm exercise.
Role of NO in the Blood Flow Responses Following Exercise
The current study also examined the impact of aging on recovery blood flow following exercise as well as the role of NO in the response. In general the magnitude and duration of postexercise muscle hyperemia are dependent on the type, intensity, and duration of the previous exercise. In young adults, blood flow during the recovery from exercise is markedly reduced during NOS inhibition (37, 43). Our results are in agreement with these aforementioned studies, in that there was a reduction in the total postexercise FBF and FVC as well a faster 50% decay rate following moderate-intensity forearm exercise during the l-NMMA trials (Fig. 4). When only comparing control conditions, older adults demonstrated a lower total postexercise FBF and FVC (Fig. 4, A and B) and faster rate of decay following moderate-intensity forearm contractions than their younger counterparts. This was likely due in part to the significantly higher steady-state FBF and FVC values during exercise in the young adults. Interestingly, the effect of NOS inhibition in reducing the total postexercise FBF and FVC was similar between young and older adults following moderate-intensity exercise. This was somewhat surprising to us since 1) our current results and those previously reported in young adults (37, 43) suggest that NO contributes to the magnitude of postexercise blood flow and 2) human aging is associated with a reduction in NO bioavailability. In this context we thought NOS inhibition would have less of an impact on muscle blood flow during recovery in older adults. However, we remain puzzled by these findings.
Experimental Considerations
The experimental design employed in the current study used relative (%MVC) workloads to compare the hyperemic and vasodilator kinetics during forearm exercise between young and older adults. Although the MVC and thus relative workload used did not significantly differ between age groups (P = 0.22–0.28), the young adults did demonstrate an ∼20% greater MVC (41 ± 5 vs 34 ± 4 kg). Therefore, it could be argued that the altered kinetic profile observed in the older adults is due to a lower absolute workload. To address this potential confound, we compared the hyperemic and vasodilator kinetic responses in a subset of young and older participants (n = 7 for each age group) to better match the groups for MVC and absolute workload across the three exercise trials. This approach effectively reduced the difference in MVC (35 ± 5 vs 34 ± 3 kg, P = 0.94) between the young and older groups and thus made the mean absolute workloads (∼3.4, 6.9, and 10.4 kg) used at each exercise intensity (10%, 20% and 30%, respectively) nearly identical between age groups.
Results from the subset analysis indicate that when matched for absolute workload the amplitude of the hyperemic and vasodilator response was not different between age groups at any level of exercise (amplitudes of the FVC responses are shown in Fig. 5A). These results would indicate that the magnitude of change in FBF and FVC for a given absolute workload is not impaired with aging and are in agreement with previous studies that have demonstrated similar hyperemic and vasodilator responses during steady-state forearm exercise in young and older adults when performed at identical workloads (17, 30). In addition to a lack of age-related differences in the amplitude parameter, the subanalysis also revealed that there was no effect of l-NMMA in either age group when compared within similar absolute workloads. Previous findings (as well as the data related to relative exercise intensity presented in this study) have demonstrated that NOS inhibition can result in modest reductions in steady-state FBF and FVC in young adults (19, 22, 42) but this effect is minimal in older adults (12, 41, 48), thus suggesting that NO-mediated vasodilation is impaired during rhythmic handgrip exercise with aging. Although the reason for the disparity between the results from the subset of subjects in the current study and those previously reported are not completely clear it may be due to the small number of subjects included in the subanalysis and thus of insufficient power to detect such differences. In contrast to the amplitude data, the timing (tau) of the hyperemic and vasodilator response was still slower in older adults after matching subjects for absolute workload (τFVC is shown in Fig. 5B). Taken together with the results derived from the relative workloads comparisons, our data suggest that aging slows (i.e., prolongs the tau parameter during on-transient kinetics) the hyperemic and vasodilator response to forearm exercise despite the magnitude of the response being similar between age groups.
Fig. 5.
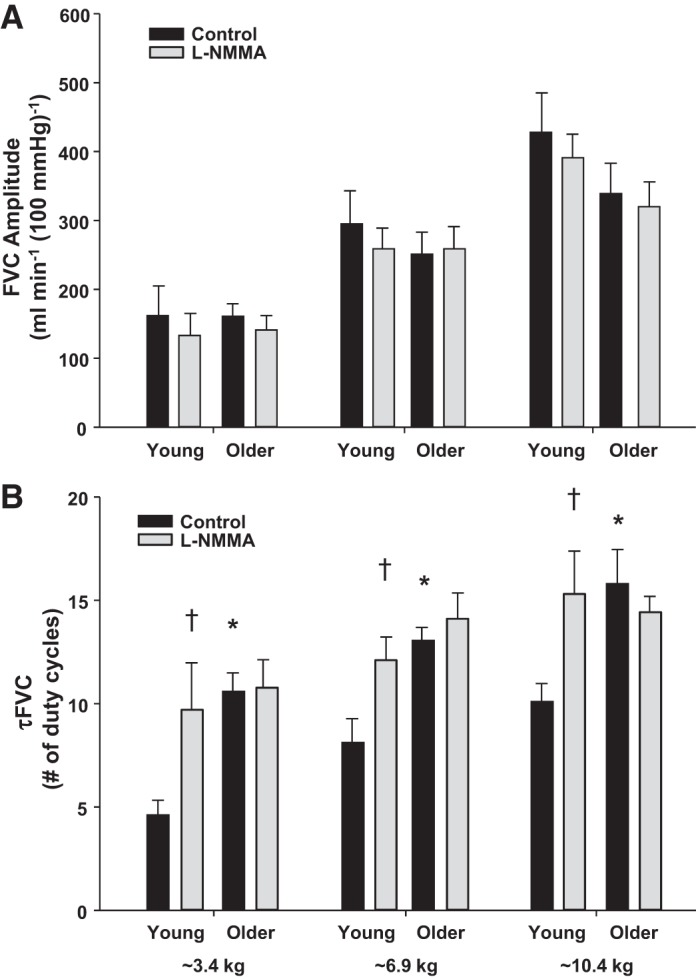
FVC amplitude (A) and τFVC (B) during dynamic forearm exercise at three separate absolute workloads (∼3.4, 6.9, and 10.4 kg) in a subset of young and older adults (n = 7 for each) under control (saline) and NOS inhibition (l-NMMA) conditions. The amplitude of the vasodilator response did not differ between age groups at any level of exercise. The vasodilator responses were slower (prolonged τFVC) in older adults at all exercise intensities. Additionally, NOS inhibition slowed τFVC in the young adults. *P < 0.05 vs. young, †P < 0.05 vs. control.
In the present study, continuous blood velocity and steady-state brachial artery diameter measurements were used to calculate the FBF and FVC for each duty cycle during each exercise trial. This approach could have resulted in an overestimation of the FBF and FVC values during the initial duty cycles. However, since the change in brachial artery diameter was similar between drug conditions and age groups within each exercise intensity (data not shown), it is likely that any overestimation would have been similar across trials and not influence the reported age and drug condition differences. Additionally our approach assumes that the onset of vasodilation at the measured conduit (i.e., brachial) artery level is similar to that in the microcirculation. Previous evidence suggests that the kinetics of flow at the capillary level is slower than those measured in the femoral artery during knee extension exercise (23). However, these conclusions were based on direct measurements of femoral blood velocity, whereas capillary flow was estimated. Interestingly, the time course of rapid vasodilation within the microcirculation itself appears to be different (38).
Last, reductions in resting FBF and FVC due to l-NMMA were observed in both young and older adults. It is possible that the shift in baseline conditions could confound the calculated vasodilator kinetic profiles during exercise. However, the monoexponential model used to calculate Amp and τ during on-kinetics takes into account the starting (baseline) value. Moreover, we previously demonstrated that the onset of vasodilation (expressed as the slope of the exercise response between rest and steady-state exercise during dynamic forearm exercise) was blunted during concurrent administration of l-NMMA and aminophylline (adenosine receptor antagonist) compared with control (saline) conditions despite statistically similar resting FBF and FVC values (5).
Conclusions
To our knowledge, this study is the first to quantify and characterize the influence of aging in the hyperemic and vasodilator kinetics during exercise. Our data suggest that the amplitude of the hyperemic and vasodilator responses were attenuated in older adults at the higher relative exercise intensity (30% MVC). However, these age-related differences no longer existed when comparing the amplitude at similar absolute workloads. Thus there do not appear to be age-related differences in the amplitude of the hyperemic or vasodilator response in the forearm when matched for workload. On the other hand, the speed of skeletal muscle blood flow and vasodilation during rhythmic forearm exercise at the same relative and absolute intensity are slower in otherwise healthy aging humans. Moreover these age-related differences in the kinetic profile (i.e., timing) appear to be due in part to a decreased NO bioavailability in older adults. Taken together with our previous data (6), aging is associated with impairments in the regulation of skeletal muscle blood flow during dynamic exercise starting with the initial contraction and continuing until steady-state levels are achieved.
GRANTS
This research was supported by National Institutes of Health Research Grants HL-105467 (to D. P. Casey) and HL-46493 and HL-119337 (to M. J. Joyner) and by CTSA UL1 TR000135. The Caywood Professorship via the Mayo Foundation also supported this research.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.P.C. and M.J.J. conception and design of research; D.P.C., S.M.R., and M.J.J. performed experiments; D.P.C. and S.M.R. analyzed data; D.P.C. and M.J.J. interpreted results of experiments; D.P.C. prepared figures; D.P.C. drafted manuscript; D.P.C., S.M.R., and M.J.J. edited and revised manuscript; D.P.C., S.M.R., and M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We are grateful to the study volunteers for participation. We thank Dr. R. Carter for statistical consultation during the review process. We also thank B. Walker, J. Taylor, P. Engrav, S. Roberts, S. Wolhart, and D. Treichler for technical assistance.
REFERENCES
- 1.Behnke BJ, Delp MD. Aging blunts the dynamics of vasodilation in isolated skeletal muscle resistance vessels. J Appl Physiol 108: 14–20, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behnke BJ, Delp MD, Dougherty PJ, Musch TI, Poole DC. Effects of aging on microvascular oxygen pressures in rat skeletal muscle. Respir Physiol Neurobiol 146: 259–268, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Brock RW, Tschakovsky ME, Shoemaker JK, Halliwill JR, Joyner MJ, Hughson RL. Effects of acetylcholine and nitric oxide on forearm blood flow at rest and after a single muscle contraction. J Appl Physiol 85: 2249–2254, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Casey DP, Joyner MJ. Influence of alpha-adrenergic vasoconstriction on the blunted skeletal muscle contraction-induced rapid vasodilation with aging. J Appl Physiol 113: 1201–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casey DP, Mohamed EA, Joyner MJ. Role of nitric oxide and adenosine in the onset of vasodilation during dynamic forearm exercise. Eur J Appl Physiol 113: 295–303, 2013. [DOI] [PubMed] [Google Scholar]
- 6.Casey DP, Walker BG, Ranadive SM, Taylor JL, Joyner MJ. Contribution of nitric oxide in the contraction-induced rapid vasodilation in young and older adults. J Appl Physiol 115: 446–455, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 8.Chavoshan B, Sander M, Sybert TE, Hansen J, Victor RG, Thomas GD. Nitric oxide-dependent modulation of sympathetic neural control of oxygenation in exercising human skeletal muscle. J Physiol 540: 377–386, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen PM, Nyberg M, Mortensen SP, Nielsen JJ, Secher NH, Damsgaard R, Hellsten Y, Bangsbo J. Leg oxygen uptake in the initial phase of intense exercise is slowed by a marked reduction in oxygen delivery. Am J Physiol Regul Integr Comp Physiol 305: R313–R321, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Clifford PS, Hellsten Y. Vasodilatory mechanisms in contracting skeletal muscle. J Appl Physiol 97: 393–403, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Crecelius AR, Kirby BS, Luckasen GJ, Larson DG, Dinenno FA. Mechanisms of rapid vasodilation after a brief contraction in human skeletal muscle. Am J Physiol Heart Circ Physiol 305: H29–H40, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeLorey DS, Wang SS, Shoemaker JK. Evidence for sympatholysis at the onset of forearm exercise. J Appl Physiol 93: 555–560, 2002. [DOI] [PubMed] [Google Scholar]
- 14.DeSouza CA, Clevenger CM, Greiner JJ, Smith DT, Hoetzer GL, Shapiro LF, Stauffer BL. Evidence for agonist-specific endothelial vasodilator dysfunction with ageing in healthy humans. J Physiol 542: 255–262, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dietz NM, Rivera JM, Eggener SE, Fix RT, Warner DO, Joyner MJ. Nitric oxide contributes to the rise in forearm blood flow during mental stress in humans. J Physiol 480: 361–368, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dinenno FA, Masuki S, Joyner MJ. Impaired modulation of sympathetic alpha-adrenergic vasoconstriction in contracting forearm muscle of ageing men. J Physiol 567: 311–321, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol 290: H272–H278, 2006. [DOI] [PubMed] [Google Scholar]
- 18.duManoir GR, DeLorey DS, Kowalchuk JM, Paterson DH. Differences in exercise limb blood flow and muscle deoxygenation with age: contributions to O2 uptake kinetics. Eur J Appl Physiol 110: 739–751, 2010. [DOI] [PubMed] [Google Scholar]
- 19.Dyke CK, Proctor DN, Dietz NM, Joyner MJ. Role of nitric oxide in exercise hyperaemia during prolonged rhythmic handgripping in humans. J Physiol 488: 259–265, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol 81: 1807–1814, 1996. [DOI] [PubMed] [Google Scholar]
- 21.Frandsenn U, Bangsbo J, Sander M, Hoffner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with N(G)-nitro-l-arginine methyl ester in humans. J Physiol 531: 257–264, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilligan DM, Panza JA, Kilcoyne CM, Waclawiw MA, Casino PR, Quyyumi AA. Contribution of endothelium-derived nitric oxide to exercise-induced vasodilation. Circulation 90: 2853–2858, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Harper AJ, Ferreira LF, Lutjemeier BJ, Townsend DK, Barstow TJ. Human femoral artery and estimated muscle capillary blood flow kinetics following the onset of exercise. Exp Physiol 91: 661–671, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, Knuuti J, Kalliokoski K, Hellsten Y. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am J Physiol Heart Circ Physiol 300: H1510–H1517, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Jasperse JL, Seals DR, Callister R. Active forearm blood flow adjustments to handgrip exercise in young and older healthy men. J Physiol 474: 353–360, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Modulation of postjunctional alpha-adrenergic vasoconstriction during exercise and exogenous ATP infusions in ageing humans. J Physiol 589: 2641–2653, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirby BS, Voyles WF, Simpson CB, Carlson RE, Schrage WG, Dinenno FA. Endothelium-dependent vasodilatation and exercise hyperaemia in ageing humans: impact of acute ascorbic acid administration. J Physiol 587: 1989–2003, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrenson L, Poole JG, Kim J, Brown C, Patel P, Richardson RS. Vascular and metabolic response to isolated small muscle mass exercise: effect of age. Am J Physiol Heart Circ Physiol 285: H1023–H1031, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Limberg JK, Evans TD, Pegelow DF, Eldridge MW, Sebranek JJ, Proctor LT, Schrage WG. Heterogeneous vascular responses to hypoxic forearm exercise in young and older adults. Eur J Appl Physiol 112: 3087–3095, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin EA, Nicholson WT, Eisenach JH, Charkoudian N, Joyner MJ. Bimodal distribution of vasodilator responsiveness to adenosine due to difference in nitric oxide contribution: implications for exercise hyperemia. J Appl Physiol 101: 492–499, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 33.Mortensen SP, Nyberg M, Winding K, Saltin B. Lifelong physical activity preserves functional sympatholysis and purinergic signalling in the ageing human leg. J Physiol 590: 6227–6236, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poole JG, Lawrenson L, Kim J, Brown C, Richardson RS. Vascular and metabolic response to cycle exercise in sedentary humans: effect of age. Am J Physiol Heart Circ Physiol 284: H1251–H1259, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl Physiol 95: 1963–1970, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Proctor DN, Shen PH, Dietz NM, Eickhoff TJ, Lawler LA, Ebersold EJ, Loeffler DL, Joyner MJ. Reduced leg blood flow during dynamic exercise in older endurance-trained men. J Appl Physiol 85: 68–75, 1998. [DOI] [PubMed] [Google Scholar]
- 37.Radegran G, Saltin B. Nitric oxide in the regulation of vasomotor tone in human skeletal muscle. Am J Physiol Heart Circ Physiol 276: H1951–H1960, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Roseguini BT, Davis MJ, Harold Laughlin M. Rapid vasodilation in isolated skeletal muscle arterioles: impact of branch order. Microcirculation 17: 83–93, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saltin B, Mortensen SP. Inefficient functional sympatholysis is an overlooked cause of malperfusion in contracting skeletal muscle. J Physiol 590: 6269–6275, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saltin B, Radegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand 162: 421–436, 1998. [DOI] [PubMed] [Google Scholar]
- 41.Schrage WG, Eisenach JH, Joyner MJ. Ageing reduces nitric-oxide- and prostaglandin-mediated vasodilatation in exercising humans. J Physiol 579: 227–236, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrage WG, Joyner MJ, Dinenno FA. Local inhibition of nitric oxide and prostaglandins independently reduces forearm exercise hyperaemia in humans. J Physiol 557: 599–611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shoemaker JK, Halliwill JR, Hughson RL, Joyner MJ. Contributions of acetylcholine and nitric oxide to forearm blood flow at exercise onset and recovery. Am J Physiol Heart Circ Physiol 273: H2388–H2395, 1997. [DOI] [PubMed] [Google Scholar]
- 44.Taddei S, Virdis A, Ghiadoni L, Salvetti G, Bernini G, Magagna A, Salvetti A. Age-related reduction of NO availability and oxidative stress in humans. Hypertension 38: 274–279, 2001. [DOI] [PubMed] [Google Scholar]
- 45.Taddei S, Virdis A, Mattei P, Ghiadoni L, Gennari A, Fasolo CB, Sudano I, Salvetti A. Aging and endothelial function in normotensive subjects and patients with essential hypertension. Circulation 91: 1981–1987, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Thomas GD, Sander M, Lau KS, Huang PL, Stull JT, Victor RG. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc Natl Acad Sci USA 95: 15090–15095, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas GD, Victor RG. Nitric oxide mediates contraction-induced attenuation of sympathetic vasoconstriction in rat skeletal muscle. J Physiol 506: 817–826, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Trinity JD, Wray DW, Witman MA, Layec G, Barrett-O'Keefe Z, Ives SJ, Conklin JD, Reese V, Richardson RS. Contribution of nitric oxide to brachial artery vasodilation during progressive handgrip exercise in the elderly. Am J Physiol Regul Integr Comp Physiol 305: R893–R899, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tschakovsky ME, Hughson RL. Rapid blunting of sympathetic vasoconstriction in the human forearm at the onset of exercise. J Appl Physiol 94: 1785–1792, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Tschakovsky ME, Sujirattanawimol K, Ruble SB, Valic Z, Joyner MJ. Is sympathetic neural vasoconstriction blunted in the vascular bed of exercising human muscle? J Physiol 541: 623–635, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wray DW, Nishiyama SK, Harris RA, Zhao J, McDaniel J, Fjeldstad AS, Witman MA, Ives SJ, Barrett-O'Keefe Z, Richardson RS. Acute reversal of endothelial dysfunction in the elderly after antioxidant consumption. Hypertension 59: 818–824, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


