Abstract
Superoxide and nitric oxide are generated by skeletal muscle, and these species are increased by contractile activity. Mitochondria have long been assumed to play the primary role in generation of superoxide in muscle, but recent studies indicate that, during contractile activity, membrane-localized NADPH oxidase(s) rapidly generate(s) superoxide that plays a role in redox signaling. This process is important in upregulation of rapid and specific cytoprotective responses that aid maintenance of cell viability following contractile activity, but the overall extent to which redox signaling contributes to regulation of muscle metabolism and homeostasis following contractile activity is currently unclear, as is identification of key redox-sensitive protein targets involved in these processes. Reactive oxygen and nitrogen species have also been implicated in the loss of muscle mass and function that occurs with aging, although recent work has questioned whether oxidative damage plays a key role in these processes. A failure of redox signaling occurs in muscle during aging and may contribute to the age-related loss of muscle fibers. Whether such changes in redox signaling reflect primary age-related changes or are secondary to the fundamental mechanisms is unclear. For instance, denervated muscle fibers within muscles from aged rodents or humans appear to generate large amounts of mitochondrial hydrogen peroxide that could influence adjacent innervated fibers. Thus, in this instance, a “secondary” source of reactive oxygen species may be potentially generated as a result of a primary age-related pathology (loss of neurons), but, nevertheless, may contribute to loss of muscle mass and function during aging.
Keywords: reactive oxygen, nitric oxide, muscle, contractions
the editor-in-chief of the Journal of Applied Physiology invited this review to accompany the presentation of the 2014 Edward F. Adolph lecture to the Environmental and Exercise section of the American Physiological Society, a lecture entitled “30 Years of chasing radicals in muscle: Redox regulation of muscle adaptations to contractile activity and aging.” My plan is to present a personal (and hence undoubtedly biased) view of how this exciting field has developed over 30 years, the key achievements that have been made, and to discuss some of the difficulties involved in studying this area. Of necessity, this is not a comprehensive description of all that has been discovered and is inevitably incomplete, since the field continues to evolve rapidly and relevant data appear on a regular basis that impact on our understanding of the area. Three key topics will be covered to which our research group have contributed a significant number of publications: 1) generation of reactive oxygen and nitrogen species in contracting skeletal muscle; 2) roles of reactive oxygen species in skeletal muscle; and 3) reactive oxygen species in muscle aging.
GENERATION OF REACTIVE OXYGEN AND NITROGEN SPECIES IN CONTRACTING SKELETAL MUSCLE
It is well established that skeletal muscle fibers generate superoxide and nitric oxide (NO), and these parent molecules can be converted to several secondary reactive oxygen species (ROS) and reactive nitrogen species (RNS). Superoxide and NO are generated from various sources within muscle fibers, and superoxide (53, 76), hydrogen peroxide (90), and NO (3, 46) are released into the interstitial space of muscle fibers (or generated on the extracellular side of the muscle plasma membrane). Contractile activity has been shown to increase the intracellular content or activities of superoxide, hydrogen peroxide, and NO (66, 75, 76, 84), while superoxide, hydrogen peroxide, hydroxyl radical, and NO have been detected in the muscle interstitial space (53, 67, 90).
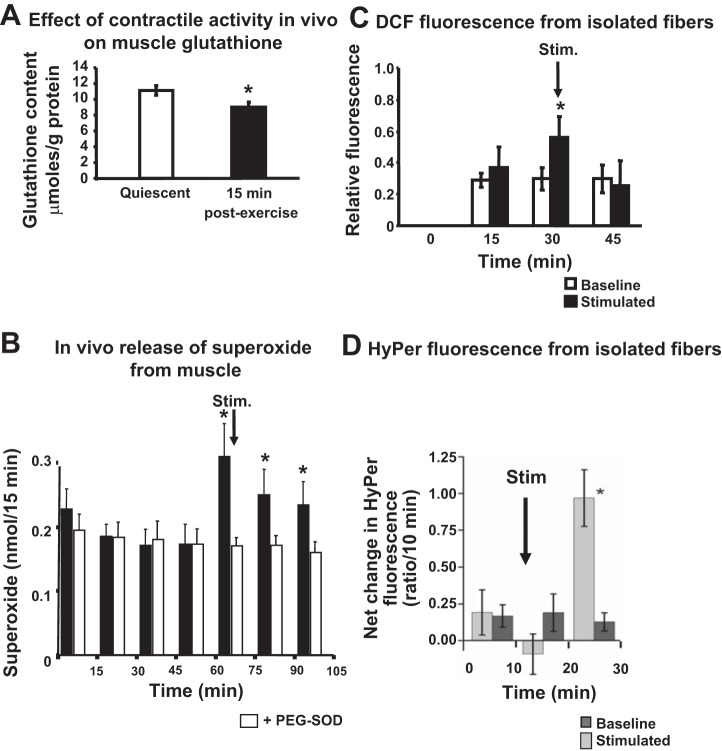
A number of different approaches have been used to demonstrate the increase in ROS that occurs during contractile activity. Although most data to date have been generated using nonspecific approaches, techniques have become increasingly sophisticated such that (for instance) new specific, genetically encoded fluorescent probes, such as HyPer, can report changes in single species in defined subcellular compartments (see Fig. 1 for examples of approaches that have been used). Much of the initial work in this area was based on the assumption that mitochondria were the main source of the ROS generated during contractile activity in muscle, but several recent publications disagree with this possibility (73). There is some debate about the precise location of NAD(P)H oxidase(s) that has been claimed as an alternative sources, but the presence of this enzyme in the skeletal muscle plasma membrane (41), sarcoplasmic reticulum (97), and the T-tubules (19) has been reported. The T-tubule localized enzyme appears to be particularly relevant, since it has been claimed to be specifically activated by contractions (19). In recent studies, we have examined the potential contribution of mitochondrial and nonmitochondrial sources to the acute increase in superoxide seen during muscle contractions (69, 79) and concluded that NADPH oxidase effects predominated over mitochondria during the short contraction periods (10–15 min) that were studied. Thus present data appear to indicate that a nonmitochondrial NADPH oxidase (likely to be the Nox2 isoform) is the major source of generation of superoxide during short-term contractile activity. The Nox4 isoform of NADPH oxidase has also been reported to be expressed in mitochondria and sarcoplasmic reticulum of skeletal muscle (79, 85), but any role in contraction-induced superoxide generation is unclear.
Fig. 1.
Examples of data derived from different approaches to study reactive oxygen species (ROS) generation in muscle or muscle fibers. A: reduction in glutathione content of muscles from wild-type mice in vivo following a 15-min period of isometric contractile activity. *Significant difference vs. quiescent. [Redrawn from Vasilaki et al. (90).] B: increase in interstitial superoxide monitored by microdialysis in the gastrocnemius muscle of mice during a 15-min period of isometric contractile activity. *Significant difference vs. polyethylene glycol (PEG)-superoxide dismutase (SOD). [Redrawn from Close et al. (11).] C: increase in intracellular 2′,7′-dichlorofluorescein (DCF) fluorescence from fibers isolated from the flexor digitorum brevis (FDB) muscle of mice and subjected to 15 min of isometric contractile activity in vitro. *Significant difference vs. baseline. [Redrawn from Palomero et al. (66).] D: increase in hydrogen peroxide content (indicated by increased HyPer fluorescence) in fibers isolated from the FDB muscle of mice and subjected to 10 min of isometric contractile activity in vitro. *Significant difference vs. baseline. [Redrawn from Pearson et al. (69).]
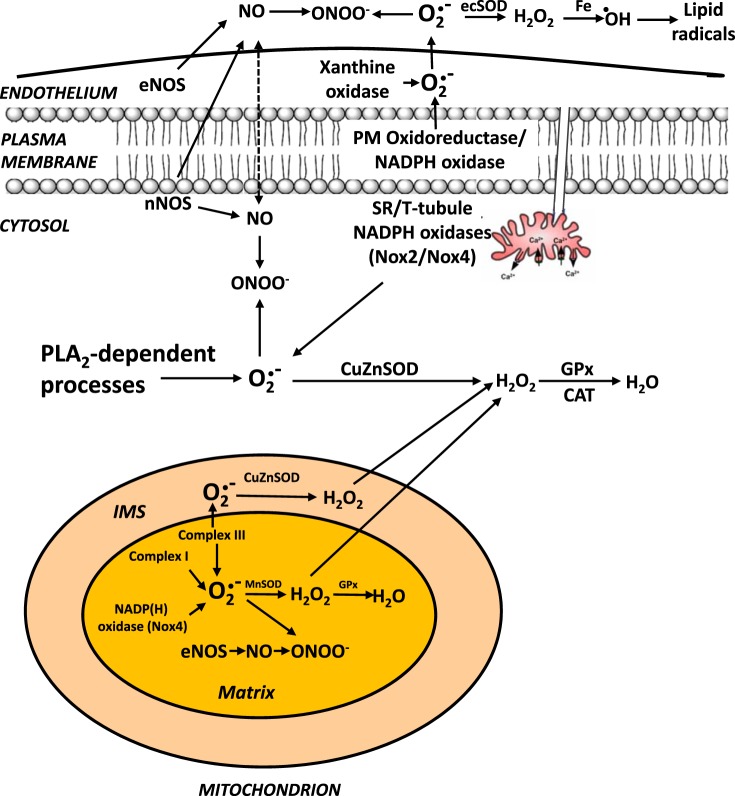
A number of specific ROS and RNS are detected in the extracellular space of skeletal muscle myotubes or isolated fibers in culture or in microdialysates from muscle interstitial fluid in vivo. It appears that muscle fibers may have generating systems for superoxide that release this species into the extracellular space (53, 76). Substantial diffusion of superoxide (or its protonated form) through the plasma membrane seems extremely unlikely (27), but other species that are detected in the muscle extracellular space (e.g., hydrogen peroxide and NO) can potentially diffuse across membranes and hence may originate from intracellular sites. Javesghani et al. (41) reported that a plasma membrane-localized NAD(P)H oxidase could release superoxide to the external face of the membrane, and Ward et al. (96) have described a stretch-activated NADPH oxidase (Nox2 isoform) that plays a major role in contraction-induced ROS generation in cardiac myocytes. This enzyme is also reported to be present in the skeletal muscle plasma membrane and appears to release superoxide to the outside of the cell. Other NAD(P)H-dependent systems have also been suggested to play a role in release of superoxide from muscle fibers (34). In muscle in vivo or intact muscle preparations ex vivo, xanthine oxidase enzymes in the endothelium may also play an important role in contraction-induced release of superoxide (24), and this enzyme has been claimed to be important in adaptations of muscle to contractile activity (22). Figure 2 summarizes our present understanding of the sites that have been identified for generation of ROS and NO in skeletal muscle fibers.
Fig. 2.
Updated working scheme for sites of ROS/reactive nitrogen species (RNS) generation by skeletal muscle demonstrating the potential role of Nox2 and Nox4 isoforms of NADPH oxidase in generating superoxide in mitochondria and cytosol and acknowledging the lack of evidence for any release of superoxide from mitochondrial during contractile activity. NO, nitric oxide; ONOO−, peroxynitrite; ecSOD, extracellular SOD; eNOS, endothelial NO synthase; nNOS, neuronal NO synthase; PLA2, phospholipase A2; GPx, glutathione peroxidase; CAT, catalase; IMS, intermembrane space. [Modified from Jackson (33).]
ROLES OF ROS IN SKELETAL MUSCLE: OXIDATIVE DAMAGE OR REDOX SIGNALING?
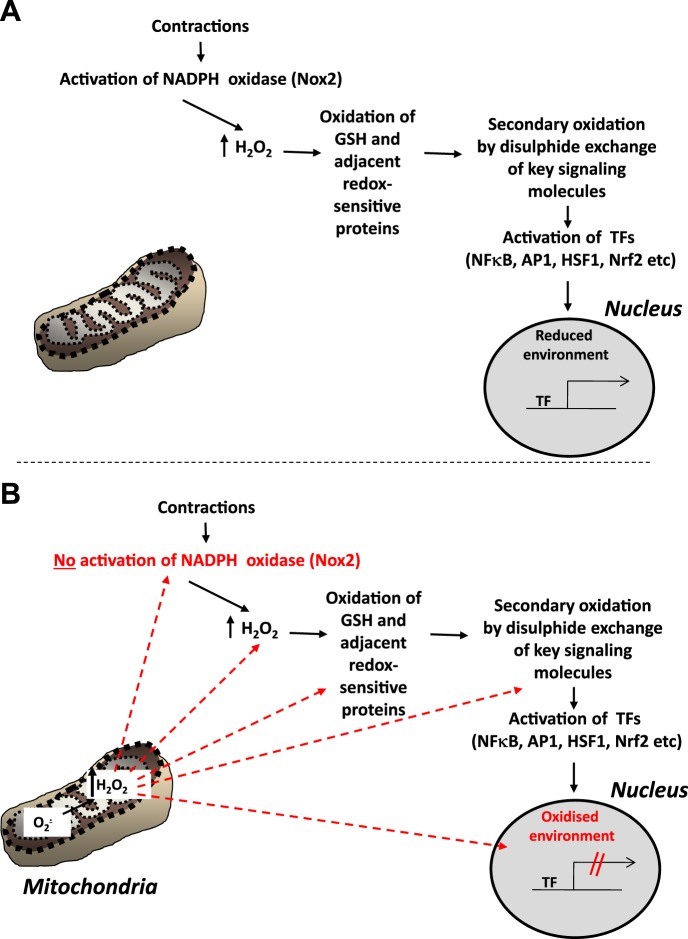
Although excess ROS can be deleterious to cells, causing oxidative damage to lipids, DNA and proteins (27), these species also appear to act as mediators of some adaptive processes following cellular stresses under normal physiological conditions. ROS mediate regulatory functions that lead to changes in cell and tissue homeostasis through modification of gene expression (17, 28, 36). Modification of specific thiol residues in proteins appears to be the major mechanism by which ROS exert such regulatory roles (40). Contractile activity increases the intracellular generation of superoxide and NO, and these species plus a number of secondary ROS and RNS (66, 73, 75) can mediate activation of a number of redox-regulated signaling pathways. The nature of these pathways has been the subject of extensive research, and redox-regulated processes (such as activation of NF-κB) have been shown to stimulate the expression of genes associated with myogenesis (2), catabolism, and mitochondrial biogenesis (4, 71, 87). Our group has been particularly interested in the role of ROS in activation of short-term cytoprotective changes in expression of regulatory enzymes and cytoprotective proteins in response to contractile activity (30, 53, 54). This appears to occur through redox-dependent activation of a number of transcriptional pathways, including the transcription factors, NF-κB, activator protein-1, heat shock factor-1 and nuclear transcription factor erythroid 2p45-related factor-2 (36, 42, 77, 91); see Fig. 3A.
Fig. 3.
A: schematic representation of the redox signaling pathways that are postulated to lead to adaptive activation of transcription factors and upregulation of the expression of cytoprotective proteins following contractile activity in skeletal muscle. TF, transcription factor. [Redrawn and updated from Jackson and McArdle (35).] B: putative sites at which the redox signaling pathway may be modified in aging leading to a failure of adaptive responses to contractile activity. Excess hydrogen peroxide generated by mitochondria in the muscle during aging may influence the pathway shown in A at multiple points: prevention of activation of NADPH oxidase; a chronic increase in cytosolic hydrogen peroxide; aberrant chronic oxidation of glutathione and other redox sensitive signaling proteins; oxidation of the nuclear environment leading to a failure of TF to activate transcription. AP1, activator protein-1; HSF1, heat shock factor-1; Nrf2, nuclear transcription factor erythroid 2p45-related factor-2.
POTENTIAL MODULATING EFFECTS OF ANTIOXIDANT SUPPLEMENTS ON ROS-STIMULATED ADAPTATIONS TO CONTRACTILE ACTIVITY
Researchers have been attempting to suppress the presumed deleterious effects of ROS and RNS generated during exercise since the first descriptions of their generation in this situation (e.g., Ref. 15). There has been little evidence of beneficial effects on muscle from such interventions, but the realization that these species play important roles in redox signaling has prompted a rethink of what antioxidants might achieve in this situation. Our group initially demonstrated that high doses of vitamin C could inhibit rapid stress responses to acute exercise (45), and this line was pursued by others who reported that high doses of antioxidants could reduce the training effects of exercise on muscle mitochondrial biogenesis, maximal O2 uptake, and improvements in insulin sensitivity (23, 77). The implication of such studies is that ROS or RNS play a key role in regulating multiple training-induced adaptations to muscle in humans and animals. Unfortunately, such findings could not be repeated by other scientists, who reported normal adaptations to exercise training, despite administration of high-dose antioxidants (e.g., Ref. 29). This difference resulted in an intense head-to-head debate in the scientific literature from the groups reporting these differing results (e.g., Ref. 31). There are a number of differences in experimental design that are likely to underlie the differences in reported outcomes, including the study of animals or humans, trained or untrained subjects, the durations and protocols for the training, the choice of markers of oxidative stress, the time points studied, the use of muscle vs. blood markers, and many more potential factors. A recent article by Paulsen et al. (68) has shed some light on this controversy, although this also illustrates the complexity of relating signaling processes to true physiological function. The study appears to confirm that these supplements do not universally inhibit major physiological adaptations to exercise training, although they did inhibit potentially relevant changes in mitochondrial proteins. A full explanation for the apparent discrepancies in the literature in this area is unlikely to appear until more is known about the scope and importance of redox signaling in muscle, but the present debate highlights the potential unintended consequences of untargeted use of high-dose antioxidant supplements.
ROS AND MUSCLE AGING
Aging leads to a reduction in muscle mass and function that contributes to physical instability and increased risk of falls (99), such that, by the age of 70 yr, skeletal muscle cross-sectional area has declined by 25–30% and muscle strength by 30–40% (72). In both humans and rodents, there is evidence that the age-related reduction in muscle mass and function is primarily due to decreased numbers of muscle fibers, and atrophy and weakening of the remaining fibers (6, 49, 50), although a recent study suggests atrophy of type II fibers without fiber loss is the major contributor to the decreased muscle mass seen in healthy elderly human subjects (61). Most of the intrinsic and extrinsic changes regulating muscle aging in humans have been observed in rodents, indicating that mice and rats can provide relevant models of human sarcopenia (14). Denervation also contributes to loss of muscle mass in humans and rodents (13, 39). The comparable changes in morphology seen in myofibers of aged rodents and humans suggest the mechanisms leading to muscle loss and atrophy at the cellular level are comparable (57). Muscle from old rodents also shows an increased proportion of more oxidative fibers (13) and an attenuation of various responses to contractile activity, including acute stress responses (91), mitochondrial biogenesis (51), and the contraction-induced increase in muscle protein synthesis (12). These are potentially important aspects of the multiple age-related deficits in muscle, including contributing to slowed reactions and an inability to fine-tune movements, while transgenic studies indicate that correction of specific attenuated responses to contractions can preserve muscle force generation in aged mice (7, 44, 52).
OXIDATIVE DAMAGE AND DEFECTIVE REDOX SIGNALING IN MUSCLE FROM OLD MICE AND HUMANS
An increase in oxidative damage has been reported in tissues (including skeletal muscle) of all aged organisms compared with levels found in young organisms (16, 81, 90). The possibility that increased oxidative damage plays a key role in age-related tissue dysfunction has received considerable attention. In nonmammalian models, interventions designed to reduce the activities of ROS, such as overexpression of CuZn, superoxide dismutase 1 (SOD1), catalase or both in Drosophila (63–65), or treatment with a MnSOD and catalase mimetic in C. elegans (56), extended lifespan and thus support the hypothesis, but these effects have not been confirmed in other studies (20). In mammals, only a small number of manipulations designed to reduce ROS activities and/or oxidative damage have increased lifespan (82, 98). It, therefore, appears that increased ROS generation is not the fundamental cause of aging (or more precisely, the fundamental determinant of lifespan). Many studies have reported that mitochondrial ROS generation is increased in skeletal muscle during aging (Refs. 55, 88 for reviews) in association with impaired function and oxidative damage to mitochondrial components (38, 81). Furthermore, other studies indicate that interventions to reduce mitochondrial hydrogen peroxide content (82) or increase cytoprotective proteins that reduce oxidative damage (7) can preserve muscle function during aging. Increased mitochondrial ROS generation has also been proposed to play a key mediating role in pathological changes in muscle in conditions such as disuse atrophy (74).
MODIFICATION OF MUSCLE ROS DURING AGING: KNOCKOUT OF KEY REGULATORY PROTEINS
A number of studies have examined the effects of deletion of regulatory enzymes for ROS, but, despite frequent observation of increased oxidative damage in these models, no clear relationship with skeletal muscle aging was seen (38). The exception to this pattern was in mice with a whole body deletion of SOD1, which show neuromuscular changes with aging that appear to reflect an accelerated skeletal muscle aging process (58). Adult SOD1 knockout (SOD1KO) mice show a decline in skeletal muscle mass, loss of muscle fibers and a decline in the number of motor units, loss of motor function and contractility, partial denervation, and mitochondrial dysfunction by 8 mo old (37, 47, 92). The fiber loss in SOD1KO mice is accompanied by degeneration of neuromuscular junctions (NMJs) (37). These changes are also seen in old wild-type (WT) mice, but not until after 22 mo of age. Hence we have proposed that SOD1KO mice are a useful model to examine the potential role of ROS in skeletal muscle aging (32).
It is relevant to consider why only the SOD1KO mice show an accelerated muscle aging phenotype, although other models with knockout of regulatory enzymes for ROS or RNS also show an increase in oxidative damage to muscle. SOD1 is expressed in both the cytosol of cells and within the mitochondrial intermembrane space, where it is likely to be present at high concentration compared with cytosolic SOD1 (43). One implication of this is that lack of SOD1 may influence redox homeostasis in the mitochondria, in addition to the cytosol, and hence that disturbances in either cytosolic or mitochondrial redox may underlie the accelerated skeletal muscle aging phenotype seen in SOD1KO mice. In our studies, we examined the nature of the reactive species that are generated in mice lacking SOD1. Some studies of aging models have suggested that the decline in tissue function that occurs with aging and the accelerated loss of skeletal muscle fibers in SOD1KO mice may be caused by superoxide toxicity (38, 56). An alternative possibility is that superoxide and NO may react chemically to form peroxynitrite, a reaction that competes with the dismutation of superoxide to hydrogen peroxide by SOD (5). In adult SOD1 null mice, the phenotype may, therefore, be associated with excess superoxide, but may also be due to increased peroxynitrite or a reduction in NO bioavailability. We demonstrated that, similar to muscle fibers from old WT mice, those from adult SOD1 knockout mice showed an increase in oxidation of the nonspecific intracellular ROS probe, 2′,7′-dichlorodihydrofluorescin-diacetate (DCFH) at rest compared with fibers from adult WT mice (92). Surprisingly, the fibers from SOD1KO mice showed no increase in DCFH oxidation following contractile activity, although an increase in DCFH oxidation was seen in muscle fibers from adult WT mice following contractile activity. The explanation for this is currently unclear, although DCFH is relatively insensitive to oxidation by superoxide, but is oxidized by other ROS, including hydrogen peroxide, hydroxyl radicals, peroxynitrite, and NO (60). Single muscle fibers from flexor digitorum brevis of WT and SOD1KO mice were, therefore, also loaded with NO-sensitive [4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM)] and superoxide-sensitive [dihydroethidium (DHE)] probes (78). These studies illustrated that a lack of SOD1 in the fibers from SOD1KO mice did not increase superoxide availability at rest, since no increase in ethidium or 2-hydroxyethidium formation from DHE was seen in fibers from SOD1KO mice compared with those from WT mice. Fibers from SOD1KO mice were found to have decreased NO availability (decreased DAF-FM fluorescence), increased 3-nitrotyrosines in muscle proteins, indicating increased peroxynitrite formation, and increased content of peroxiredoxin V (a peroxynitrite reductase) compared with WT mice. Following contractile activity, muscle fibers from SOD1KO mice also showed substantially reduced generation of superoxide compared with fibers from WT mice. Inhibition of NO synthase to reduce NO availability, and hence the potential for formation of peroxynitrite, did not affect DHE oxidation in fibers from WT or SOD1KO at rest or during contractions. In contrast, fibers isolated from neuronal NO synthase transgenic mice showed increased DAF-FM fluorescence and reduced DHE oxidation in resting muscle fibers. These data appear to indicate that peroxynitrite is formed in muscle fibers as a consequence of lack of SOD1 in SOD1KO mice and may, therefore, contribute to fiber loss in this model. More generally, these data also support the hypothesis that NO regulates superoxide availability and peroxynitrite formation in muscle fibers (78).
RELATIVE ROLE OF A LACK OF SOD1 IN MUSCLE OR IN MOTONEURONS IN THE ACCELERATED AGING PHENOTYPE SEEN IN SOD1KO MICE.
To specifically examine how changes in muscle SOD1 might influence age-related changes in muscle, mice with muscle-specific deletion of SOD1 (mSOD1KO mice) were examined (100), but these mice show no evidence of premature NMJ degeneration or loss of muscle fibers and surprisingly showed some muscle hypertrophy (100). We examined whether the changes in ROS generation observed in the global knockout model (SOD1KO mice) were also seen in mSOD1KO mice. In brief, the multiple changes in markers of oxidative damage and adaptation seen in SOD1KO mice and described above were not observed in the mSOD1KO mice, including no evidence for the increases in 3-nitrotyrosines and peroxiredoxin V previously reported in muscles of SOD1KO mice (79, 100).
To determine the role of motoneurons in the loss of muscle mass and function seen in SOD1KO mice, a transgenic SOD1KO mouse in which human SOD1 is expressed in neurons under control of a synapsin 1 promoter (nSOD1Tg-SOD1KO mice) was established (80). These “nerve rescue” mice expressed SOD1 in central and peripheral neurons, but not other tissues. Sciatic nerve CuZnSOD content in nSOD1Tg-SOD1KO mice was ∼20% that of WT control mice, but they showed no loss of muscle mass or maximum isometric specific force production at 8–12 mo of age, when significant reductions were seen in SOD1KO mice (80). Thus these data appeared to demonstrate that at least 20% of WT CuZnSOD levels in neurons is essential in preserving skeletal muscle and NMJ structure and function in SOD1KO mice and implicated a lack of SOD1 specifically in motoneurons in the pathogenesis of the accelerated muscle aging phenotype seen in the whole body SOD1 null mice.
Adult mice lacking SOD1, therefore, replicate many of the features seen in old WT mice, and it appears that further examination of this model and variants of the model with tissue-specific modification of SOD1 content could identify key mechanisms leading to loss of muscle fibers and function that are relevant to aging of WT mice. The initiating role for the motoneuron in this model provides a means of determining mechanisms by which disruption of redox homeostasis in the motoneuron can cause loss of muscle fibers, and we speculate that this may also be important for aging in WT mice. Although SOD1KO mice are a model in which fundamental questions about mechanisms that are highly relevant to understanding muscle aging can be addressed, it is reiterated that there is no evidence that a simple lack of SOD1 contributes to aging-related loss of muscle in WT mice or humans.
POTENTIAL PRIMARY AND SECONDARY SOURCES OF ROS DURING AGING
Increased ROS generation by mitochondria has been implicated in aging of muscle and other tissues for a considerable period of time. This process was originally claimed to have a primary role in the aging process (38, 81), but the recent work of Pérez et al. (70) and Gems and Doonan (20) argues strongly against a primary role for oxidative damage in skeletal muscle in aging. Other recent data also indicate that not all mitochondria isolated from aging muscle show increased ROS generation (21, 25). Despite these contrasting data, some interventions that specifically reduce mitochondrial ROS (mice overexpressing catalase in mitochondria, mCAT mice; Ref. 82) or protect against oxidative damage (mice overexpressing heat shock protein 10, HSP10Tg mice; Ref. 44) appear to preserve muscle mass and function. We have previously proposed that excess generation of hydrogen peroxide by mitochondrial from aged mice could act to attenuate the ability of muscle fibers from aged mice to adapt to contractile activity (35) as shown schematically in Fig. 3B. It is, therefore, relevant to consider whether increased mitochondrial ROS might play a secondary role in aging processes and be a consequence of more direct effects of aging. Potential examples of this may be the increase in muscle mitochondrial ROS that appears to occur secondarily to other age-related changes in the SOD1KO mouse studies described above, and also by the observation that experimental denervation leads to a very large sustained increase in muscle mitochondrial ROS generation (59). Data from both of these situations support the possibility that functional denervation of individual muscle fibers may lead to a fiber-specific increase in mitochondrial ROS generation.
There is extensive evidence that some denervation of muscle fibers occurs with aging. In humans, an ∼25% reduction in the number of motoneurons occurs with aging, and, although the causes of this loss are unknown, small motoneurons (which tend to innervate type I fibers) are preserved relative to large motoneurons. Over time, the loss of large motoneurons appears to be partially compensated by a sprouting phenomenon through which small motoneurons innervate those type II fibers that have become temporarily denervated, and hence these fibers acquire a slower phenotype. This process is thought to be incomplete and eventually the new “giant” motor units are lost (13). Studies to determine whether the age-related loss of muscle fibers is associated with loss of motor units in humans and rodents indicate that substantial net loss of whole motor units occurs with increasing age in both species (8, 18, 48). Atrophy and loss of axons has been reported in older individuals (93), together with additional abnormalities in peripheral nerves, including segmental demyelination (1, 83), swollen demyelinated and remyelinated axons, and denervated Schwann cell columns (26). A variety of changes have been reported in NMJs of aged mice, including axonal swelling and sprouting, withdrawal of axons from postsynaptic sites, and fragmentation of the postsynaptic structures (10, 86), and there is evidence from older postmortem studies that such changes are seen in elderly humans (62). Recent data from rodents also indicate that, despite the loss of peripheral axons that occurs with aging, the number of motoneuron cell bodies in the lumbar spinal cord are unchanged, suggesting that changes may predominantly occur in peripheral regions of motor units (10). Thus it appears that motor axon and NMJ loss with aging occurs in parallel with loss of muscle fibers and diminished muscle function (9, 49, 50) in both humans and animals, but it is currently unclear whether either of these is the primary event (48, 95).
Thus we speculate that a feasible integrating mechanism based on the present data relating to the age-related changes in ROS activities and redox signaling in muscle is that denervation of individual muscle fibers leads to a large increase in mitochondrial ROS generation in the affected fibers. Since the key ROS generated in mitochondria of the denervated fibers appears to be hydrogen peroxide or other peroxides, such species are membrane permeable and could diffuse to adjacent innervated fibers, leading to redox-related changes in oxidative damage and redox signaling.
CONCLUSIONS
In conclusion, recent data indicate that membrane-localized NADPH oxidase(s) are the source of the superoxide generated in skeletal muscle during contractile activity that play an important role in redox signaling, and that these pathways upregulate cytoprotective responses that aid maintenance of cell viability following contractile activity. A failure of this redox signaling pathway appears to occur in muscle during aging and may contribute to the loss muscle fibers, but whether these changes are primary or secondary events in aging is unclear. One possible explanation that provides an explanation for the present data is that a small number of denervated muscle fibers within the muscle may generate large amounts of hydrogen peroxide from mitochondria, and that this can influence redox signaling in adjacent innervated fibers, and thus provides a secondary source of ROS that may contribute to loss of muscle mass and function during aging.
GRANTS
This work has also been supported by many funding agencies, including the Biotechnology and Biological Sciences Research Council, Medical Research Council, Arthritis Research UK, Research into Ageing, Wellcome Trust, and US National Institute on Aging.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.J.J. conception and design of research; M.J.J. performed experiments; M.J.J. analyzed data; M.J.J. interpreted results of experiments; M.J.J. prepared figures; M.J.J. drafted manuscript; M.J.J. edited and revised manuscript; M.J.J. approved final version of manuscript.
ACKNOWLEDGMENTS
The author acknowledges the many collaborators and colleagues who have contributed to this work over 30 years, with particular thanks to his mentors, the late Professor Richard H. T. Edwards and Professor John F. Faulkner (University of Michigan), who inspired his work on skeletal muscle and aging.
REFERENCES
- 1.Adinolfi AM, Yamuy J, Morales FR, Chase MH. Segmental demyelination in peripheral nerves of old cats. Neurobiol Aging 12: 175–179, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M, Acharyya S, Rudnicki MA, Hollenbach AD, Guttridge DC. IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis. J Cell Biol 180: 787–802, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol 77: 2519–2521, 1994. [DOI] [PubMed] [Google Scholar]
- 4.Bar-Shai M, Carmeli E, Reznick AZ. The role of NF-kappaB in protein breakdown in immobilization, aging, and exercise: from basic processes to promotion of health. Ann N Y Acad Sci 1057: 431–447, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Beckman JS, Koppenol WH. Nitric oxide, superoxide, peroxynitrite: the good, the bad, ugly. Am J Physiol Cell Physiol 271: C1424–C1437, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broome C, Kayani AC, Palomero J, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Effect of lifelong overexpression of HSP70 in skeletal muscle on age-related oxidative stress and adaptation following non-damaging contractile activity. FASEB J 20: 1549–1551, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Brown WF, Strong MJ, Snow R. Methods for estimating numbers of motor units in biceps-brachialis muscles and losses of motor units with aging. Muscle Nerve 11: 423–432, 1988. [DOI] [PubMed] [Google Scholar]
- 9.Campbell MJ, Mccomas AJ, Petito F. Physiological changes in ageing muscles. J Neurol Neurosurg Psychiatry 36: 174–182, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chai RJ, Vukovic J, Dunlop S, Grounds MD, Shavlakadze T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLos One 6: e28090, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Close GC, Ashton T, McArdle A, Jackson MJ. Microdialysis studies of extracellular reactive oxygen species in skeletal muscle: factors influencing the reduction of cytochrome c and hydroxylation of salicylate. Free Radic Biol Med 39: 1460–1467, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J 19: 422–424, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Delbono O. Neural control of aging skeletal muscle. Aging Cell 2: 21–9, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Demontis F, Piccirillo R, Goldberg AL, Perrimon N. The influence of skeletal muscle on systemic aging and lifespan. Aging Cell 12: 943–949, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dillard CJ, Litov RE, Savin WM, Dumelin EE, Tappel AL. Effects of exercise, vitamin E, and ozone on pulmonary function and lipid peroxidation. J Appl Physiol 45: 927–932, 1978. [DOI] [PubMed] [Google Scholar]
- 16.Drew B, Phaneuf S, Dirks A, Selman C, Gredilla R, Lezza A, Barja G, Leeuwenburgh C. Effects of aging and caloric restriction on mitochondrial energy production in gastrocnemius muscle and heart. Am J Physiol Regul Integr Comp Physiol 284: R474–R480, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Dröge W. Free radicals in the physiological control of cell function. Physiol Rev 82: 47–95, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Einsiedel LJ, Luff AR. Alterations in the contractile properties of motor units within the ageing rat medial gastrocnemius. J Neurol Sci 112: 170–177, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Espinosa A, Leiva A, Pena M, Muller M, Debandi A, Hidalgo C, Carrasco MA, Jaimovich E. Myotube depolarization generates reactive oxygen species through NAD(P)H oxidase; ROS-elicited Ca2+ stimulates ERK, CREB, early genes. J Cell Physiol 209: 379–388, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Gems D, Doonan R. Antioxidant defense and aging in C. elegans: is the oxidative damage theory of aging wrong? Cell Cycle 8: 1681–1687, 2009. [DOI] [PubMed] [Google Scholar]
- 21.Ghosh S, Lertwattanarak R, Lefort N, Molina-Carrion M, Joya-Galeana J, Bowen BP, Garduno-Garcia JJ, Abdul-Ghani M, Richardson A, DeFronzo RA, Mandarino L, Van Remmen H, Musi N. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes 60: 2051–60, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomez-Cabrera MC, Borras C, Pallardo FV, Sastre J, Ji LL, Vina J. Decreasing xanthine oxidase-mediated oxidative stress prevents useful cellular adaptations to exercise in rats. J Physiol 567: 113–120, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gomez-Cabrera MC, Domenech E, Romagnoli M, Arduini A, Borras C, Pallardo FV, Sastre J, Viña J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am J Clin Nutr 87: 142–149, 2008. [DOI] [PubMed] [Google Scholar]
- 24.Gomez-Cabrera MC, Close GL, Kayani A, McArdle A, Jackson MJ. Effect of xanthine oxidase-generated extracellular superoxide on skeletal muscle force generation. Am J Physiol Regul Integr Comp Physiol 298: R2–R8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gouspillou G, Sgarioto N, Kapchinsky S, Purves-Smith F, Norris B, Pion CH, Barbat-Artigas S, Lemieux F, Taivassalo T, Morais JA, Aubertin-Leheudre M, Hepple RT. Increased sensitivity to mitochondrial permeability transition and myonuclear translocation of endonuclease G in atrophied muscle of physically active older humans. FASEB J 28: 1621–1633, 2014. [DOI] [PubMed] [Google Scholar]
- 26.Grover-Johnson N, Spencer PS. Peripheral nerve abnormalities in aging rats. J Neuropathol Exp Neurol 40: 155–165, 1981. [DOI] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JMC. Free Radical Biology and Medicine. Oxford, UK: Oxford University Press, 1989. [Google Scholar]
- 28.Haddad JJ. Antioxidant and pro-oxidant mechanisms in the regulation of redox(y)-sensitive transcription factors. Cell Signal 14: 879–897, 2002. [DOI] [PubMed] [Google Scholar]
- 29.Higashida K, Kim SH, Higuchi M, Holloszy JO, Han DH. Normal adaptations to exercise despite protection against oxidative stress. Am J Physiol Endocrinol Metab 301: E779–E784, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hollander JM, Lin KM, Scott BT, Dillmann WH. Overexpression of PHGPx and HSP60/10 protects against ischemia/reoxygenation injury. Free Radic Biol Med 35: 742–751, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Holloszy JO. Response to letter to the editor by Gomez-Cabrera et al. Am J Physiol Endocrinol Metab 302: E478–E479, 2012. [DOI] [PubMed] [Google Scholar]
- 32.Jackson MJ. Lack of CuZnSOD activity: a pointer to the mechanisms underlying age-related loss of muscle function, a commentary on “absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy”. Free Radic Biol Med 40: 1900–1902, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Jackson MJ. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid Redox Signal 15: 2477–2486, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jackson MJ. Free radicals generated by contracting muscle: by-products of metabolism or key regulators of muscle function? Free Radic Biol Med 44: 132–141, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Jackson MJ, McArdle A. Age-related changes in skeletal muscle reactive oxygen species generation and adaptive responses to reactive oxygen species. J Physiol 589: 2139–2145, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson MJ, Papa S, Bolanos J, Bruckdorfer R, Carlsen H, Elliott RM, Flier J, Griffiths HR, Heales S, Holst B, Lorusso M, Lund E, Oivind Moskaug J, Moser U, Di Paola M, Polidori MC, Signorile A, Stahl W, Vina-Ribes J, Astley SB. Antioxidants, reactive oxygen and nitrogen species, gene induction and mitochondrial function. Mol Aspects Med 23: 209–285, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Jang YC, Lustgarten MS, Liu Y, Muller FL, Bhattacharya A, Liang H, Salmon AB, Brooks SV, Larkin L, Hayworth CR, Richardson A, Van Remmen H. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J 24: 1376–1390, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jang YC, Van Remmen H. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol 44: 256–260, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Jang YC, Van Remmen H. Age-associated alterations of the neuromuscular junction. Exp Gerontol 46: 193–198, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Janssen-Heininger YM, Mossman BT, Heintz NH, Forman HJ, Kalyanaraman B, Finkel T, Stamler JS, Rhee SG, van der Vliet A. Redox-based regulation of signal transduction: principles, pitfalls, and promises. Free Radic Biol Med 45: 1–17, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Javesghani D, Magder SA, Barreiro E, Quinn MT, Hussain SN. Molecular characterization of a superoxide-generating NAD(P)H oxidase in the ventilatory muscles. Am J Respir Crit Care Med 165: 412–418, 2002. [DOI] [PubMed] [Google Scholar]
- 42.Ji LL, Gomez-Cabrera MC, Steinhafel N, Vina J. Acute exercise activates nuclear factor (NF)-kappaB signaling pathway in rat skeletal muscle. FASEB J 18: 1499–1506, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Kawamata H, Manfredi G. Import, maturation, and function of SOD1 and its copper chaperone CCS in the mitochondrial intermembrane space. Antioxid Redox Signal 13: 1375–1384, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kayani AC, Close GL, Dillmann WH, Mestril R, Jackson MJ, McArdle A. Overexpression of HSP10 in skeletal muscle of transgenic mice prevents the age-related fall in maximum tetanic force generation and muscle cross-sectional area. Am J Physiol Regul Integr Comp Physiol 299: R268–R276, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khassaf M, McArdle A, Esanu C, Vasilaki A, McArdle F, Griffiths RD, Brodie DA, Jackson MJ. Effect of vitamin C supplements on antioxidant defence and stress proteins in human lymphocytes and skeletal muscle. J Physiol 549: 645–652, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobzik L, Reid MB, Bredt DS, Stamler JS. Nitric oxide in skeletal muscle. Nature 372: 546–548, 1994. [DOI] [PubMed] [Google Scholar]
- 47.Larkin LM, Davis CS, Sims-Robinson C, Kostrominova TY, Van Remmen H, Richardson A, Feldman EL, Brooks SV. Skeletal muscle weakness due to deficiency of CuZn-superoxide dismutase is associated with loss of functional innervation. Am J Physiol Regul Integr Comp Physiol 301: R1400–R1407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Larsson L, Ansved T. Effects of ageing on the motor unit. Prog Neurobiol 45: 397–458, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Lexell J, Downham D, Sjostrom M. Distribution of different fibre types in human skeletal muscles. Fibre type arrangement in m vastus lateralis from three groups of healthy men between 15 and 83 years. J Neurol Sci 72: 211–222, 1986. [DOI] [PubMed] [Google Scholar]
- 50.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988. [DOI] [PubMed] [Google Scholar]
- 51.Ljubicic V, Hood DA. Kinase-specific responsiveness to incremental contractile activity in skeletal muscle with low and high mitochondrial content. Am J Physiol Endocrinol Metab 295: E195–E204, 2008. [DOI] [PubMed] [Google Scholar]
- 52.McArdle A, Dillmann WH, Mestril R, Faulkner JA, Jackson MJ. Overexpression of HSP70 in mouse skeletal muscle protects against muscle damage and age-related muscle dysfunction. FASEB J 18: 355–357, 2004. [DOI] [PubMed] [Google Scholar]
- 53.McArdle A, Pattwell D, Vasilaki A, Griffiths RD, Jackson MJ. Contractile activity-induced oxidative stress: cellular origin and adaptive responses. Am J Physiol Cell Physiol 280: C621–C627, 2001. [DOI] [PubMed] [Google Scholar]
- 54.McArdle F, Spiers S, Aldemir H, Vasilaki A, Beaver A, Iwanejko L, McArdle A, Jackson MJ. Preconditioning of skeletal muscle against contraction-induced damage: the role of adaptations to oxidants in mice. J Physiol 561: 233–244, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Melov S. Mitochondrial oxidative stress. Physiologic consequences and potential for a role in aging. Ann N Y Acad Sci 908: 219–225, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Melov S, Ravenscroft J, Malik S, Gill MS, Walker DW, Clayton PE, Wallace DC, Malfroy B, Doctrow SR, Lithgow GJ. Extension of life-span with superoxide dismutase/catalase mimetics. Science 289: 1567–1569, 2000. [DOI] [PubMed] [Google Scholar]
- 57.Miller RA. “Accelerated aging”: a primrose path to insight? Aging Cell 3: 47–51, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Muller FL, Song W, Liu Y, Chaudhuri A, Pieke-Dahl S, Strong R, Huang TT, Epstein CJ, Roberts LJ 2nd, Csete M, Faulkner JA, Van Remmen H. Absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic Biol Med 40: 1993–2004, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Van Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 293: R1159–R1168, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Murrant CL, Reid MB. Detection of reactive oxygen and reactive nitrogen species in skeletal muscle. Microsc Res Tech 55: 236–248, 2001. [DOI] [PubMed] [Google Scholar]
- 61.Nilwik R, Snijders T, Leenders M, Groen BB, Van Kranenburg J, Verdijk LB, Van Loon LJ. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp Gerontol 48: 492–498, 2013. [DOI] [PubMed] [Google Scholar]
- 62.Oda K. Age changes of motor innervation and acetylcholine receptor distribution on human skeletal muscle fibres. J Neurol Sci 66: 327–338, 1984. [DOI] [PubMed] [Google Scholar]
- 63.Orr WC, Sohal RS. Effects of Cu-Zn superoxide dismutase overexpression of life span and resistance to oxidative stress in transgenic Drosophila melanogaster. Arch Biochem Biophys 301: 34–40, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Orr WC, Sohal RS. Extension of life-span by overexpression of superoxide dismutase and catalase in Drosophila melanogaster. Science 263: 1128–1130, 1994. [DOI] [PubMed] [Google Scholar]
- 65.Orr WC, Sohal RS. Does overexpression of Cu,Zn-SOD extend life span in Drosophila melanogaster? Exp Gerontol 38: 227–230, 2003. [DOI] [PubMed] [Google Scholar]
- 66.Palomero J, Pye D, Kabayo T, Spiller DG, Jackson MJ. In situ detection and measurement of intracellular reactive oxygen species in single isolated mature skeletal muscle fibres by real-time fluorescence microscopy. Antioxid Redox Signal 10: 1463–1474, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pattwell DM, McArdle A, Morgan JE, Patridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Radic Biol Med 37: 1064–1072, 2004. [DOI] [PubMed] [Google Scholar]
- 68.Paulsen G, Cumming KT, Holden G, Hallén J, Rønnestad BR, Sveen O, Skaug A, Paur I, Bastani NE, Østgaard HN, Buer C, Midttun M, Freuchen F, Wiig H, Ulseth ET, Garthe I, Blomhoff R, Benestad HB, Raastad T. Vitamin C and E supplementation hampers cellular adaptation to endurance training in humans: a double-blind randomized controlled trial. J Physiol 592: 1887–1901, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pearson T, Kabayo T, Ng R, Chamberlain J, McArdle A, Jackson MJ. Skeletal muscle contractions induce acute changes in cytosolic superoxide, but slower responses in mitochondrial superoxide and cellular hydrogen peroxide. PLos One 9: e96378, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pérez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim Biophys Acta 1790: 1005–1014, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peterson JM, Guttridge DC. Skeletal muscle diseases, inflammation, and NF-kappaB signaling: insights and opportunities for therapeutic intervention. Int Rev Immunol 27: 375–387, 2008. [DOI] [PubMed] [Google Scholar]
- 72.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: structure, function and adaptability. Scand J Med Sci Sports 5: 129–142, 1995. [DOI] [PubMed] [Google Scholar]
- 73.Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev 88: 1243–1276, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Powers SK, Smuder AJ, Criswell DS. Mechanistic links between oxidative stress and disuse muscle atrophy. Antioxid Redox Signal 15: 2519–2528, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pye D, Kabayo T, Palmero J, Jackson MJ. Real-time measurements of nitric oxide in mature skeletal muscle fibres during contractions. J Physiol 581: 309–318, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reid MB, Shoji T, Moody MR, Entman ML. Reactive oxygen in skeletal muscle. II. Extracellular release of free radicals. J Appl Physiol 73: 1805–1809, 1992. [DOI] [PubMed] [Google Scholar]
- 77.Ristow M, Zarse K, Oberbach A, Klöting N, Birringer M, Kiehntopf M, Stumvoll M, Kahn CR, Blüher M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc Natl Acad Sci U S A 106: 8665–8670, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sakellariou GK, Pye D, Vasilaki A, Zibrik L, Palomero J, Kabayo T, McArdle F, Van Remmen H, Richardson A, Tidball JG, McArdle A, Jackson MJ. Role of superoxide-nitric oxide interactions in the accelerated age-related loss of muscle mass in mice lacking Cu,Zn superoxide dismutase. Aging Cell 10: 749–760, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ. Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18: 603–621, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sakellariou GK, Davis CS, Shi Y, Ivannikov MV, Zhang Y, Vasilaki A, Macleod GT, Richardson A, Van Remmen H, Jackson MJ, McArdle A, Brooks SV. Neuron-specific expression of CuZnSOD prevents the loss of muscle mass and function that occurs in homozygous CuZnSOD-knockout mice. FASEB J 28: 1666–1681, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sastre J, Pallardo FV, Vina J. The role of mitochondrial oxidative stress in aging. Free Radic Biol Med 35: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- 82.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 308: 1909–1911, 2005. [DOI] [PubMed] [Google Scholar]
- 83.Sharma AK, Bajada S, Thomas PK. Age changes in the tibial and plantar nerves of the rat. J Anat 130: 417–428, 2008. [PMC free article] [PubMed] [Google Scholar]
- 84.Silveira LR, Pereira-Da-Silva L, Juel C, Hellsten Y. Formation of hydrogen peroxide and nitric oxide in rat skeletal muscle cells during contractions. Free Radic Biol Med 35: 455–464, 2003. [DOI] [PubMed] [Google Scholar]
- 85.Sun QA, Wang B, Miyagi M, Hess DT, Stamler JS. Oxygen-coupled redox regulation of the skeletal muscle ryanodine receptor/Ca2+ release channel (RyR1): sites and nature of oxidative modification. J Biol Chem 288: 22961–22971, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Valdez G, Tapia JC, Kang H, Clemenson GD Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A 107: 14863–14868, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Gammeren D, Damrauer JS, Jackman RW, Kandarian SC. The IkappaB kinases IKKalpha and IKKbeta are necessary and sufficient for skeletal muscle atrophy. FASEB J 23: 362–370, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Van Remmen H, Jones DP. Current thoughts on the role of mitochondria and free radicals in the biology of aging. J Gerontol A Biol Sci Med Sci 64: 171–174, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Vasilaki A, Csete M, Pye D, Lee S, Palomero J, McArdle F, Van Remmen H, Richardson A, McArdle A, Faulkner JA, Jackson MJ. Genetic modification of the MnSOD/GPx1 pathway influences intracellular ROS generation in quiescent, but not contracting myotubes. Free Radic Biol Med 41: 1719–1725, 2006. [DOI] [PubMed] [Google Scholar]
- 90.Vasilaki A, Mansouri A, Remmen H, van der Meulen JH, Larkin L, Richardson AG, McArdle A, Faulkner JA, Jackson MJ. Free radical generation by skeletal muscle of adult and old mice: effect of contractile activity. Aging Cell 5: 109–117, 2006. [DOI] [PubMed] [Google Scholar]
- 91.Vasilaki A, McArdle F, Iwanejko LM, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev 127: 830–839, 2006. [DOI] [PubMed] [Google Scholar]
- 92.Vasilaki A, Van Der Meulen JH, Larkin L, Harrison DC, Pearson T, Van Remmen H, Richardson A, Brooks SV, Jackson MJ, Mcardle A. The age-related failure of adaptive responses to contractile activity in skeletal muscle is mimicked in young mice by deletion of Cu,Zn superoxide dismutase. Aging Cell 9: 979–990, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst 5: 191–208, 2000. [DOI] [PubMed] [Google Scholar]
- 94.Viña J, Gimeno A, Sastre J, Desco C, Asensi M, Pallardó FV, Cuesta A, Ferrero JA, Terada LS, Repine JE. Mechanism of free radical production in exhaustive exercise in humans and rats; role of xanthine oxidase and protection by allopurinol. IUBMB Life 49: 539–544, 2005. [DOI] [PubMed] [Google Scholar]
- 95.Wang ZM, Zheng Z, Messi ML, Delbono O. Extension and magnitude of denervation in skeletal muscle from ageing mice. J Physiol 565: 757–764, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ward CW, Prosser BL, Lederer WJ. Mechanical stretch-induced activation of ROS/RNS signaling in striated muscle. Antioxid Redox Signal 20: 929–936, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xia R, Webb JA, Gnall LL, Cutler K, Abramson JJ. Skeletal muscle sarcoplasmic reticulum contains a NADH-dependent oxidase that generates superoxide. Am J Physiol Cell Physiol 285: C215–C221, 2003. [DOI] [PubMed] [Google Scholar]
- 98.Yoshida T, Nakamura H, Masutani H, Yodoi J. The involvement of thioredoxin and thioredoxin binding protein-2 on cellular proliferation and aging process. Ann N Y Acad Sci 1055: 1–12, 2005. [DOI] [PubMed] [Google Scholar]
- 99.Young A, Skelton DA. Applied physiology of strength and power in old age. Int J Sports Med 15: 149–151, 1994. [DOI] [PubMed] [Google Scholar]
- 100.Zhang Y, Davis C, Sakellariou GK, Shi Y, Kayani AC, Pulliam D, Bhattacharya A, Richardson A, Jackson MJ, McArdle A, Brooks SV, Van Remmen H. CuZnSOD gene deletion targeted to skeletal muscle leads to loss of contractile force but does not cause muscle atrophy in adult mice. FASEB J 27: 3536–3548, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]