Abstract
Previous work in intact awake and sleeping goats has found that unilateral blockade of excitatory inputs in the ventral respiratory column (VRC) elicits changes in the concentrations of multiple neurochemicals, including serotonin (5-HT), substance P, glycine, and GABA, while increasing or having no effect on breathing. These findings are consistent with the concept of interdependence between neuromodulators, whereby attenuation of one modulator elicits compensatory changes in other modulators to maintain breathing. Because there is a large degree of redundancy and multiplicity of excitatory inputs to the VRC, we herein tested the hypothesis that combined unilateral blockade of muscarinic acetylcholine (mACh), neurokinin-1 (NK1, the receptor for substance P), and 5-HT2A receptors would elicit changes in multiple neurochemicals, but would not change breathing. We unilaterally reverse-dialyzed a cocktail of antagonists targeting these receptors into the VRC of intact adult goats. Breathing was continuously monitored while effluent fluid from dialysis was collected for quantification of neurochemicals. We found that neither double blockade of mACh and NK1 receptors, nor triple blockade of mACh, NK1, and 5-HT2A receptors significantly affected breathing (P ≥ 0.05) in goats that were awake or in non-rapid eye movement (NREM) sleep. However, both double and triple blockade increased the effluent concentration of substance P (P < 0.001) and decreased GABA concentrations. These findings support our hypothesis and, together with past data, suggest that both in wakefulness and NREM sleep, multiple neuromodulator systems collaborate to stabilize breathing when a deficit in one or multiple excitatory neuromodulators exists.
Keywords: neuromodulation, muscarinic receptor, neurokinin-1 receptor, 5-HT2A receptor
the control of breathing is dependent upon the balance of excitatory and inhibitory neuromodulators acting on their respective receptors at multiple sites in the brainstem (10, 40). Currently, the mechanisms underlying the orchestrated release of multiple neuromodulators, and whether or how each modulator can affect the release of other neurochemicals, are not fully understood. These topics continue to be of fundamental biological and clinical interest.
Acetylcholine (ACh), substance P (SP), and serotonin (5-HT) are neurochemicals previously shown to have excitatory effects on the central control of breathing in multiple models utilizing reduced preparations and whole animals (3, 5, 11, 16, 17, 28, 32, 34, 35, 42, 46). For example, in brainstem slices containing the pre-Bötzinger Complex (preBötC), a site critical for the generation of inspiratory rhythm (36), application of exogenous ACh or the cholinergic agonists carbachol and muscarine depolarizes inspiratory neurons, induces a long-lasting inward current, and increases respiratory frequency (34). These effects were blocked by atropine (ATR), a nonselective muscarinic ACh (mACh) receptor antagonist (34). Likewise, the importance of SP neuromodulation has been demonstrated in rats and goats, wherein neurons expressing the neurokinin-1 receptor (an SP receptor) were selectively destroyed (16, 28, 46), resulting in abnormal breathing patterns and a reduced ventilatory CO2 chemoreflex. Additionally, work in slice preparations has shown that co-localized 5-HT and SP, presumably from the raphé nucleus, provide tonic excitation to preBötC neurons via 5-HT2 receptors and NK1 receptors, respectively (11, 32). In agreement with this evidence, elimination of central serotonergic neurons (and thus all co-expressed neuropeptides) results in severe apnea, hypoventilation, and high mortality rates in transgenic neonatal mice (17), presumably in part through reduced neuromodulation in the respiratory network.
Despite a large body of work demonstrating the importance of ACh, 5-HT, and SP as sources of excitation for respiratory output, studies in our laboratory on intact goats while they were awake and during sleep have found that unilateral dialysis of antagonists of mACh receptors or NK1 receptors in the ventral respiratory column (VRC) either increase or do not change breathing (25–27). However, in these studies, we found that receptor antagonism alters the concentration of one or more neuromodulators in the effluent mock cerebrospinal fluid (mCSF). Compensation can be defined as a means of counteracting variation or neutralizing the effect of variations. Thus we hypothesize that these alterations in neurochemical levels likely compensated for the disruption in excitatory transmission in the VRC during receptor blockade, thereby preventing a decrease in breathing. These data support the concept of interdependence among neuromodulators, whereby attenuation of one source of excitation may be compensated for by other modulator systems to preserve a normal level of breathing. Therefore, in awake and sleep states, breathing may decrease only with attenuation of multiple (rather than individual) receptor types.
Herein, we first sought to determine the effects on breathing and effluent neurochemical concentrations of blockade of 5-HT2A receptors with the highly selective antagonist MDL 11939 (MDL) in the VRC of adult goats. On the basis of previous findings with unilateral dialysis of mACh receptor and NK1 receptor antagonists (25–27), we believed that a logical next step would be to further explore the concept of neuromodulator interdependence by testing the hypothesis that unilateral double blockade of mACh and NK1 receptors, or triple blockade of receptors of mACh, NK1, and 5-HT2A would not alter breathing, but would elicit compensatory changes in multiple neurochemicals. We also tested the hypothesis that there would be sleep state-dependent effects on breathing of dialysis of a triple cocktail of antagonists; thus these studies were also conducted at night while goats were awake and during natural non-rapid eye movement (NREM) sleep.
MATERIALS AND METHODS
Goats.
All animals studied herein (n = 13) were adult, nonpregnant, female goats with an average weight of 43.1 ± 2.5 kg. Animals were housed and studied in an environmental chamber with a 12:12-h light-dark cycle (lights-on at 6:00 A.M.). Goats were permitted ad libitum access to food and water, except during a 24-h fasting period immediately before all surgeries. Animals were acclimated to the environment for ≥3 days before any procedures were initiated. All aspects of the studies reported herein were reviewed and approved by the Medical College of Wisconsin's Institutional Animal Care and Use Committee before commencing any procedures or protocols.
Surgical procedures.
Before all surgeries, goats were anesthetized with ketamine (5.0 ml, 100 mg/ml, iv), intubated, and mechanically ventilated (2% isoflurane in a background of 100% oxygen). Flunixin meglumine (1 mg/kg, im), a nonsteroidal anti-inflammatory analgesic, was given once perioperatively. Throughout each surgery and immediately thereafter, rectal body temperature (Tr), heart rate, blood oxygen saturation, and respiratory rate were recorded at regular intervals. Ceftiofur sodium (4 mg/kg, im) was administered daily and all surgical sites were treated daily with triple antibiotic for ≥7 days after surgery. Buprenorphine hydrochloride (0.005 mg/kg, im) was given twice daily for 2 days postoperatively to alleviate pain. All surgeries were performed under sterile conditions.
Electroencephalographic (EEG) and electrooculographic (EOG) electrodes were implanted in the midline cranium and superior orbital ridge, respectively, for monitoring and scoring sleep state. Goats were allowed to recover for ≥14 days after instrumentation with EEG and EOG electrodes before undergoing craniotomy, in which stainless steel microtubules (MTs, 70.0 mm length, 1.27 mm OD, 0.84 mm ID) were chronically implanted (unilaterally or bilaterally) into medullary tissue targeting the preBötC. During these procedures, after an occipital craniotomy was created, the dura mater was excised, and the posterior cerebellum and dorsal medulla were exposed to visualize obex, which served as a physical landmark for implantation of MTs. As determined in previous studies (20, 47), the goat preBötC is approximately 2.5–3.5 mm rostral from obex, 4.0–5.0 mm lateral to midline, and 4.0–6.0 mm from the dorsal surface of the medulla (ventral to nucleus ambiguus). To avoid rupturing blood vessels on the medullary surface, the actual site of implantation in some goats was adjusted. MTs were permanently secured with dental acrylic. Stylets of the same length as the MTs were inserted into the MTs such that they did not protrude into or disrupt medullary tissue. The skin was then sutured closed and treated with triple antibiotic.
Physiological variables.
Animals were trained to become accustomed to all equipment and study protocols. To measure respiratory parameters, a custom-made plastic mask with a one-way breathing valve was secured to the goat's snout. Inspiratory and expiratory tubing were attached to either side of the valve. A pneumotachograph, attached in series to the inspiratory side of the breathing valve, was used to measure inspiratory flow (or minute ventilation, VI, liters/min), breathing frequency (breaths/min), and tidal volume (VT, liters/breath). Ventilatory parameters were continuously recorded on a Windaq data acquisition system for later offline analysis. For accurate calculation of ventilation and for assurance that the goats were in good health, a thermocouple was inserted into the rectum to record Tr at regular intervals throughout all studies. Tr remained within normal range (38.5–39.9°C) for the vast majority of studies. For night studies, data were collected in an identical manner, except EEG and EOG cables were connected to the animal's head to monitor sleep state.
In vivo dialysis.
A dialysis probe (70.0 mm shaft, 2.0 mm membrane, 0.5 mm membrane diameter, 20 kDa cutoff, 3 μl internal volume; Harvard Apparatus, Holliston, MA) was inserted unilaterally into one MT. The dimensions of the probe allowed only the membrane tip to penetrate the tissue. The vehicle solution for all studies was mCSF (in mM: 124 NaCl, 2.0 KCl, 2.0 MgCl2, 1.3 K2PO4, 2.0 CaCl2, 11 glucose, and 26 NaHCO3−, pH 7.32, in sterile distilled water) alone or with 1.25% DMSO (vol/vol), as appropriate. All antagonists were dissolved in mCSF or mCSF with DMSO. Solutions were warmed in a tonometer to 39°C and equilibrated with 6.4% carbon dioxide and 21% oxygen (nitrogen-balanced) before being loaded into a syringe and attached to a syringe pump (Harvard Apparatus). The syringe was connected to the probe via polypropylene tubing. Solutions were delivered at a rate of 25 μl/min. The length of the polypropylene tubing (150 or 180 cm) resulted in an approximate delay of 20 min between initiation of dialysis and when the solution in the syringe reached the probe.
Each dialysis study proceeded in the following order: a dialysis probe was inserted unilaterally into one MT and the animal was given 30 min to stabilize; predialysis data were collected for 30 min; mCSF was dialyzed for 60 min (hour 1); antagonist solution was dialyzed for 60 min (hour 2); and mCSF was dialyzed for 60 min (hour 3). In time-control studies, mCSF was dialyzed during hour 2. The dialysate (effluent flow from the probe after solute exchange at the probe tip) was collected at the end of each hour of dialysis in modified cryotubes, aliquoted, and then frozen at −80°C for later analysis of neurochemical content.
The following antagonists were used: ATR (Sigma-Aldrich, St. Louis, MO), a nonselective mACh receptor antagonist; Spantide I (SPA, U.S. Biological), an NK1 receptor antagonist; and MDL (Santa Cruz Biotechnology, Santa Cruz, CA), a 5-HT2 receptor antagonist with greater selectivity for the 5-HT2A receptor over the 5-HT2C receptor. The following groups of animals were studied (goat numbers listed according to the inset table in Fig. 1), differing only in the solution dialyzed during hour 2 and day/night condition: Group 1, day 0.5 mM MDL in 1.25% DMSO (n = 6, goats 1, 2, 3, 7, 8, and 10); Group 2, day double cocktail (5 mM ATR and 267 μM SPA, n = 5, goats 1 and 7–10); Group 3, day triple cocktail (5 mM ATR, 500 μM SPA, and 0.5 mM MDL in 1.25% DMSO, n = 6, goats 2–6 and 11); and Group 4, night triple cocktail (5 mM ATR, 500 μM SPA, and 0.5 mM MDL in 1.25% DMSO, n = 6, goats 2–6 and 11). Goats 12 and 13 were studied, but their data were not included in group means or statistical analyses. In general, daytime dialysis studies were completed between 9:00 A.M. and 2:00 P.M., whereas night studies were completed between 8:00 P.M. and 2:00 A.M. For any individual animal, ≥36 h separated all studies.
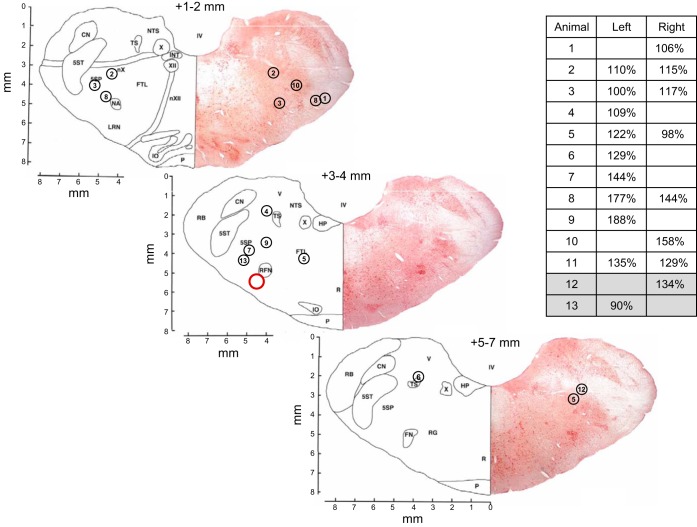
Fig. 1.
Microtubule (MT) placement (dialysis site) of each goat studied, excluding goats in time-control studies. Sections are taken from the goat brainstem atlas (7) and, for simplicity, MT placements are illustrated on three representative sections only. Red circle indicates approximate location of the goat pre-Bötzinger Complex (preBötC). Distance rostral from obex is indicated above each section. Circled numbers represent each goat studied and the left/right placement of the MTs in which studies were carried out. The corresponding N-methyl-d-aspartic acid (NMDA) injection (100 mM, 500 nl) response for each dialysis site, expressed as a percent of control, is also indicated (inset table, blank entries indicate sides not included in analysis). Gray shading (inset table) indicates animals anecdotally mentioned in the discussion but excluded from group means and statistical analyses. Tissue from goat 11 was not usable for Nissl staining and is not represented on the sections.
Mock CSF and N-methyl-d-aspartic acid injections.
Because application of glutamate receptor agonists to the preBötC elicits a tachypneic ventilatory response (20, 24, 37, 47), functional assays utilizing injections of mCSF and a glutamate receptor agonist into the MTs were performed to approximate MT placement relative to the preBötC. The injection tube was inserted only up to the distal end of the MT to avoid tissue damage. In these injection studies, breathing was measured as described above. After collection of control data (30 min), mCSF or N-methyl-d-aspartic acid (NMDA, 100 mM) was unilaterally injected (500 nl) into an MT. Injections were repeated on the contralateral MT after breathing returned to baseline levels.
Neurochemical analysis.
Neurochemical analyses were performed by a core assay laboratory using methods identical to those used by Muere et al. (25). These protocols are thus quoted from Muere et al. (25): “Glycine [glutamine, (GLN)] and γ-aminobutyric acid (GABA) were measured by reverse-phase high-performance liquid chromatography with fluorescent detection using the following parameters: Waters Resolve C18 column (150 × 3.9), a fluorescent detector with excitation at 229 nm and emission at 470 nm, β-alanine internal standard, and o-phthaldialdehyde derivitization. For detection and measurement of 5-HT, electrochemical detection with the same type of column was used, but with a potential setting of 0.6 volts vs. Ag/AgCl reference electrode and a N-methylserotonin internal standard. . . . A commercially available immunoassay (Assay Designs 900-018, range 9.76–10,000 pg/ml) was used to measure SP via a microplate reader at 405 nm.”
Data analysis.
Inspiratory flow was calibrated at the end of all experiments and data were analyzed as per past studies (25–27). Breath-by-breath analysis of ventilatory data was carried out using custom-made programs. For night studies, EEG and EOG activity was used to visually score each breath according to the awake, rapid-eye-movement (REM), or NREM sleep states. Scoring was performed by the same investigator for all studies. Although REM was frequently observed, there were not enough data for ventilatory analysis during the REM state.
As in past reports (26, 27), we compared data from this investigation to established time-control studies (25) in which mCSF was dialyzed in all 3 h. The present studies were carried out under the same conditions using identical methodologies as those in time-control studies. Accordingly, we utilized these control data for two important reasons: 1) to minimize the number of goats used because repeating control experiments may violate policies regarding using the smallest number of animals to address scientific questions; and 2) we believe that direct comparisons between time-control data and data from the studies herein are necessary for interpretation and for framing results in context with previous findings.
All ventilatory data were binned in 15-min bins. Two-way repeated-measures (RM) ANOVAs (one-factor repetition, group and time/bin as factors, Holm-Sidak test post hoc when appropriate) were performed for each ventilatory parameter. We thus used the P value of the interaction term from the results of two-way RM ANOVAs to determine whether the effect on breathing over time of antagonist dialysis was significantly different from that of time-control studies. When the P value of the interaction term was significant, we report results of the post hoc test. To minimize noise, we limited the period of analysis to the last nine bins of the study [i.e., the last 15-min bin in hour 1 (bin 1), the four bins in hour 2 (bins 2–5), and the four bins in hour 3 (bins 6–9)]. For night studies, two-way RM ANOVAs (two-factor repetition, state and time/bin as factors, Holm-Sidak post hoc test when appropriate) were also performed to determine whether there were state-dependent effects of antagonist dialysis on breathing. One animal did not have sufficient NREM sleep in hour 3 and was excluded from analysis by the statistical software; thus for ventilatory variables, the sample size for night studies was five.
As with the ventilatory data, for each group, two-way RM ANOVAs (one-factor repetition, group and hour as factors, Holm-Sidak test post hoc when appropriate) were performed for each neurochemical. We used the P value of the interaction term from the results of two-way RM ANOVAs to determine whether the effect on effluent neurochemical concentration over time of antagonist dialysis was significantly different from that of time-control studies. For night triple-cocktail studies, one-way RM ANOVAs (Holm-Sidak test post hoc when appropriate) were performed because time-control studies were not conducted at night. When the P value of the interaction term or one-way RM ANOVA was significant, we report results of the post hoc test.
For any animal, data from replicate studies were averaged before inclusion in group comparisons. All statistical analyses were performed using SigmaPlot (version 12.0 or 12.5) and all results are presented as means ± SE; values of P < 0.05 were considered statistically significant.
Histological analysis.
To determine MT placement, animals were sedated with ketamine (2.3 ml, 100 mg/ml, iv), cerebral circulation was isolated, and the goat was killed via injection of 10.0 ml (iv) of B-euthanasia. The cranial vasculature was perfused with PBS via a catheter placed in a carotid artery, followed by perfusion with 4% paraformaldehyde in PBS. The brainstem was harvested and processed for sectioning and Nissl-staining. Nissl-stained images were captured (scanned at 4,000 dots per inch; Nikon Super Coolscan 9000) and MT placements were approximated using Metamorph software. Because the MT was inserted from the dorsal surface, the distal extent of the MT tract from the dorsal surface was considered to be the dorsal coordinate of MT placement; the center of this tract was considered to be the medial-lateral coordinate. The rostral-caudal coordinate relative to obex was determined according to the tissue section on which the distal tip of the MT tract was identified.
RESULTS
Histological assessment and NMDA injection responses.
The preBötC in the goat is approximately 2.5–3.5 mm rostral from obex, 4.0–5.0 mm lateral from the midline, and 4.0–6.0 mm from the dorsal surface of the medulla, ventral to nucleus ambiguus (20, 47). Figure 1 is a simplified illustration of the MT placements of the goats studied herein (excluding those in time-control studies) and their respective responses to NMDA injections (100 mM, 500 nl, Fig. 1 inset table), which has previously been used as a physiological marker of the preBötC (26, 27). Goats 12 and 13 (gray shading, Fig. 1 inset table) are discussed here as examples of animals with unusual respiratory responses to cocktail dialysis (see Fig. 10 and discussion) but their data were excluded from group means and statistical analyses. In all of our microdialysis studies, there has been variation in MT placement and the corresponding NMDA responses (26, 27). In the present study, 11 of the 16 analyzed dialysis sites in Fig. 1 showed breathing frequency responses to NMDA injection of 15% or more, suggesting that the MT was within the preBötC region (26, 27). At five sites there was no or minimal response to NMDA injection and histological assessment suggested that the MTs were outside the targeted preBötC region. Because the injection tube was inserted only up to the distal end of the MT, a lack of response to NMDA was likely due to an extended distance between the injection site and the preBötC. However, during dialysis, the membrane tip of the probe extends 2 mm beyond the distal end of the MT, thus the diffusion distance of dialyzed antagonists was likely not identical to that of injected NMDA. Nevertheless, in this and our previous studies (25–27), the concentration of neuromodulators (specifically, SP) in the effluent mCSF has been remarkably consistent, irrespective of the NMDA response and histological identification of MT placement. Thus we have not excluded data from goats in which there was no or minimal response to NMDA. Finally, due to variation in the NMDA response and histological identification of MT placement, we refer to observed effects within the VRC and not specifically to the preBötC.
Fig. 10.
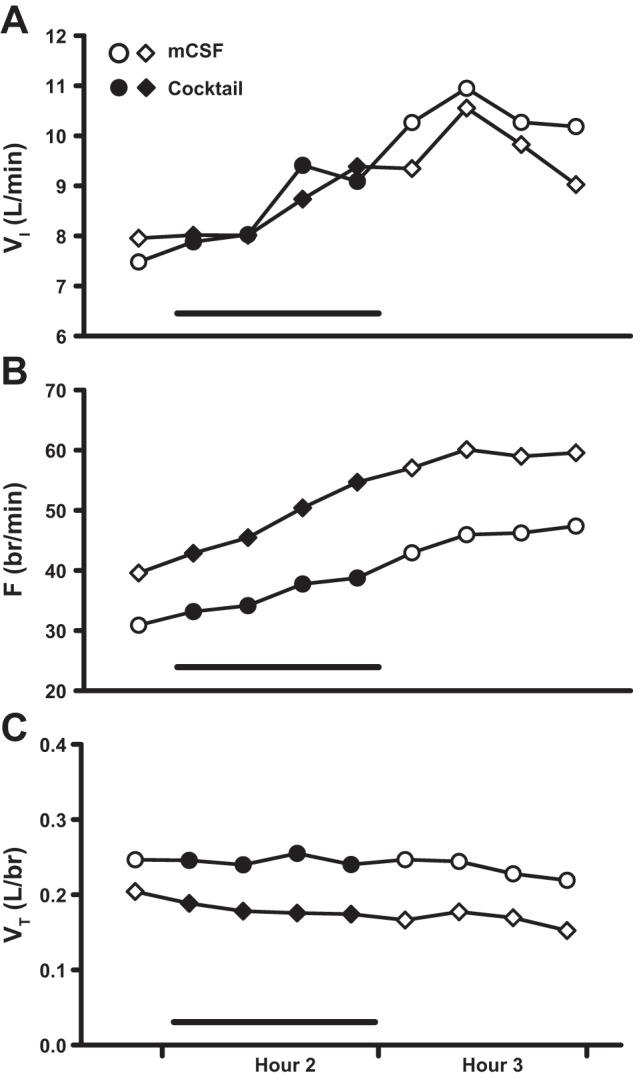
Examples of an abnormal respiratory response to daytime dialysis of a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL). These animals were not included in either the group means or statistical analyses. A: minute volume (VI, liters/min); B: frequency (F, breaths/min); C: tidal volume (VT, liters/breath). Diamonds (goat 12) and circles (goat 13) represent two different animals (see Fig. 1). The first data point represents the last 15-min bin of hour 1. Closed symbols and bar indicate period of cocktail dialysis; open symbols indicate mCSF dialysis.
Blockade of 5-HT2A receptors in the VRC has modest effects on breathing and neuromodulator concentration.
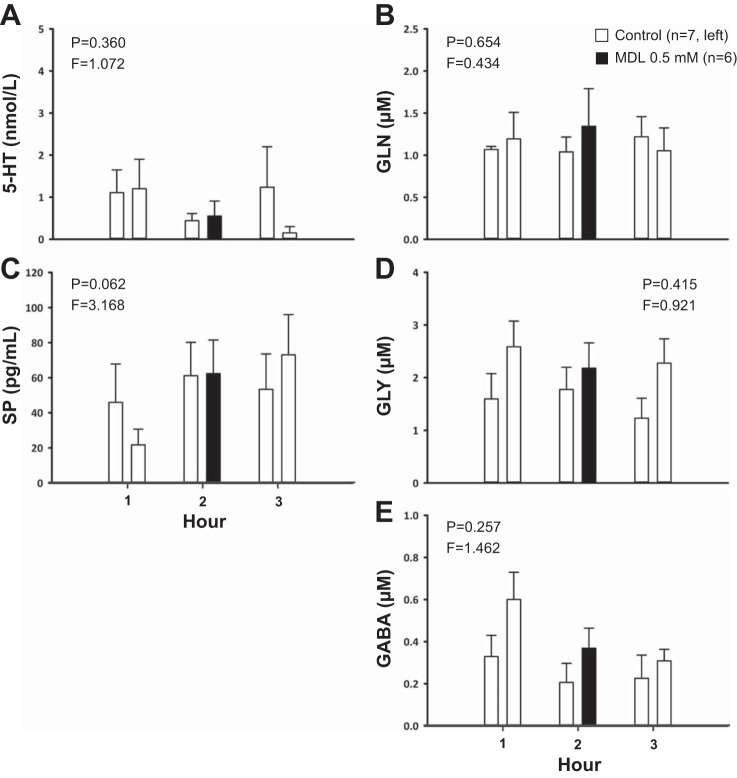
Comparisons between time-control studies (n = 7) and those of 0.5 mM MDL dialysis (n = 6) found a modestly significant interaction effect only for VI (P = 0.046, Fig. 2A), and for which post hoc analysis indicated a significant difference (P < 0.05) between groups in the third bin of hour 2. There were no significant interaction effects for any other ventilatory parameter (≥0.160, Fig. 2, B and C). Dialysis of MDL did not significantly affect the effluent concentration of any neuromodulator measured (P ≥ 0.062, Fig. 3, A–E) relative to the time-control studies.
Fig. 2.
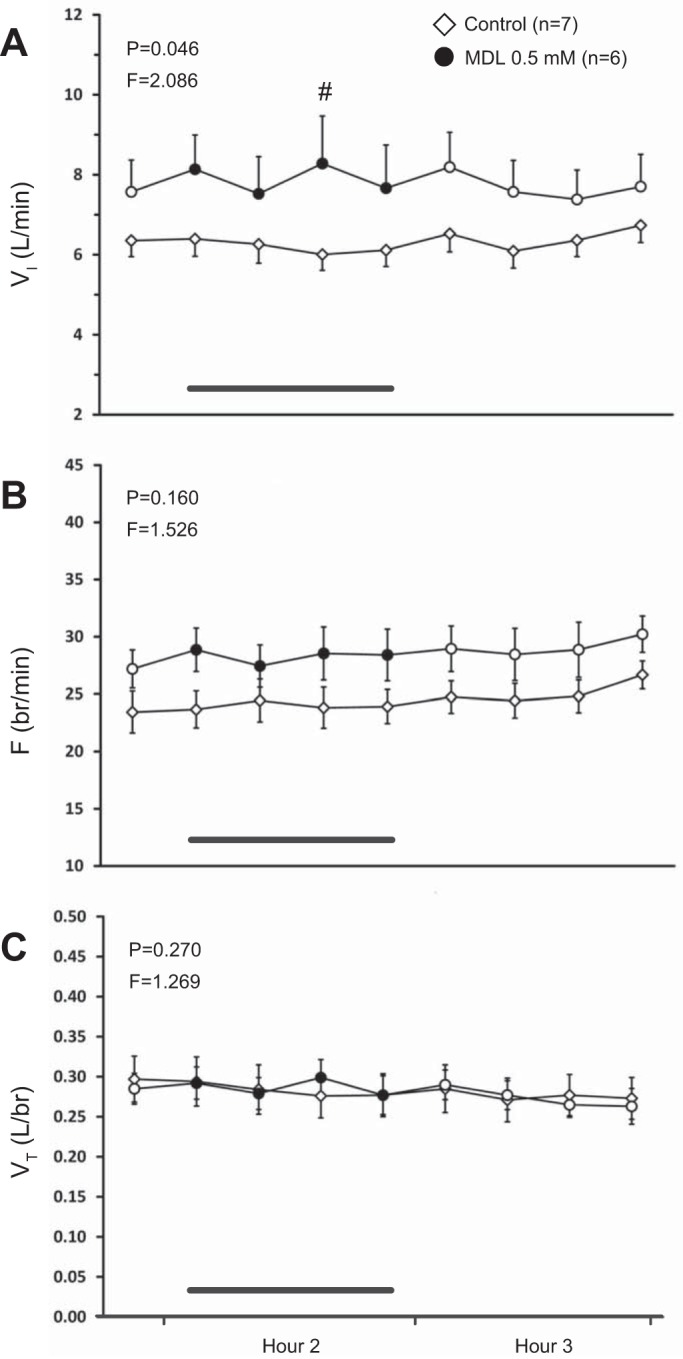
Dialysis of 0.5 mM of the antagonist MDL 11939 (MDL) (n = 6) in the ventral respiratory column (VRC) had no significant effect (P ≥ 0.160) on frequency (F, breaths/min, B) or tidal volume (VT, liters/breath, C) relative to time-control studies (n = 7). However, MDL had a modestly significant effect on minute ventilation (VI, liters/min, P = 0.046, A). The first data point represents the last 15-min bin of hour 1. Closed symbols and bar indicate period of MDL dialysis; open symbols indicate mock cerebral spinal fluid (mCSF) dialysis. F and P indicate values for interaction term from two-way repeated-measures (RM) ANOVA. #P ≤ 0.05 between groups per post hoc analysis.
Fig. 3.
Dialysis of 0.5 mM MDL (n = 6) in the VRC had no significant effect (P ≥ 0.062) on the effluent concentrations of serotonin (5-HT, A), glutamine (GLN, B), substance P (SP, C), glycine (D), or GABA (E) relative to time-control studies (n = 7). The x-axes indicate hour of dialysis; y-axes indicate effluent neurochemical concentration. Filled bars indicate dialysis of MDL; open bars indicate dialysis of mCSF. Bars on the left are data from time-control studies; bars on the right are data from MDL studies. F and P indicate values for interaction term from two-way RM ANOVA.
Effects on breathing and neuromodulator concentration of blockade of mACh, NK1, and 5-HT2A receptors.
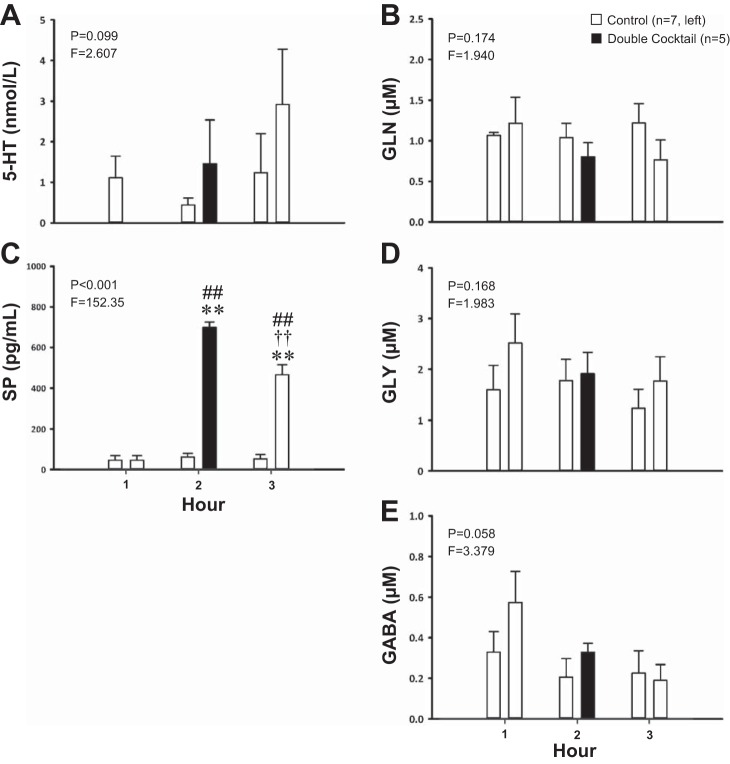
Combined blockade of mACh receptors and NK1 receptors in the VRC via dialysis of a double cocktail of antagonists (5 mM ATR and 267 μM SPA, n = 5) had no significant effect on any ventilatory parameter (P ≥ 0.084, Fig. 4, A–C) relative to the time-control studies. Double blockade of mACh receptors and NK1 receptors significantly increased the effluent concentration of SP (P < 0.001, Fig. 5C) when it was significantly elevated during hours 2 and 3 compared with both baseline and time-control values (P < 0.001) as determined by post hoc analysis. The double cocktail had no significant effect on the levels of any other neuromodulator (P ≥ 0.058; Fig. 5, A, B, D, and E).
Fig. 4.
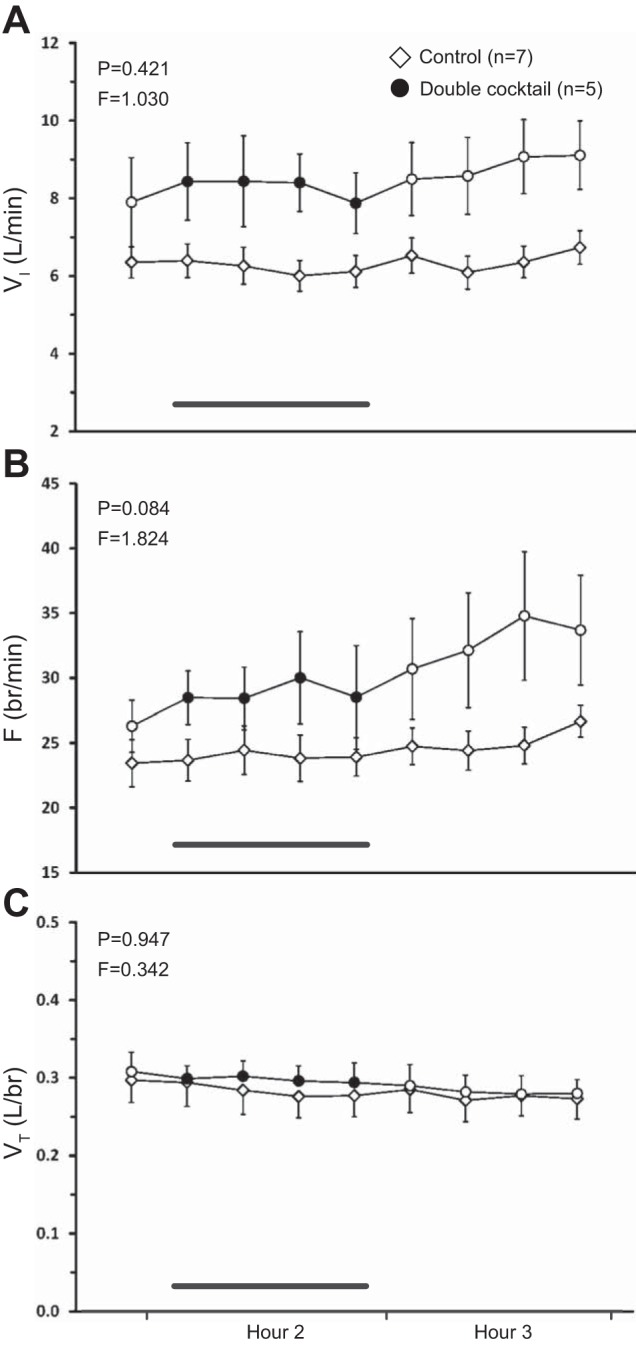
Dialysis of a double cocktail of antagonists [5 mM atropine (ATR), 267 μM Spantide I (SPA, an NK1 receptor antagonist), n = 5] in the VRC had no significant effect (P ≥ 0.084) on any respiratory parameter relative to time-control studies (n = 7). A: minute ventilation (VI, liters/min); B: frequency (F, breaths/min); C: tidal volume (VT, liters/breath). The first data point represents the last 15-min bin of hour 1. Closed symbols and bar indicate period of cocktail dialysis; open symbols indicate mCSF dialysis. F and P indicate values for interaction term from two-way repeated-measures ANOVA.
Fig. 5.
Dialysis of a double cocktail of antagonists (5 mM ATR, 267 μM SPA, n = 5) in the VRC significantly increased the effluent SP concentration (P < 0.001, C) relative to time-control studies (n = 7) but had no significant effect (P > 0.05) on serotonin (5-HT, A), glutamine (GLN, B), glycine (D), or GABA (E), although the effect on GABA was close to significant. The x-axes indicate hour of dialysis; y-axes indicate effluent neurochemical concentration. Filled bars indicate dialysis of cocktail; open bars indicate dialysis of mCSF. Bars on the left are data from time-control studies; bars on the right are data from cocktail studies. F and P indicate values for interaction term from two-way RM ANOVA. **P ≤ 0.001 vs. hour 1; ††P ≤ 0.001 vs. hour 2; ##P ≤ 0.001 vs. time-control value per post hoc analysis.
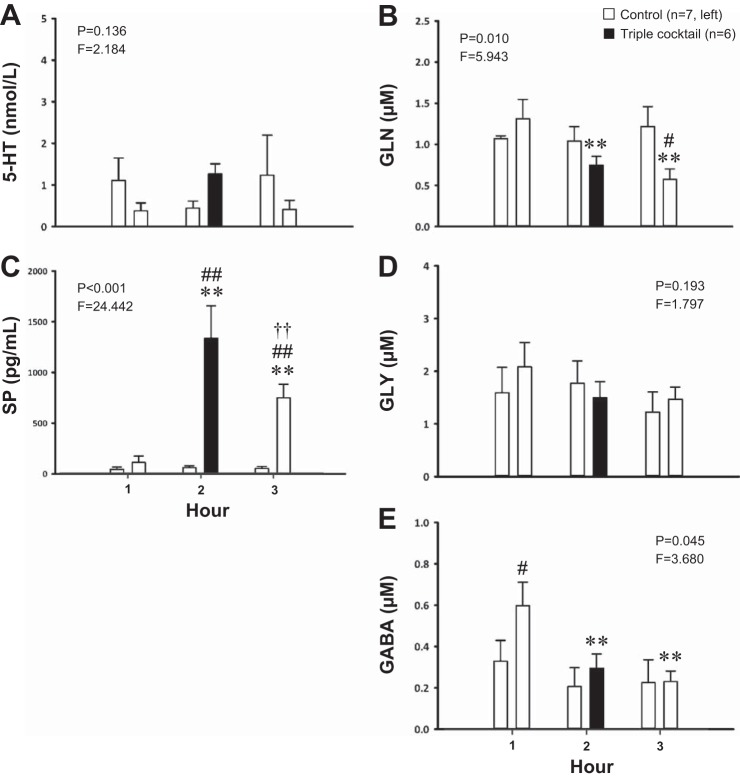
Similarly, blockade of receptors of mACh, NK1, and 5-HT2A with a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 6) during the day had no significant effect on any respiratory variable (P ≥ 0.509, Fig. 6, A–C). However, there was a significant increase in effluent SP concentration (P < 0.001, Fig. 7C). As with the double cocktail, post hoc analysis showed that SP remained significantly elevated during hours 2 and 3 with triple cocktail dialysis compared with both baseline and time-control values (P ≤ 0.001). There were significant decreases in effluent glutamine (P = 0.010, Fig. 7B) and GABA (P = 0.045, Fig. 7E) concentrations. Post hoc analysis indicated that glutamine decreased over time with triple cocktail dialysis (P ≤ 0.001) and was significantly different than time-control values in hour 3 (P < 0.05). Effluent GABA concentration also decreased over time (P ≤ 0.001) as revealed by post hoc testing, however, there was also a significant difference in baseline GABA concentration between the cocktail and time-control groups (P < 0.05). Blockade with a triple cocktail did not significantly affect the effluent concentrations of 5-HT or glycine (P ≥ 0.136, Fig. 7, A and D).
Fig. 6.
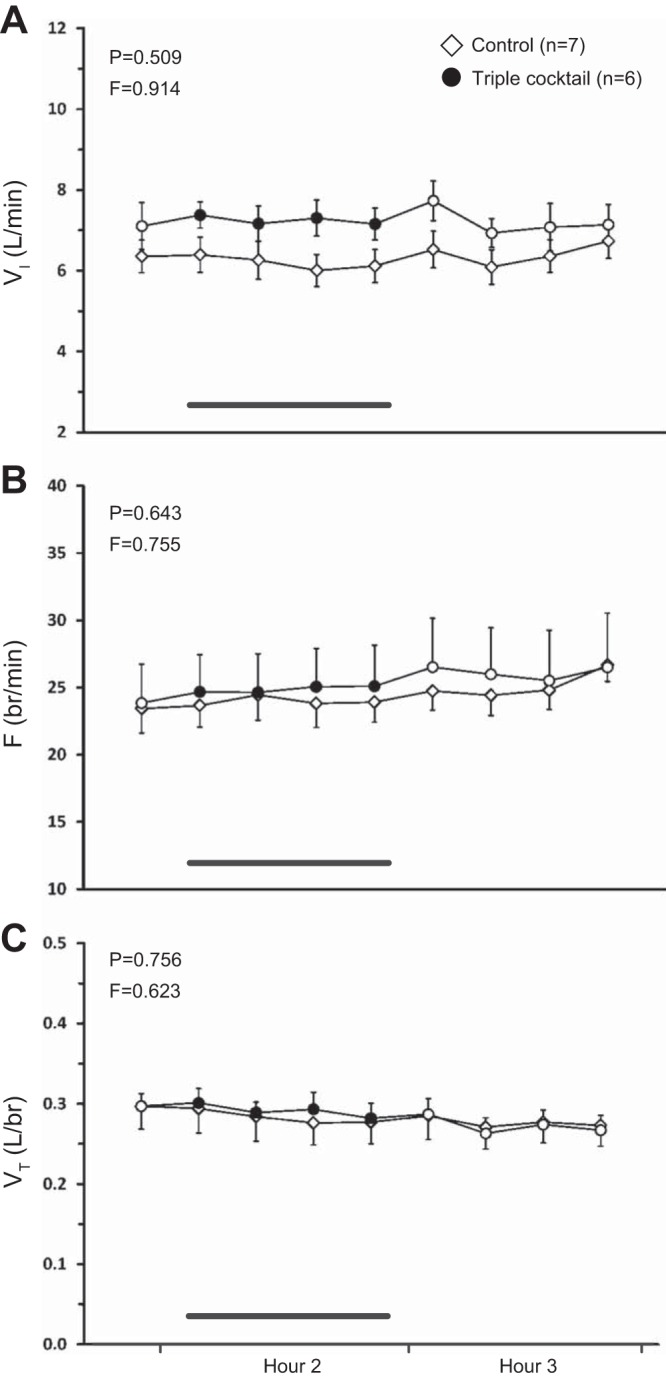
Daytime dialysis of a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 6) in the VRC had no significant effect (P ≥ 0.509) on any respiratory parameter relative to time-control studies (n = 7). A: minute ventilation (VI, liters/min); B: frequency (F, breaths/min); C: tidal volume (VT, liters/breath). The first data point represents the last 15-min bin of hour 1. Closed symbols and bar indicate period of cocktail dialysis; open symbols indicate mCSF dialysis. F and P indicate values for interaction term from two-way RM ANOVA.
Fig. 7.
Daytime dialysis of a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 6) in the VRC significantly increased the effluent concentration of substance P (SP, P < 0.001, C) and significantly decreased (P ≤ 0.045) the effluent concentrations of glutamine (GLN, B) and GABA (E); cocktail dialysis had no significant effect (P ≥ 0.136) on serotonin (5-HT, A) or glycine (GLY, D) concentration. The x-axes indicate hour of dialysis; y-axes indicate effluent neurochemical concentration. Filled bars indicate dialysis of cocktail; open bars indicate dialysis of mCSF. Bars on the left are data from time-control studies; bars on the right are data from cocktail studies. F and P indicate values for interaction term from two-way repeated-measures ANOVA. **P ≤ 0.001 vs. hour 1; ††P ≤ 0.001 vs. hour 2; #P < 0.05; ##P ≤ 0.001 vs. time-control value per post hoc analysis.
Nighttime effects on breathing and neuromodulator concentration of blockade of mACh, NK1, and 5-HT2A receptors.
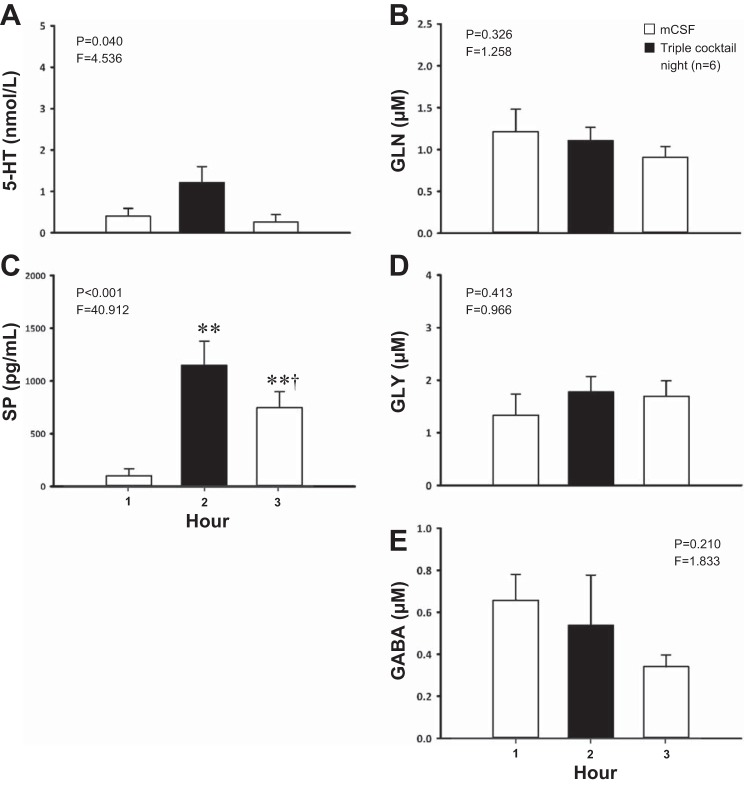
Dialysis of the same triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 5 for ventilatory data, n = 6 for neurochemical data) at night had no state-dependent effects on any ventilatory variable (P ≥ 0.134, Fig. 8, A–C) as determined by the interaction term for sleep state and time from two-way RM ANOVAs. However, there were significant increases in effluent 5-HT (P = 0.040, Fig. 9A) and SP (P < 0.001, Fig. 9C) concentrations, although post hoc analysis of 5-HT data did not identify which hours were significantly different. SP was significantly elevated during hours 2 and 3 (P < 0.001, post hoc). Triple-cocktail dialysis at night had no significant effect on the effluent concentration of any of the three amino acids (P ≥ 0.210; Fig. 9, B, D, and E).
Fig. 8.
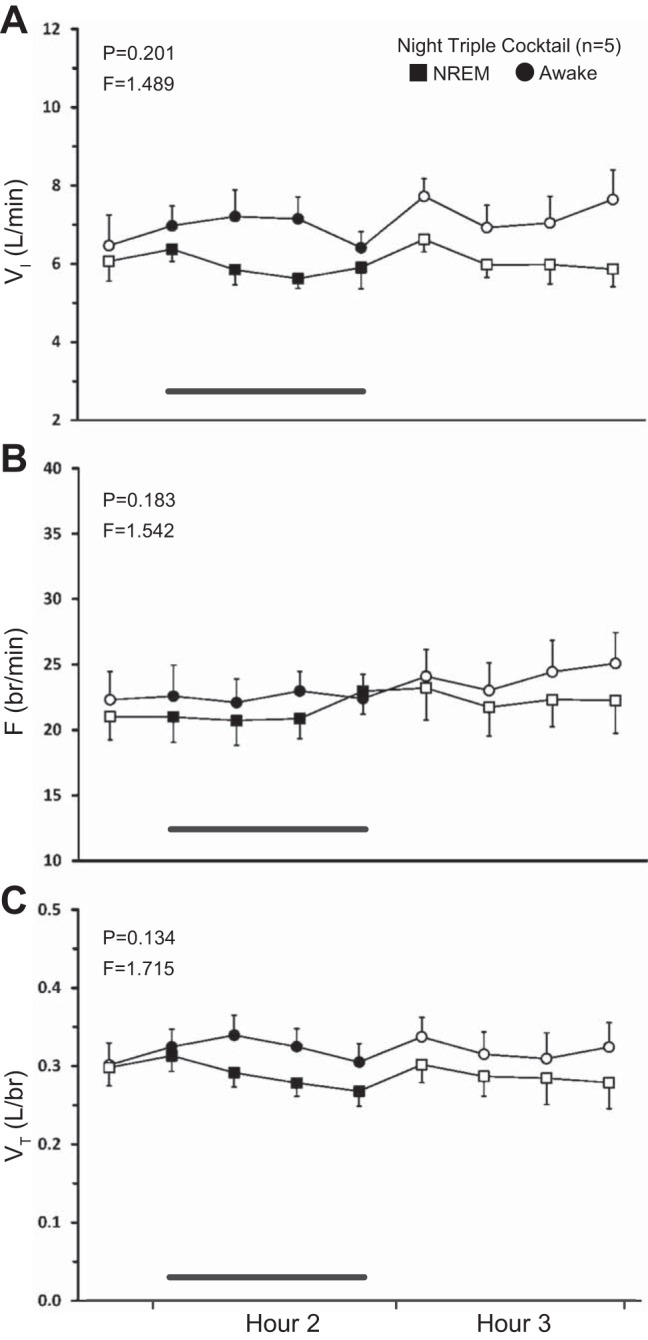
Nighttime dialysis of a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 5) in the VRC did not have significant state-dependent effects on any respiratory parameter (P ≥ 0.134). A: minute ventilation (VI, liters/min); B: frequency (F, breaths/min); C: tidal volume (VT, liters/breath). Squares represent breathing during non-rapid eye movement sleep; circles represent breathing during wakefulness. The first data point represents the last 15-min bin of hour 1. Closed symbols and bar indicate period of cocktail dialysis; open symbols indicate mCSF dialysis. F and P indicate values for interaction term from two-way RM ANOVA.
Fig. 9.
Nighttime dialysis of a triple cocktail of antagonists (5 mM ATR, 500 μM SPA, and 0.5 mM MDL, n = 6) in the VRC significantly increased the effluent concentrations of serotonin (5-HT, P = 0.040, A) and substance P (SP, P < 0.001, C) while having no significant effect (P ≥ 0.210) on glutamine (GLN, B), glycine (GLY, D), or GABA (E). The x-axes indicate hour of dialysis; y-axes indicate effluent neurochemical concentration. Filled bars indicate dialysis of cocktail; open bars indicate dialysis of mCSF. F and P indicate values from one-way RM ANOVA. **P ≤ 0.001 vs. hour 1; †P ≤ 0.05 vs. hour 2 per post hoc analysis.
Animals excluded from statistical analyses.
In two additional animals, day (Fig. 10) and night (data not shown) dialysis studies of the same triple cocktail of antagonists were completed. These goats were not included in either the group means or statistical analyses because they had an abnormally strong response to the cocktail, characterized by a large, sustained increase in VI (Fig. 10A) driven by an increase in breathing frequency (Fig. 10B). VT did not appear to be much affected by triple cocktail dialysis (Fig. 10C).
DISCUSSION
No effect of combined receptor blockade on breathing.
Many studies have previously demonstrated the contributions to breathing of muscarinic, NK1, and 5-HT receptors in various models (3, 5, 11, 16, 17, 28, 32, 34, 35, 42, 46). However, the large array of excitatory and inhibitory inputs to the respiratory network (10) suggests that neuromodulators likely act in concert to regulate breathing. Furthermore, their effects might not necessarily be additive, and the effects of one modulator may be dependent on the presence or absence of another modulator (9). The importance of understanding the effects of multiple receptor types on breathing has also been pointed out by Doi and Ramirez (10), who noted that “future studies need to address the combinatory effects of different neuromodulators. For example, by simultaneously blocking muscarinic, nicotinic, 5-HT2 and NK1 receptors. . . . to learn whether the contribution of each neuromodulator is cumulative and whether the drive of all neuromodulators is necessary for maintaining baseline frequency.”
We hypothesized that simultaneous blockade of muscarinic, NK1, and 5-HT2A receptors would not alter breathing, but it would alter multiple neuromodulators. The hypothesis was confirmed, because we found that combined blockade of receptors of mACh, NK1, and 5-HT2A did not significantly affect breathing, but it consistently increased the effluent concentrations of SP while significantly decreasing or tending to decrease the effluent concentrations of GABA.
We previously found that unilateral dialysis into the VRC of 50 mM ATR increased 5-HT and SP in the effluent mCSF (25), which we postulate may have overcompensated for a reduction in muscarinic excitation to thereby increase breathing. In that study, the inhibitory neuromodulators GABA and glycine also increased, possibly in response to overcompensation in 5-HT and SP release. Importantly, these changes in neuromodulators occurred specifically at the site of dialysis, not in the contralateral VRC, indicating that neuromodulator interdependence likely occurs locally and not globally (25). In subsequent studies, including the present, we unilaterally dialyzed a lower concentration of ATR (26), and antagonists to NK1 (27) and 5-HT2A receptors individually, and we also dialyzed these excitatory receptor antagonists simultaneously. Overall, none of these treatments altered breathing, but SP consistently increased, and GABA either tended to decrease in the dialysate, or it did so significantly. In the present study, the changes in SP and GABA may have compensated for the presumed reduction in neuronal excitation with blockade of 5-HT2A, NK1 and muscarinic receptors, resulting in no change in breathing.
Although neuromodulator interdependence appears to be mediated locally, other potential contributing mechanisms to maintain stable breathing could be through anatomical connectivity with the contralateral preBötC (41), or through reflex compensation independent of changes in neuromodulators in the contralateral preBötC. Indeed, we have shown that bilateral neurotoxic destruction of the preBötC in awake goats results in terminal apnea (47). However, unilateral destruction did not induce apnea, but it did induce hypoventilation for several hours. Additionally, St-Jacques and St-John (38) found in decerebrate, vagotomized rats that attenuation of preBötC activity with unilateral injection of muscimol, the inhibitory GABA agonist, resulted in several minutes of respiratory apnea. Moreover, in anesthetized mice, unilateral injections into the preBötC of an excitatory receptor antagonist or a cocktail of antagonists caused a significant decrease in respiratory frequency (11). These examples of hypoventilation/apnea resulting from unilateral perturbations are in contrast to our findings that unilateral dialysis of antagonists to excitatory receptors appears to elicit a rapid and precise response to maintain stable breathing. Accordingly, our unilateral dialysis studies demonstrate an important fundamental characteristic of the respiratory control network: that is, its ability to compensate for fluctuations in excitatory input. Furthermore, neuromodulator interdependence does not appear to be unique to the preBötC region because we found changes in neuromodulators irrespective of the site of dialysis in the VRC (Fig. 1). Moreover, in previous studies, we found that dialysis of ATR into the pontine Kölliker-Fuse nucleus elicited increases in SP comparable to ATR dialysis into the VRC (unpublished data). Finally, we speculate that neuromodulator interdependence may not be unique to the respiratory control network nor even to mammals, because it likely occurs in other central-pattern generators such as those controlling mastication, locomotion, and the crustacean stomatogastric ganglion and cardiac ganglia, which generate motor patterns (4, 9, 23, 31).
Potential mechanisms stabilizing breathing in the face of ubiquitous neuromodulation or attenuation of multiple neuromodulation.
In a recent overview of neuromodulation of neuronal circuits, Dr. Eve Marder (23) addressed two pertinent questions. First, why don't neural circuits important for behavior become overmodulated? And second, what mechanisms might protect against overmodulation? She thereby proposed five potential stability mechanisms: 1) modulators that coordinately act on opposing processes by acting on both inward and outward currents; 2) voltage-dependence of modulator actions; 3) convergence of many modulators onto the same voltage-dependent current (or, as we additionally suggest, convergence onto the same second-messenger pathway); 4) saturation of postsynaptic action; and 5) modulators act coordinately on multiple targets to keep systems functionally matched (i.e., coordinated modulation of the central pattern generator, the neuromuscular junction, and muscle activity) (23). One or more of these protective mechanisms against overmodulation may also be protective against the reverse situation of excessive attenuation of excitatory neuromodulator receptor activity due to antagonist dialysis. For example, receptors targeted by our antagonist cocktails couple to G protein-coupled receptors of the Gq/11 subtype (10), thereby converging on the same second-messenger system, the phospholipase C (PLC) pathway. This commonality might explain why 5 mM ATR (26), SPA (27), and MDL each had the same effect on breathing (i.e., no effect) when dialyzed individually or in combination. It is also possible that simultaneous blockade of mACh, NK1, and 5-HT2A receptors is still not enough to significantly depress activity in the PLC pathway to a degree such that breathing is affected. This may be due to the multitude of other receptors that couple to the Gq/11 subtype (10), such as the α-1 adrenergic and P2Y purinergic receptors (10, 13), both of which can modulate respiratory network activity (21, 43).
Alternatively, potentiation of signaling in parallel pathways might partially account for the maintenance of breathing during antagonist challenge. The most consistent finding in these and past studies is an antagonist-induced increase in effluent SP concentration (25–27). It is possible that the excess SP may be binding and activating NK2 and NK3 receptors, negating the antagonist effects on NK1 receptors (6, 33, 39). NK2 and NK3 receptors have previously been shown to provide excitatory modulation in the rabbit preBötC, likely through activation by SP (2), which can also exert effects through these receptors (6). Another example is the 5-HT4A receptor, which is coupled to the Gs G protein-coupled receptor subtype. Activation of this receptor leads to stimulation of adenylyl cyclase, the protein kinase A pathway, and inactivation of potassium channels (10). The 5-HT4A receptor is known to be expressed in the preBötC and has excitatory respiratory effects capable of reversing fentanyl-induced respiratory depression (22). Thus any 5-HT in the extracellular space, presumably blocked from interacting with 5-HT2A receptors during MDL or cocktail dialysis, may instead be acting on 5-HT4A receptors to stimulate breathing without any necessary increases in 5-HT concentration. In summary, the point we wish to emphasize is that there may be adequate redundancy and multiplicity of excitatory inputs to the VRC, such that blocking a subset of these receptors with double or triple cocktails of antagonists would be insufficient to significantly decrease breathing.
Finally, the most consistent effect on neuromodulator concentration of cocktail dialysis was an increase in SP and a tendency for GABA to decrease, although the changes in GABA were not all significant. If NK1 receptors and 5-HT2A receptors are blocked, it is possible that the decrease in effluent GABA concentration may have offset reduced activation of excitatory receptors, preventing an attenuation of breathing with cocktail dialysis. Due to involvement of glutamine in the reuptake and recycling of GABA (1), the observed decreases in glutamine we report may reflect decreases in GABA release.
Additional neuromodulatory inputs with the potential for compensation.
It is important to note that neuromodulators not measured herein may have had effects that contributed to the maintenance of breathing observed in the present studies. These neuromodulators likely include orexin (8), histamine (10), ATP (13, 15, 21), prostaglandins (19), and catecholamines (43). Evidence from past work shows that the modulatory environment can reversibly affect the number and type of pacemaker cells in a population of respiratory neurons (43), and that physiological state (e.g., hypoxia) can influence the dependence of the respiratory network on different modulators/pacemaker currents. Examples of modulators that illustrate these points are norepinephrine (NE) and 5-HT. In mouse brainstem slices containing the ventral respiratory group (43), application of NE was found to increase fictive respiratory frequency via the α-1 receptor. In addition to differentially modulating burst shape and frequency of distinct pacemaker neurons, NE also induced conditional expression of cadmium-sensitive pacemaker properties in what were originally nonpacemaker neurons (43). Conversely, pacemaker properties of cadmium-insensitive neurons are instead dependent on 5-HT2A receptors (30). Whereas bursting in cadmium-sensitive pacemakers is dependent on the nonspecific cation current (ICAN), bursting properties in cadmium-insensitive pacemakers depend on the persistent sodium current (INaP) (14, 29, 45). Finally, during hypoxia and subsequent gasping behavior, the respiratory network is believed to reconfigure into one that is selectively dependent on INaP (10, 12, 14, 29). Thus subtle changes in (or complete blockade of) NE or 5-HT may have altered the relative expression of these current types or the dependence of the respiratory network on a given modulator/current type. Reconfiguration of the network in this manner during antagonist dialysis may partially account for the stability of breathing.
A neuromodulator may also have different effects on breathing depending on the modulatory/physiological state of the network, as previously proposed by others (11). This concept has been recently demonstrated by Zanella et al. (49). In those studies, NE, which can act to stabilize respiratory activity in reduced preparations (44), was found to actually increase respiratory irregularity in vitro and in vivo, specifically after exposure to acute intermittent hypoxia (49). Similarly, prostaglandin E2 (PGE2) when applied at low concentrations has been shown by Koch et al. (19) to selectively increase sigh frequency, but not eupneic frequency, although it increases both sigh and eupneic frequency at higher concentrations. Using inhibitors of the INaP and ICAN, the study further suggests that the contrasting effects of PGE2 concentration on distinct respiratory behaviors may be due to differential modulation of INaP at low concentrations vs. ICAN at higher concentrations. Thus the effect of a modulator is context-dependent, and changes in the balance of excitatory and inhibitory modulators may drastically alter the net output of a network. This phenomenon has been previously demonstrated in other neural networks; specifically, the cardiac sac motor pattern in the lobster, wherein the effect of proctolin is dependent upon exposure of the network to red-pigment-concentrating hormone (9).
Relevant to the preceding discussion on modulatory environment are findings on two goats that displayed large, sustained increases in breathing frequency (+20 breaths/min) with dialysis of the triple cocktail of antagonists, both in the day (Fig. 10) and night (data not shown), while awake and in NREM sleep. The responses of these animals were clearly different from those of the rest of the group; as such, they were excluded from group means and statistical analysis. An interesting distinguishing feature common to these two goats was a higher-than-average baseline breathing frequency (≥30 breaths/min). Daytime baseline levels of 5-HT and SP were within normal range and changes in these neuromodulators with dialysis of the triple cocktail were comparable to those of the rest of the group. However, one animal had lower-than-average baseline levels of glycine and GABA, which may have partially contributed to an overall higher baseline breathing frequency. Although it is difficult to determine the precise cause of the elevated baseline breathing, these observations suggest that the response to receptor blockade may be dependent upon the initial modulatory or physiological state of the animal.
Indeed, the dependence of a drug effect on baseline respiratory activity has been demonstrated in other studies. In particular, studies in anesthetized mice found that hemilateral injection of a NK1 receptor antagonist (RP67580) into the preBötC region significantly decreased respiratory frequency (11). However, the inhibitory effect of RP67580 was significantly correlated with the baseline breathing frequency of the animal: mice with lower starting frequencies showed a larger decrease in frequency with NK1 receptor blockade (11). The same studies found that when both α-1 NE and 5-HT2 receptors were blocked, the inhibitory effect of RP67580 was further accentuated. The authors therefore concluded that breathing depends on NK1 receptor activation more so at low breathing frequencies or under conditions of reduced excitatory transmission. The contrasting effects of receptor blockade on breathing in these mouse studies (decrease) vs. our observations in goats (increase) may be due to the use of anesthesia. Despite this caveat, both models suggest that the effect of an antagonist on breathing may depend on the respiratory/modulatory state of the animal.
Limitations.
Technical limitations and sample availability prevented measurement of ACh/choline and thyrotropin-releasing hormone in the present studies. The limitations of the use of dialysis in our animal model to investigate the control of breathing have been discussed in greater detail in previous publications (25, 26); only a brief summary is given here. First, the precise boundaries of the goat preBötC remain unclear (20), rendering MT placement difficult in some animals. Second, determination of the diffusion area of dialyzed substances can be problematic. Accordingly, we cannot precisely identify the site affected by NMDA injections or dialysis of antagonists, thus we believe it most appropriate to state that our observed effects were within the VRC. Third, the length of the infusion line connecting the syringe in the dialysis pump to the probe (i.e., dead space in delivery) required ∼20 min for fresh solution to reach the probe, thus antagonist delivery continued for 20 min into the third hour of mCSF dialysis. This dead space in our delivery system likely explains why SP consistently remained elevated during hour 3. Fourth, pertinent to our night studies, we were unable to separate neurochemicals collected during different sleep states. Additionally, sleep state-associated changes in fractional interstitial volume (48) have been previously reported, which would affect both modulator and drug concentrations in the tissue. Finally, repeated probe insertions in one MT may have caused potential tissue damage around the site of dialysis.
Conclusion.
Combined unilateral blockade of mACh, NK1, and 5-HT2A receptors in the goat VRC does not affect breathing. This lack of effect may be due to a number of compensatory mechanisms, including redundancy/multiplicity of excitatory inputs to the VRC, activation of parallel signaling pathways, the contribution of other neuromodulators not measured herein, the effects of changes in the modulatory milieu on the number and type of pacemakers in the VRC, or alterations in the dependence of the respiratory network on different modulators/current types. Our data suggest that many interacting systems may need to be substantially impaired for the emergence or complete manifestation of respiratory deficits. If such is the case, it would be highly relevant to disease conditions in which multiple modulator systems are affected. An example of such is Rett syndrome, in which abnormalities in glutamate, GABA, brain-derived neurotrophic factor, NE, and 5-HT have all been implicated in the respiratory pathologies associated with the disorder (18). Our conclusions emphasize the need to adopt a multiform approach in treating such conditions or alleviating associated respiratory defects. Furthermore, anecdotal evidence and the published work of others (11) suggest that the effectiveness of a treatment may be dependent on the initial respiratory status of the patient.
GRANTS
This project was funded by National Heart, Lung, and Blood Institute Grants HL-25739, HL-112996, and HL-007852; and by the Department of Veterans Affairs.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.M., M.R.H., and H.V.F. conception and design of research; C.M., S.E.N., S.J.O., J.R.M., T.L., L.G.P., and H.V.F. performed experiments; C.M. and H.V.F. analyzed data; C.M., M.R.H., and H.V.F. interpreted results of experiments; C.M. and S.E.N. prepared figures; C.M. drafted manuscript; C.M., S.E.N., S.J.O., J.R.M., T.L., M.R.H., L.G.P., and H.V.F. edited and revised manuscript; C.M., S.E.N., S.J.O., J.R.M., T.L., M.R.H., L.G.P., and H.V.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Lisa Henderson, Jenifer Phillips, and Camille Torres for their help with neurochemical quantification.
Present address of J. Miller: Zablocki Veterans Affairs Medical Center, Milwaukee, WI 53295.
REFERENCES
- 1.Bak LK, Schousboe A, Waagepetersen HS. The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98: 641–653, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bongianni F, Mutolo D, Cinelli E, Pantaleo T. Neurokinin receptor modulation of respiratory activity in the rabbit. Eur J Neurosci 27: 3233–3243, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Bonis JM, Neumueller SE, Krause KL, Kiner T, Smith A, Marshall BD, Qian B, Pan LG, Forster HV. A role for the Kolliker-Fuse nucleus in cholinergic modulation of breathing at night during wakefulness and NREM sleep. J Appl Physiol 109: 159–170, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brezina V. Beyond the wiring diagram: signalling through complex neuromodulator networks. Philos Trans R Soc Lond B Biol Sci 365: 2363–2374, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burton MD, Nouri K, Baichoo S, Samuels-Toyloy N, Kazemi H. Ventilatory output and acetylcholine: perturbations in release and muscarinic receptor activation. J Appl Physiol 77: 2275–2284, 1994. [DOI] [PubMed] [Google Scholar]
- 6.Datar P, Srivastava S, Coutinho E, Govil G. Substance P: structure, function, therapeutics. Curr Top Med Chem 4: 75–103, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Dean C, Geiger LK, Sprtel BM, Ohtake PJ, Forster HV. An anatomic atlas of the medulla oblongata of the adult goat. J Appl Physiol 87: 1220–1229, 1999. [DOI] [PubMed] [Google Scholar]
- 8.Dias MB, Li A, Nattie E. The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol 170: 96–102, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dickinson PS, Fairfield WP, Hetling JR, Hauptman J. Neurotransmitter interactions in the stomatogastric system of the spiny lobster: one peptide alters the response of a central pattern generator to a second peptide. J Neurophysiol 77: 599–610, 1997. [DOI] [PubMed] [Google Scholar]
- 10.Doi A, Ramirez JM. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doi A, Ramirez JM. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. J Neurosci 30: 8251–8262, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Funk GD, Greer JJ. The rhythmic, transverse medullary slice preparation in respiratory neurobiology: contributions and caveats. Respir Physiol Neurobiol 186: 236–253, 2013. [DOI] [PubMed] [Google Scholar]
- 13.Funk GD, Huxtable AG, Lorier AR. ATP in central respiratory control: a three-part signaling system. Respir Physiol Neurobiol 164: 131–142, 2008. [DOI] [PubMed] [Google Scholar]
- 14.Garcia AJ 3rd, Zanella S, Koch H, Doi A, Ramirez JM. Chapter 3–networks within networks: the neuronal control of breathing. Prog Brain Res 188: 31–50, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436: 108–111, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 168: 101–108, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch H, Caughie C, Elsen FP, Doi A, Garcia AJ 3rd, Zanella S, Ramirez JM. Prostaglandin E2 differentially modulates the central control of eupnoea, sighs and gasping in mice. J Physiol 593: 305–319, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krause KL, Forster HV, Kiner T, Davis SE, Bonis JM, Qian B, Pan LG. Normal breathing pattern and arterial blood gases in awake and sleeping goats after near total destruction of the presumed pre-Bötzinger complex and the surrounding region. J Appl Physiol 106: 605–619, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD. P2Y1 receptor modulation of the pre-Bötzinger complex inspiratory rhythm generating network in vitro. J Neurosci 27: 993–1005, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science 301: 226–229, 2003. [DOI] [PubMed] [Google Scholar]
- 23.Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron 76: 1–11, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCrimmon DR, Monnier A, Hayashi F, Zuperku EJ. Pattern formation and rhythm generation in the ventral respiratory group. Clin Exp Pharmacol Physiol 27: 126–131, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Atropine microdialysis within or near the pre-Bötzinger Complex increases breathing frequency more during wakefulness than during NREM sleep. J Appl Physiol 114: 694–704, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muere C, Neumueller S, Miller J, Olesiak S, Hodges MR, Pan L, Forster HV. Evidence for respiratory neuromodulator interdependence after cholinergic disruption in the ventral respiratory column. Respir Physiol Neurobiol 205: 7–15, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muere C, Neumueller S, Olesiak S, Miller J, Hodges MR, Pan L, Forster HV. Blockade of neurokinin-1 receptors in the ventral respiratory column does not affect breathing but alters neurochemical release. J Appl Physiol 118: 732–741, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nattie E, Li A. Neurokinin-1 receptor-expressing neurons in the ventral medulla are essential for normal central and peripheral chemoreception in the conscious rat. J Appl Physiol 101: 1596–1606, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Pena F, Parkis MA, Tryba AK, Ramirez JM. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prinz AA, Bucher D, Marder E. Similar network activity from disparate circuit parameters. Nat Neurosci 7: 1345–1352, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphé neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quartara L, Maggi CA. The tachykinin NK1 receptor. Part II: Distribution and pathophysiological roles. Neuropeptides 32: 1–49, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol 83: 1243–1252, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao XM, Feldman JL. Cholinergic neurotransmission in the preBötzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 130: 1069–1081, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Solomon IC, Edelman NH, Neubauer JA. Patterns of phrenic motor output evoked by chemical stimulation of neurons located in the pre-Bötzinger complex in vivo. J Neurophysiol 81: 1150–1161, 1999. [DOI] [PubMed] [Google Scholar]
- 38.St-Jacques R, St-John WM. Transient, reversible apnoea following ablation of the pre-Bötzinger complex in rats. J Physiol 520, Pt 1: 303–314, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev 94: 265–301, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stornetta RL. Identification of neurotransmitters and co-localization of transmitters in brainstem respiratory neurons. Respir Physiol Neurobiol 164: 18–27, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan W, Pagliardini S, Yang P, Janczewski WA, Feldman JL. Projections of preBötzinger complex neurons in adult rats. J Comp Neurol 518: 1862–1878, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Telgkamp P, Cao YQ, Basbaum AI, Ramirez JM. Long-term deprivation of substance P in PPT-A mutant mice alters the anoxic response of the isolated respiratory network. J Neurophysiol 88: 206–213, 2002. [DOI] [PubMed] [Google Scholar]
- 43.Viemari JC, Ramirez JM. Norepinephrine differentially modulates different types of respiratory pacemaker and nonpacemaker neurons. J Neurophysiol 95: 2070–2082, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Viemari JC, Roux JC, Tryba AK, Saywell V, Burnet H, Peña F, Zanella S, Bévengut M, Barthelemy-Requin M, Herzing LB, Moncla A, Mancini J, Ramirez JM, Villard L, Hilaire G. Mecp2 deficiency disrupts norepinephrine and respiratory systems in mice. J Neurosci 25: 11521–11530, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viemari JC, Tryba AK. Bioaminergic neuromodulation of respiratory rhythm in vitro. Respir Physiol Neurobiol 168: 69–75, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah T, Davis S, Forster HV. Small reduction of neurokinin-1 receptor-expressing neurons in the pre-Bötzinger complex area induces abnormal breathing periods in awake goats. J Appl Physiol 97: 1620–1628, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wenninger JM, Pan LG, Klum L, Leekley T, Bastastic J, Hodges MR, Feroah TR, Davis S, Forster HV. Large lesions in the pre-Bötzinger complex area eliminate eupneic respiratory rhythm in awake goats. J Appl Physiol 97: 1629–1636, 2004. [DOI] [PubMed] [Google Scholar]
- 48.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O'Donnell J, Christensen DJ, Nicholson C, Iliff JJ, Takano T, Deane R, Nedergaard M. Sleep drives metabolite clearance from the adult brain. Science 342: 373–377, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanella S, Doi A, Garcia AJ 3rd, Elsen F, Kirsch S, Wei AD, Ramirez JM. When norepinephrine becomes a driver of breathing irregularities: how intermittent hypoxia fundamentally alters the modulatory response of the respiratory network. J Neurosci 34: 36–50, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]