Abstract
While home dialysis is being promoted, there are few comparative effectiveness studies of home-based modalities to guide patient decisions. To address this, we matched 1116 daily home hemodialysis (DHD) patients by propensity scores to 2784 contemporaneous USRDS patients receiving home peritoneal dialysis (PD), and compared hospitalization rates from cardiovascular, infectious, access-related or bleeding causes (prespecified composite), and modality failure risk. We performed similar analyses for 1187 DHD patients matched to 3173 USRDS patients receiving in-center conventional hemodialysis (CHD). The composite hospitalization rate was significantly lower with DHD than with PD (0.93 vs. 1.35/patient-year, hazard ratio=0.73 (95% CI=0.67–0.79)). DHD patients spent significantly fewer days in hospital than PD patients (5.2 vs. 9.2 days/patient-year), and significantly more DHD patients remained admission-free (52% DHD vs. 32% PD). In contrast, there was no significant difference in hospitalizations between DHD and CHD (DHD vs. CHD: 0.93 vs. 1.10/patient-year, hazard ratio 0.92 (0.85–1.00)). Cardiovascular hospitalizations were lower with DHD than with CHD (0.68 (0.61–0.77)), while infectious and access hospitalizations were higher (1.15 (1.04–1.29) and 1.25 (1.08–1.43), respectively). Significantly more PD than DHD patients switched back to in-center HD (44% vs. 15% 3.4 (2.9–4.0)). In this prevalent cohort, home DHD was associated with fewer admissions and hospital days than PD, and a substantially lower risk of modality failure.
Keywords: cardiovascular disease, hemodialysis, peritoneal dialysis
Home-based dialysis modalities are being increasingly promoted internationally as preferred renal replacement therapy options when transplantation is not immediately feasible.1, 2, 3, 4, 5, 6 Both home peritoneal dialysis (PD) and home hemodialysis (HD) are associated with similar or better survival than in-center HD,7 while offering better patient autonomy and quality of life,8, 9 at lower cost.10, 11 PD remains the dominant home dialysis therapy, comprising 15% of dialysis in developed countries.12 In contrast, <2% of patients with end-stage renal disease receive home HD.13 Yet, although the use of PD has been steadily decreasing in developed countries,12 the prevalence of home HD is increasing.13, 14
Many advocate home HD in order to facilitate the delivery of more intensive dialysis, delivered as more frequent (at least 5 days per week) and/or longer (at least 6 h per treatment) sessions. However, whether such intensive HD therapies improve hard outcomes over conventional three times per week HD or PD is as yet unknown. Prior observational studies suggested that home frequent HD was associated with better survival than in-center three times per week HD.15, 16, 17, 18, 19 Despite rigorous methods used in some of these studies to match groups on known prognostic variables,15, 16, 17 these studies are difficult to interpret, as they compared home patients with in-center patients. Compared with patients receiving in-center HD, patients performing their own treatments at home may have better health literacy, social support, financial resources, cognitive function, and motivation. It is thus unclear whether the observed improvements in survival in these studies were related to greater HD frequency and duration, or to these other unmeasured factors. A recent observational study comparing home intensive with home CHD found no difference in survival between groups,20 and the Frequent Hemodialysis Network Nocturnal Randomized Trial surprisingly noted significantly increased mortality with six nights per week home HD compared with three days per week home HD.21
Nevertheless, the recent growth in home HD has likely been facilitated by options for more frequent and/or longer treatments, the possibility of night-time treatments, the recent development of easier-to-use machines, and better funding models.13, 22 As home HD becomes more widely available, patients needing dialysis are now faced with a multitude of choices: hospital or home-based therapy; if home-based, PD or home HD; if HD, what frequency and duration? Few comparative effectiveness studies of these options exist to help guide these choices. Given that patients opting for home daily HD often also have the choice of doing PD instead, studies comparing the effects of these two therapies on clinically important, patient-centered outcomes are highly relevant. Acknowledging differences in technique, training time, and available home care support between home daily HD and PD, appropriately matched comparisons of home daily HD with PD would have the added advantage of being less subject to selection bias introduced by self-care ability, as both therapies are performed at home. This may be particularly true of home HD with newer devices that have improved the ease of training for home daily HD.23
With these considerations, we performed an observational matched retrospective cohort study to compare dialysis-related hospitalization risk associated with DHD versus PD. We hypothesized that DHD would result in less cardiovascular, infectious, and bleeding admissions, but similar access-related hospitalizations compared with PD. This hypothesis was based on the theoretical benefits of DHD over PD, including higher daily ultrafiltration capacity, greater small solute clearance with potentially improved hemostasis and immune function, but an equal tendency to vascular access or PD catheter–related complications. Notwithstanding the limitations of home versus in-center analyses discussed above, we also compared hospitalization risk between home DHD and in-center three times per week CHD to serve as a reference point in the context of previous studies.
RESULTS
Study sample, baseline characteristics, and dialysis prescription
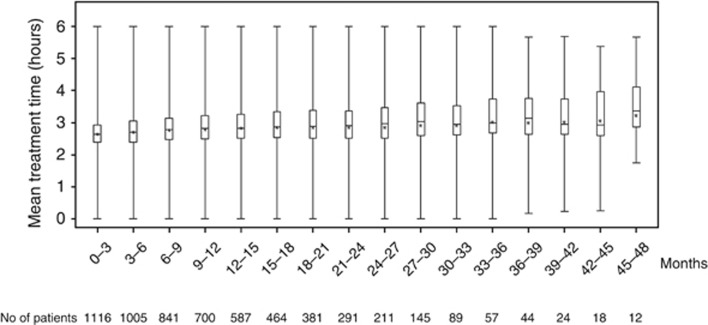
Of 1386 potentially eligible DHD subjects, 1116 (81%) were matched to 2784 PD controls, and 1187 (86%) were matched to 3173 in-center CHD controls (Table 1). Distribution of baseline variables was similar between DHD and comparator groups with standardized differences of <10% for all variables (Table 2). The mean treatment time received by DHD subjects was 2.7 h (s.d.=0.6, interquartile range=2.4-2.9 h) during month 1 and 2.9 h (s.d.=0.6, interquartile range=2.5–3.4 h) by 24 months (Figure 1). Eighty-nine percent of DHD patients used low dialysate flow rates of 300 ml/min; the remaining received ⩾900 ml/min. Mean percent reduction in urea was 40% per treatment (s.d.=10, interquartile range=35–43). Of PD controls, 68% received continuous ambulatory PD, whereas 32% used a cycler.
Table 1. Patient flow.
| Home DHD | Home PD | Home DHD | In-center CHD | |
|---|---|---|---|---|
| No of patients aged⩾ 18 years | 2501 | 195,465 | 2501 | 1,519,609 |
| Exclusions | ||||
| Not in the time windowa | 0 | 133,908 | 0 | 109,623 |
| Not Medicare before index | 922 | 41,280 | 922 | — |
| Nonindependent living | 50 | 828 | 50 | 65,282 |
| Missing race | 0 | 1 | 0 | 573 |
| Missing comorbidity | 0 | 1 | 0 | 59 |
| BMI>50 or <16 or missing | 87 | 634 | 87 | 116,201 |
| Albumin <1.0 g/dl or hemoglobin<5 g/dl | 10 | 81 | 10 | 6057 |
| Prior transplants>2 | 2 | 5 | 2 | 30 |
| Follow-up<30 days | 44 | 618 | 44 | 41,537 |
| Total no excluded | 1115 | 177,356 | 1115 | 339,362 |
| No eligible | 1386 | 18,109 | 1386 | 1,180,247 |
| No matched | 1116 | 2784 | 1187 | 3173 |
| Follow-up time (years) | 1506 | 4923 | 1614 | 6885 |
| (25/50/75th percentile) | (0.6/1.2/2.0) | (0.7/1.4/2.6) | 0.6/1.2/2.0) | (0.9/1.9/3.2) |
| Range | 0.1–4.8 | 0.1–5.4 | 0.1–4.8 | 0.1–7.9 |
Abbreviations: BMI, body mass index; CHD, conventional hemodialysis; DHD, daily home hemodialysis; HD, home hemodialysis; No, number; PD, peritoneal dialysis; USRDS, United States Renal Data System.
The time window was defined as having started renal replacement therapy after 1995, as end-stage renal disease patients were more completely captured in the USRDS after this date. In addition, only patients who started PD between 2004 and 2009 were eligible to be selected as PD controls, as DHD subjects also began HD during this period.
Table 2. Baseline characteristics.
| Variable | Home DHD n=1116 | Home PD n=2784 | Standardized difference | Home DHD n=1187 | In-center CHD n=3173 | Standardized difference |
|---|---|---|---|---|---|---|
| Age (years) | 50.5 (15.8) | 50.9 (15.6) | 2.1 | 50.3 (15.9) | 50.8 (15.7) | 2.9 |
| 18–29 years (%) | 10.5 | 9.2 | 4.4 | 10.6 | 9.7 | 3.0 |
| 30–39 years (%) | 18.2 | 16.2 | 5.3 | 19.2 | 19.0 | 0.4 |
| 40–49 years (%) | 18.6 | 17.9 | 1.8 | 17.4 | 17.7 | 1.0 |
| 50–59 years (%) | 19.4 | 20.8 | 3.5 | 20.3 | 20.1 | 0.5 |
| 60–69 years (%) | 20.8 | 22.2 | 3.3 | 19.8 | 20.6 | 2.0 |
| >70 years (%) | 12.6 | 13.9 | 3.7 | 12.8 | 12.8 | 0.2 |
| Male sex (%) | 67.3 | 66.9 | 0.9 | 67.6 | 67.6 | 0.1 |
| Smoker (%) | 7.4 | 6.9 | 1.9 | 7.2 | 7.3 | 0.6 |
| BMI (kg/m2; %) | 29.0 (6.8) | 28.6 (6.2) | 6.0 | 29.6 (7.2) | 29.8 (7.1) | 1.8 |
| Race | ||||||
| White (%) | 70.9 | 73.1 | 1.9 | 68.8 | 68.0 | 1.9 |
| Black (%) | 26.6 | 25.1 | 3.4 | 28.1 | 29.1 | 2.1 |
| Others (%) | 2.5 | 1.2 | 9.7 | 3.0 | 3.0 | 0.4 |
| ESRD start date | ||||||
| 1977–1994 (%) | 0.6 | 0.6 | 0.4 | 0.6 | 0.4 | 3.5 |
| 1995–1999 (%) | 8.1 | 7.6 | 1.6 | 8.3 | 6.7 | 6.1 |
| 2000–2004 (%) | 21.2 | 22.1 | 2.0 | 23.0 | 22.5 | 1.2 |
| 2005–2009 (%) | 70.1 | 69.8 | 0.6 | 68.0 | 70.4 | 5.1 |
| ESRD duration (years) | 3.3 (2.9) | 3.3 (3.2) | 2.3 | 3.5 (2.9) | 3.5 (2.7) | 0.7 |
| 1–3 months (%) | 0.9 | 1.0 | 1.4 | 0.9 | 1.0 | 1.1 |
| 3–6 months (%) | 7.4 | 8.4 | 4.0 | 7.5 | 8.4 | 3.4 |
| 6–12 months (%) | 10.7 | 11.7 | 3.3 | 10.8 | 12.0 | 3.8 |
| 12–24 months (%) | 18.0 | 19.6 | 4.0 | 18.5 | 20.3 | 4.8 |
| 24–48 months (%) | 32.4 | 34.4 | 4.4 | 31.1 | 30.8 | 0.6 |
| 48–72 months (%) | 15.9 | 13.9 | 5.5 | 15.1 | 14.3 | 2.4 |
| 72–96 months (%) | 5.9 | 4.2 | 8.0 | 7.5 | 6.7 | 3.0 |
| >96 months (%) | 8.9 | 6.8 | 8.2 | 8.6 | 6.4 | 8.3 |
| Access type | ||||||
| AV fistula (%) | 10.9 | — | — | 10.6 | 9.4 | 3.9 |
| AV graft (%) | 2.1 | — | — | 2.0 | 2.1 | 0.6 |
| Catheter (%) | 33.8 | — | — | 34.9 | 33.4 | 3.1 |
| Unknown (%) | 53.2 | — | — | 52.5 | 55.0 | 5.1 |
| Wait-listed for transplant (%) | 40.6 | 40.1 | 1.0 | 39.0 | 38.1 | 2.9 |
| Prior transplant (%) | ||||||
| 0 | 88.7 | 90.8 | 6.8 | 91.3 | 93.5 | 8.4 |
| 1 | 10.1 | 8.6 | 5.4 | 8.4 | 6.4 | 7.8 |
| 2 | 1.1 | 0.7 | 5.0 | 0.2 | 0.1 | 3.9 |
| Comorbidities | ||||||
| Diabetes (%) | 24.1 | 24.9 | 2.0 | 22.9 | 23.5 | 1.4 |
| Hypertension (%) | 82.0 | 82.2 | 0.3 | 82.1 | 81.6 | 1.3 |
| CHF (%) | 16.5 | 16.1 | 1.0 | 18.1 | 18.3 | 0.6 |
| IHD (%) | 6.3 | 7.4 | 4.6 | 5.5 | 5.8 | 1.5 |
| CVD (%) | 2.6 | 2.1 | 3.7 | 4.8 | 5.5 | 3.3 |
| PVD (%) | 7.8 | 8.4 | 2.1 | 8.3 | 9.5 | 4.4 |
| COPD (%) | 5.1 | 5.39 | 1.3 | 5.3 | 5.26 | 0.2 |
| Cancer (%) | 3.3 | 2.51 | 4.8 | 5.8 | 3.56 | 10.4 |
| Lab values | ||||||
| Albumin (g/dl; %) | 3.3 (0.71) | 3.3 (0.71) | 2.8 | 3.3 (0.7) | 3.3 (0.7) | 2.9 |
| Hemoglobin (g/dl; %) | 10.1 (1.77) | 10.0 (1.77) | 4.5 | 10.0 (1.8) | 10.0 (1.8) | 3.4 |
Abbreviations: AV, arteriovenous; BMI, body mass index; CHD, conventional hemodialysis; CHF, congestive heart failure; COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; DHD, daily hemodialysis; ESRD, end-stage renal disease; IHD, ischemic heart disease; PD, peritoneal dialysis; PVD, peripheral vascular disease. Results are presented as mean (s.d.) or percentage as indicated.
Figure 1.
Treatment time received by daily hemodialysis patients over time. No, number.
DHD versus PD
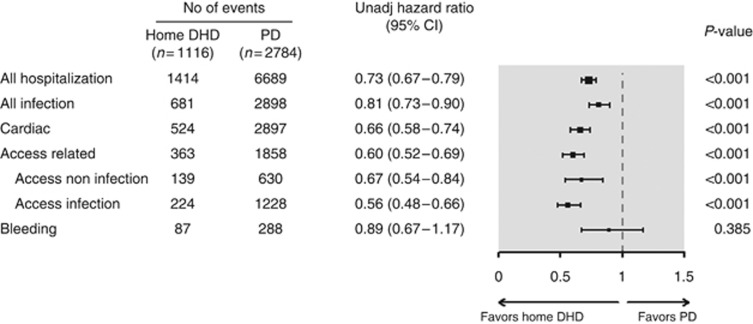
There were 2443/3900 patients who had 8103 hospitalizations during 6429 patient-years (mean follow-up 1.6 years, s.d.=1.3). The composite hospitalization rate was significantly lower for DHD compared with PD (DHD: 0.94/patient-year, PD: 1.36/patient-year; hazard ratio (HR)=0.73 (95% confidence interval (CI)=0.67–0.79); P<0.001).
Results were similar when we included only up to three hospitalizations per patient, excluded recurrent hospitalizations occurring within 14 days of a previous one, and restricted the analysis to the subgroup whose index date was <1 year after start of end-stage renal disease. The subdistribution HR of time to first hospitalization with DHD versus PD, accounting for the competing events of death and transplantation, was similar to the primary analysis (HD=0.64, (95% CI=0.58–0.71); P<0.001).
Patients receiving DHD spent a mean of 5.2 days per patient-year in hospital, compared with PD patients who spent 9.2 days per patient-year (P<0.001). Fifty-two percent of DHD versus 32% of PD patients remained admission-free (P=<0.001) during follow-up. Hospitalization rates owing to the prespecified individual causes were each significantly lower with DHD than with PD (Figure 2).
Figure 2.
Relative hazard of hospitalization associated with home daily home hemodialysis (DHD) versus peritoneal dialysis (PD). CI, confidence interval; No, number.
DHD versus in-center CHD
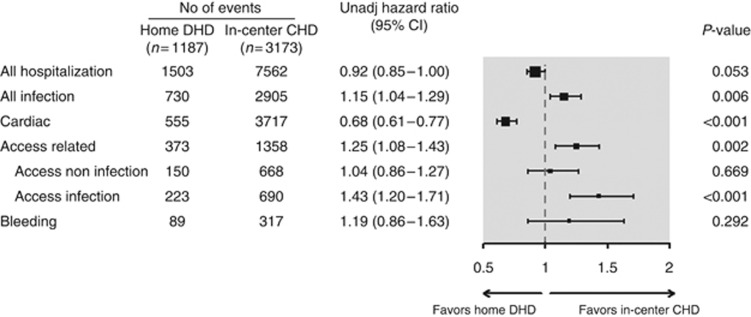
There were 2629/4360 patients who had 9065 hospitalizations over 8299 patient-years (mean follow-up 1.9 years, s.d.=1.4 years). There was no significant difference in the composite hospitalization rate between home DHD and in-center CHD (DHD: 0.93/patient-year, CHD: 1.10/patient-year; HR=0.92 (95% CI=0.85–1.00); P=0.053).
Results were similar when we included only up to three hospitalizations per patient, excluded recurrent hospitalizations occurring within 14 days of a previous one, and restricted the analysis to the subgroup whose index date was <1 year after the start of end-stage renal disease. The subdistribution HR of time to first hospitalization with DHD versus CHD, accounting for the competing events of death and transplantation, was similar to the primary analysis (HR=0.90, (95% CI 0.71–1.19); P=0.357).
Patients receiving home DHD spent fewer days in hospital than patients receiving in-center CHD (5.2 vs. 7.0 days per patient-year, respectively, P=0.011). However, there was no significant difference between groups in the proportion of patients remaining admission-free during follow-up (DHD 51% vs. CHD 50%, P=0.62). Cardiovascular hospitalizations were significantly lower with DHD than with CHD (HR=0.68 (95% CI=0.61–0.77), P<0.001), whereas infectious and access-related hospitalizations were significantly higher (HR=1.15 (95% CI=1.04–1.29); P=0.006, and HR=1.25 (95% CI=1.08–1.43); P=0.002), respectively (Figure 3). Of the latter, access infections appeared to account for the higher observed HR (Figure 3).
Figure 3.
Relative hazard of hospitalization associated with daily home hemodialysis (DHD) versus in-center conventional hemodialysis (CHD). CI, confidence interval; No, number.
Dialysis modality switches
Approximately 1% (9/1162) of DHD patients switched to PD, whereas 25% (691/2784) of PD patients switched to home HD (defined as ‘cross-overs'). During follow-up, 15% (172/1116) of the DHD group compared with 44% (1233/2784) of the PD group switched back to in-center CHD (defined as ‘modality failure'). In both groups, >80% of the modality failures occurred within the first year, whereas >90% occurred by 2 years. The hazard of switching back to in-center CHD with PD relative to DHD was 3.4 (95% CI=2.9–4.0, P<0.001).
DISCUSSION
Home dialysis offers patients independence, scheduling flexibility, less time spent in dialysis facilities, and autonomy to choose the dialysis modality they prefer. Most nephrologists feel that home dialysis is underutilized,24 and the importance of patient education in improving home dialysis uptake is recognized.2, 25, 26, 27 Home HD and PD are viewed as complementary therapies, with modality choice predominantly determined by patient preference.1, 2 Although PD remains the dominant home therapy, the use of home HD is increasing. How home HD compares in efficacy with PD on patient-centered outcomes is unclear. We conducted this study to address this knowledge gap.
In this retrospective cohort of predominantly prevalent patients receiving in-center HD, we found that patients who switched to home daily HD had a reduced risk of hospitalization because of cardiovascular, infectious, access-related, and bleeding causes, compared with those who switched to PD. Compared with PD patients, daily HD patients spent 43% fewer days in hospital owing to the prespecified causes, and significantly more daily HD patients than PD patients remained admission-free during follow-up. We also observed that home daily HD was a significantly more sustainable therapy than PD: ~15% of patients in the daily HD group went back to in-center conventional HD by 2 years, compared with almost 50% of PD patients during the same time period.
Previous studies comparing HD and PD have focused on in-center conventional HD. In an analysis of United States Renal Data System (USRDS) data, PD was associated with a significantly increased risk of hospitalization after adjustment for baseline factors (RR=1.14, 95% CI=1.13–1.15).28 This association was confirmed by a prospective Canadian study, with relative risks in the range of 1.10–1.26 in favor of HD.29 Two smaller, more recent prospective studies found no significant differences in admission rates between groups.30, 31 It is difficult to compare our results with these studies, as we evaluated specific rather than all-cause hospitalizations. Interestingly, Lafrance observed 52% more infectious hospitalizations in patients receiving PD than propensity score–matched patients receiving in-center conventional HD;32 we observed a 23% increase with PD compared with home DHD.
What possible mechanisms underlie our findings? All patients received home DHD using a single manufacturer's device. DHD with this device typically uses lower dialysate flow rates than conventional in-center HD (15–30 rather than 90–120 l per treatment), yet it still provides greater weekly clearance than does PD (mean urea standard Kt/V 2.5 vs. 1.8 per week).33 Uremia has been linked to impaired immune function.34, 35 Whether more dialysis improves these parameters has never been shown, but if true could explain the reduced risk of infection and access infection–related admissions that we observed with DHD than with PD. As DHD allows more ultrafiltration and phosphate removal than PD,33 it is plausible that these factors contributed to reduced cardiovascular admissions with DHD. Finally, the possibility that PD patients were hospitalized more frequently because they were more frail than home DHD patients despite propensity-score matching cannot be excluded.36 However, we did exclude patients living in institutions or in assisted-living situations to reduce this likelihood.
The PD discontinuation rates that we observed are concerning. Jaar et al.37 found PD discontinuation rates of 25% during the first year of starting PD, whereas ours were more than double that (55% during the first year). Even if we do not count patients who crossed over from PD to DHD, as they still were able to continue on a home therapy, the proportion of patients who switched from PD to in-center HD was still higher than those observed by Jaar et al.37 (37% during the first year). The high PD discontinuation rates that we observed may be related to the fact that ours was a prevalent rather than incident sample—~80% of patients in our study had been on dialysis for >1 year. Prevalent patients are more likely to have lost residual renal function, which is a significant predictor of PD failure.38 Alternatively, patients already established on in-center CHD who switch to PD may have more barriers to PD than those who start PD from the outset. Whether increased availability of home HD would allow more PD patients who are failing PD to do home HD rather than in-center HD is not known. Further study is needed into the factors resulting in significantly poorer long-term success of PD compared with home DHD.
Patients switching from in-center CHD to home DHD had a similar risk of the composite outcome as patients continuing in-center CHD, as a significant reduction in cardiovascular hospitalizations with DHD was offset by a concomitant increase in admissions from infections (both general and access-related). More frequent ultrafiltration likely affords better control of euvolemia, potentially reducing cardiovascular risk—a finding consistent with the Frequent Hemodialysis Network Daily Trial, which showed significantly improved left ventricular mass with six times versus three times per week HD.39 However, why DHD patients had more infectious admissions than CHD patients is puzzling. Any residual confounding from comparing home with in-center patients would have biased our results in the opposite direction, suggesting that the observed association is in fact real. Our data on treatment time and urea clearance confirm that DHD patients received the prescribed therapy, reducing issues of noncompliance. Perhaps DHD patients had increased bacterial exposure owing to more frequent contact of the blood with foreign materials (dialyzer, water, needles and so on) and more frequent access cannulation, resulting in increased and/or more severe infections requiring hospital admission. Home DHD patients may also have had higher rates of buttonhole rather than rope-ladder cannulation, a factor observed to cause infection.40 Alternatively, this finding may simply be a consequence of logistics: whereas patients receiving in-center CHD have the opportunity to receive intravenous antibiotics during their HD sessions when needed, patients dialyzing at home may have required hospital admission to receive intravenous antibiotics. Further study is needed to distinguish between these possibilities. It should be mentioned that unlike the FHN Daily Trial we did not find any increased risk of noninfection-related access complications with more frequent HD. Finally, as there is little difference in weekly urea clearance with DHD using low dialysate flows and CHD,33 it is not surprising that bleeding admissions were similar between these groups.
Our study is the first to address the comparative clinical effectiveness of home DHD versus PD, and has several methodological strengths. We analyzed data from a complete data set of consecutive patients receiving DHD from a single provider, eliminating the bias that arises from inclusion of only prevalent long-term survivors. We obtained baseline variables and outcomes data from a single well-validated data source to avoid information bias. Our choice of the composite outcome was hypothesis-based, and we used standard USRDS definitions to classify hospitalizations. We used rigorous methods to match patients on all known baseline characteristics. Most importantly, the comparison of home DHD with home PD not only addresses an important clinical question but substantially reduces the risk of confounding owing to factors often associated with the ability to dialyze at home.
We recognize the limitations of observational studies using administrative data sets, the most important being that analysis is limited to the data actually collected. Consequently, we were unable to match on potentially important prognostic variables, such as frailty index, help of an unpaid caregiver to perform home dialysis, vascular access type (relevant for the two HD comparisons), residual renal function, or the reasons that DHD or PD were started, and were are thus unable to completely exclude residual confounding or indication bias. We were unable to match exactly on the index date. However, given how closely the groups were matched at the start of end-stage renal disease, there is no reason to believe that the accumulation of comorbidities between the start of end-stage renal disease (or match date) and the index date would differ substantially between groups. Moreover, when we restricted our analysis to the subgroup of patients whose index date was <1 year after the start of end-stage renal disease, we obtained similar HRs as for the main analysis, suggesting that our results are robust. We do not have data on the mechanisms underlying our observations, and we did not have any information on the reasons for PD discontinuation. It is also important to note that our results cannot be extrapolated to frequent HD therapies using other DHD dialysis devices that provide high dialysate flows. Finally, due to having to restrict our cohort to patients with Medicare as their primary insurer, ours was mostly a prevalent cohort with mean time on dialysis of over 3 years; as such these results may not apply to incident patients newly starting renal replacement therapy in whom PD is often promoted as the initial dialysis modality.3, 5, 6
In summary, our study provides timely, highly relevant information for prevalent patients considering home dialysis. Our results suggest that in prevalent patients hospitalization risk is equal between home DHD and in-center CHD, but that PD is associated with higher risks of hospitalization and a substantially greater risk of modality failure. Given the negative impact of hospitalizations on cost-effectiveness and quality of life, these results have implications for programs and patients. Well-conducted prospective studies are needed to confirm these findings, and to better determine whether the observed associations are causal by identifying the underlying mechanisms. Future studies should also evaluate the reasons for the higher rates of modality failure in prevalent patients choosing PD versus those who choose DHD.
MATERIALS AND METHODS
Data sources
We identified patients receiving DHD, a large US dialysis provider's administrative database, which contains detailed information for all patients receiving dialysis within its facilities. We selected control patients receiving PD and in-center CHD from the USRDS. The USRDS is an integrated national database that includes data on demographics, diagnoses, biochemistry, dialysis claims, treatment history, hospitalizations, and vital status for all patients with end-stage renal disease in the US since 1995.41 We obtained detailed dialysis prescription and treatment information for DHD patients from the provider's database. To avoid information bias, we obtained all other data for all study cohorts (including baseline variables, hospitalizations, modality switches, and deaths) from the USRDS. The provider supplied the data and allowed linkage to USRDS for a fee; the company had no input into the study design or analyses. All analyses adhered to a detailed, predefined protocol, and we prepared this manuscript according to STROBE guidelines.30
Study sample
We included all adults ⩾18 years old who began DHD (>5 days/week, 1.5–4.5 h/day) between January 2004 and December 2009 in the provider's facilities. All DHD patients used a single dialysis device;33 >90% received DHD using low dialysate flows (<300 ml/min). We selected two comparator groups of adults ⩾18 years old receiving PD and CHD from USRDS from the same time period. As complete hospitalization data are only available from USRDS for patients who are insured by Medicare as primary payor, we excluded patients from both groups who did not meet this criteria. We also excluded DHD patients who received PD, and PD controls who received DHD at any time. To avoid elimination of potentially ‘healthier' patients from the CHD group, CHD controls who received PD first were not excluded (114/3173; 3.6% of the final cohort). Other exclusions are listed in Table 1.
Primary outcome, index date, and follow-up
The primary outcome was the composite of all hospitalizations from the index date to the end of follow-up owing to the prespecified causes of cardiovascular, infectious, access-related, and bleeding.
Study variables
All diagnoses were classified as in the USRDS 2012 Annual Report using the ICD-9/10 systems.41 We defined the index date as DHD or PD start date, and then matched DHD and PD patients by duration of end-stage renal disease (vintage) before this date to avoid immortal time bias.42 Because CHD controls did not have a ‘natural' index date, the index date was calculated as dialysis start date plus the matched DHD's subject's vintage; only patients who were alive and receiving CHD on the index date were thus eligible to serve as controls. To reduce bias from informative censoring for patients who discontinued DHD or PD, we attributed all events occurring up to 90 days after dialysis modality switches to the prior modality.
Statistical analysis
Derivation of propensity scores
We constructed a logistic regression model to predict the receipt of DHD versus PD using the following covariates available at the start of end-stage renal disease: age, sex, race, body mass index, diabetes, hypertension, congestive heart failure, cerebrovascular disease, peripheral vascular disease, chronic obstructive pulmonary disease, cancer, prior transplant, medical insurance coverage, albumin, and hemoglobin. From this model, we calculated a propensity score for each patient, which is the estimated probability of being assigned DHD over PD given the observed covariates in our logistic regression model.43 Because DHD and PD patients with the same propensity score will have similar distributions of observed baseline covariates, matching on propensity score reduces the impact of selection bias.44, 45 We used a similar approach to calculate the propensity score of receiving DHD versus CHD.
Matching procedures
Using the ‘greedy match' macro,46 we matched between one and three PD patients to each DHD patient by the following: the propensity score (caliper width=0.02), first renal replacement therapy start date (5-year intervals from 1995 to 2009), duration of end-stage renal disease before index date (index date minus first renal replacement therapy start date: 1–3 months, 3–6 months, 6–12 months, 12–24 months, 24–48 months, 48–72 months, 72–96 months, >96 months), age (3-year intervals), sex, race, body mass index (5 kg/m2 intervals), congestive heart failure, cancer, and cerebrovascular disease. We evaluated various caliper widths for the propensity score iteratively, and chose the width that produced the most matches while maintaining between-group standardized differences of <10%. Matching on first renal replacement therapy start date and vintage were prespecified to control for era effects and survivor bias, whereas the other variables were added later owing to variable imbalance after propensity-score matching.47, 48
Similarly, we matched 1–3 CHD patients to each DHD patient by propensity score (caliper width=0.02), first renal replacement therapy start date, age (3-year intervals), sex, race, body mass index (5 kg/m2 bins), albumin (1.5 g/dl intervals), congestive heart failure, diabetes, and prior transplant. Duration of end-stage renal disease was matched by definition, given the method by which we calculated the index date for CHD patients (see above).
Baseline characteristics
We reported standardized differences in baseline characteristics between daily and conventional groups; differences >10% were considered important.49, 50 When estimating descriptive statistics and standardized differences, each control patient was weighted by the inverse of the number of control patients within the matched set.51
Primary outcome
We used an Andersen–Gill model,52 accounting for matched strata, to evaluate time to all hospitalizations owing to the prespecified causes, and to estimate the relative change in the hazard of hospitalization owing to DHD compared with PD, and owing to DHD compared with CHD. A robust sandwich covariance matrix structure was used to account for intra-individual correlation between repeated events for the same individual. Events of transplantation, death, recovery of renal function, and loss to follow-up were censored; switches from DHD or PD to an alternative dialysis modality were censored at 90 days after the switch to reduce bias from informative censoring.
Sensitivity analyses
To ensure that a small group of patients with multiple readmissions were not primarily responsible for the observed results, we repeated the primary analysis excluding hospitalizations after the 3rd, and any occurring within 14 days of a previous one. Given that patients may have accumulated comorbidities between start of end-stage renal disease when matching variables were available and the index date, we repeated the primary analysis in the subgroup of patients whose index date was <1 year from the start of end-stage renal disease. Finally, recognizing the potential for informative censoring due to the competing events of death and transplantation, we performed a competing risk analysis for time to first hospitalization owing to the prespecified causes using the approach of Fine for stratified data.53, 54
Secondary outcomes
We used the approach described above for individual components of the composite outcome. We compared the number of days spent in the hospital between groups using a negative binomial regression with generalized estimating equation to account for the correlation within each matched strata.55 We compared the proportion of patients remaining admission-free during follow-up using conditional logistic regression.
We compared modality failure (defined as switch to in-center conventional HD) for home DHD and PD using the stratified two-sided log-rank test.56 Events of death, transplantation, and loss to follow-up were censored. PD outcomes were weighted by the inverse of the number of PD patients per matched set. We used a Cox proportional hazards model, stratified by matched sets, to estimate the relative change in the hazard of modality failure owing to DHD compared with PD.
All HRs are presented as unadjusted. We performed all analyses using SAS 9.2 (Cary, NC), except for the stratified competing risk analysis, which was performed in R (3.0.2, R foundation for statistical computing). We used two-sided P-values and considered P<0.05 statistically significant.
Acknowledgments
We thank Elizabeth Forest and Paul Eggers for providing data linkage with the United States Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government. We thank the dialysis provider for allowing us to use their data for a fee. This study was funded by a peer-reviewed grant from the Baxter Extramural Grant Program. RSS is supported by a Clinician-Investigator Award from the Fonds de la recherche en santé du Québec (FRSQ).
All the authors declared no competing interests.
References
- Thodis ED, Oreopoulos DG. Home dialysis first: a new paradigm for new ESRD patients. J Nephrol. 2011;24:398–404. doi: 10.5301/JN.2011.8374. [DOI] [PubMed] [Google Scholar]
- Burkart J. Role of peritoneal dialysis in the era of the resurgence of home hemodialysis. Hemodial Int. 2008;12:S51–S54. doi: 10.1111/j.1542-4758.2008.00297.x. [DOI] [PubMed] [Google Scholar]
- Burkart J. The future of peritoneal dialysis in the United States: optimizing its use. Clin J Am Soc Nephrol. 2009;4:S125–S131. doi: 10.2215/CJN.04760709. [DOI] [PubMed] [Google Scholar]
- Chow J, Fortnum D, Moodie JA, et al. The HOME network: an Australian national initiative for home therapies. J Ren Care. 2013;39:56–61. doi: 10.1111/j.1755-6686.2013.00339.x. [DOI] [PubMed] [Google Scholar]
- Heaf J. Underutilization of peritoneal dialysis. JAMA. 2004;291:740–742. doi: 10.1001/jama.291.6.740. [DOI] [PubMed] [Google Scholar]
- Covic A, Bammens B, Lobbedez T, et al. Educating end-stage renal disease patients on dialysis modality selection: clinical advice from the European Renal Best Practice (ERBP) Advisory Board. Nephrol Dial Transplant. 2010;25:1757–1759. doi: 10.1093/ndt/gfq206. [DOI] [PubMed] [Google Scholar]
- Vonesh EF, Snyder JJ, Foley RN, et al. Mortality studies comparing peritoneal dialysis and hemodialysis: what do they tell us. Kidney Int. 2006;103:S3–11. doi: 10.1038/sj.ki.5001910. [DOI] [PubMed] [Google Scholar]
- Ginieri-Coccossis M, Theofilou P, Synodinou C, et al. Quality of life, mental health and health beliefs in haemodialysis and peritoneal dialysis patients: investigating differences in early and later years of current treatment. BMC Nephrol. 2008;9:14. doi: 10.1186/1471-2369-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanouzas D, Ng KP, Fallouh B, et al. What influences patient choice of treatment modality at the pre-dialysis stage. Nephrol Dial Transplant. 2012;27:1542–1547. doi: 10.1093/ndt/gfr452. [DOI] [PubMed] [Google Scholar]
- Komenda P, Gavaghan MB, Garfield SS, et al. An economic assessment model for in-center, conventional home, and more frequent home hemodialysis. Kidney Int. 2012;81:307–313. doi: 10.1038/ki.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karopadi ANRE, Ronco C.Relative Cost of PD and HD: Data from 44 Countries. World Congress of Nephrology 2013 (Abstract). Accessed on 1 May 2014. Available from: : http://www.abstracts2view.com/wcn/view.php?nu=WCN13L_499 .
- Jain AK, Blake P, Cordy P, et al. Global trends in rates of peritoneal dialysis. J Am Soc Nephrol. 2012;23:533–544. doi: 10.1681/ASN.2011060607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagg CR. The renaissance of home hemodialysis: where we are, why we got here, what is happening in the United States and elsewhere. Hemodial Int. 2008;12:S2–S5. doi: 10.1111/j.1542-4758.2008.00287.x. [DOI] [PubMed] [Google Scholar]
- Agar JW, Hawley CM, George CR, et al. Home haemodialysis in Australia–is the wheel turning full circle. Med J Aust. 2010;192:403–406. doi: 10.5694/j.1326-5377.2010.tb03565.x. [DOI] [PubMed] [Google Scholar]
- Weinhandl ED, Liu J, Gilbertson DT, et al. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol. 2012;23:895–904. doi: 10.1681/ASN.2011080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesrallah GE, Lindsay RM, Cuerden MS, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen KL, Zhang R, Huang Y, et al. Survival and hospitalization among patients using nocturnal and short daily compared to conventional hemodialysis: a USRDS study. Kidney Int. 2009;76:984–990. doi: 10.1038/ki.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellstrand CM, Buoncristiani U, Ting G, et al. Short daily haemodialysis: survival in 415 patients treated for 1006 patient-years. Nephrol Dial Transplant. 2008;23:3283–3289. doi: 10.1093/ndt/gfn210. [DOI] [PubMed] [Google Scholar]
- Blagg CR, Kjellstrand CM, Ting GO, et al. Comparison of survival between short-daily hemodialysis and conventional hemodialysis using the standardized mortality ratio. Hemodial Int. 2006;10:371–374. doi: 10.1111/j.1542-4758.2006.00132.x. [DOI] [PubMed] [Google Scholar]
- Marshall MR, Hawley CM, Kerr PG, et al. Home hemodialysis and mortality risk in Australian and New Zealand populations. Am J Kidney Dis. 2011;58:782–793. doi: 10.1053/j.ajkd.2011.04.027. [DOI] [PubMed] [Google Scholar]
- Chertow GMLN, Beck GJ, Eggers PW, The FHN Trial Group et al. Effects of randomization to frequent in-center hemodialysis on long- term mortality: frequent hemodialysis daily trial (abstract) J Am Soc Nephrol 2013. FR-PO342.
- Young BA, Chan C, Blagg C, et al. How to overcome barriers and establish a successful home HD program. Clin J Am Soc Nephrol. 2012;7:2023–2032. doi: 10.2215/CJN.07080712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. Portable home hemodialysis for kidney failure. Issues Emerg Health Technol. 2007;108:1–4. [PubMed] [Google Scholar]
- Mendelssohn DC, Mullaney SR, Jung B, et al. What do American nephologists think about dialysis modality selection. Am J Kidney Dis. 2001;37:22–29. doi: 10.1053/ajkd.2001.20635. [DOI] [PubMed] [Google Scholar]
- Golper TA, Mehrotra R, Schreiber MS. Is Dorothy correct? The role of patient education in promoting home dialysis. Semin Dial. 2013;26:138–142. doi: 10.1111/sdi.12086. [DOI] [PubMed] [Google Scholar]
- Davis JS, Zuber K. Implementing patient education in the CKD clinic. Adv Chronic Kidney Dis. 2013;20:320–325. doi: 10.1053/j.ackd.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Agraharkar M, Patlovany M, Henry S, et al. Promoting use of home dialysis. Adv Perit Dial. 2003;19:163–167. [PubMed] [Google Scholar]
- Bloembergen WE, Port FK, Mauger EA, et al. A comparison of cause of death between patients treated with hemodialysis and peritoneal dialysis. J Am Soc Nephrol. 1995;6:184–191. doi: 10.1681/ASN.V62184. [DOI] [PubMed] [Google Scholar]
- Murphy SW, Foley RN, Barrett BJ, et al. Comparative hospitalization of hemodialysis and peritoneal dialysis patients in Canada. Kidney Int. 2000;57:2557–2563. doi: 10.1046/j.1523-1755.2000.00115.x. [DOI] [PubMed] [Google Scholar]
- Harris SA, Lamping DL, Brown EA, et al. North Thames Dialysis Study G. Clinical outcomes and quality of life in elderly patients on peritoneal dialysis versus hemodialysis. Perit Dial Int. 2002;22:463–470. [PubMed] [Google Scholar]
- Quinn RR, Ravani P, Zhang X, et al. Impact of modality choice on rates of hospitalization in patients eligible for both peritoneal dialysis and hemodialysis. Perit Dial Int. 2014;34:41–48. doi: 10.3747/pdi.2012.00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafrance JP, Rahme E, Iqbal S, et al. Association of dialysis modality with risk for infection-related hospitalization: a propensity score-matched cohort analysis. Clin J Am Soc Nephrol. 2012;7:1598–1605. doi: 10.2215/CJN.00440112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohn OF, Coe FL, Ing TS. Solute kinetics with short-daily home hemodialysis using slow dialysate flow rate. Hemodial Int. 2010;14:39–46. doi: 10.1111/j.1542-4758.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- Lim WH, Kireta S, Leedham E, et al. Uremia impairs monocyte and monocyte-derived dendritic cell function in hemodialysis patients. Kidney Int. 2007;72:1138–1148. doi: 10.1038/sj.ki.5002425. [DOI] [PubMed] [Google Scholar]
- Eleftheriadis T, Antoniadi G, Liakopoulos V, et al. Disturbances of acquired immunity in hemodialysis patients. Semin Dial. 2007;20:440–451. doi: 10.1111/j.1525-139X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Johansen KL, Chertow GM, Kutner NG, et al. Low level of self-reported physical activity in ambulatory patients new to dialysis. Kidney Int. 2010;78:1164–1170. doi: 10.1038/ki.2010.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaar BG, Plantinga LC, Crews DC, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol. 2009;10:3. doi: 10.1186/1471-2369-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesnyk I, Dekker FW, Boeschoten EW, et al. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int. 2010;30:170–177. doi: 10.3747/pdi.2008.00277. [DOI] [PubMed] [Google Scholar]
- FHNT Group, Chertow GM, Levin NW, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae JM, Ahmed SB, Atkar R, et al. A randomized trial comparing buttonhole with rope ladder needling in conventional hemodialysis patients. Clin J Am Soc Nephrol. 2012;7:1632–1638. doi: 10.2215/CJN.02730312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center USRDSC USRDS 2012 Researcher's Guide to the USRDS Database 2012. Accessed 27 May 2014. Available from: : http://www.usrds.org/2012/rg/A_intro_sec_1_12.pdf .
- Shariff SZ, Cuerden MS, Jain AK, et al. The secret of immortal time bias in epidemiologic studies. J Am Soc Nephrol. 2008;19:841–843. doi: 10.1681/ASN.2007121354. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- Austin PC, Grootendorst P, Anderson GM. A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: a Monte Carlo study. Stat Med. 2007;26:734–753. doi: 10.1002/sim.2580. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- General SAS Macros. Accessed on 12 September 2013. Available from: : http://www.mayo.edu/research/departments-divisions/department-health-sciences-research/division-biomedical-statistics-informatics/software/locally-written-sas-macros#general .
- Austin PC. An Introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. doi: 10.1080/00273171.2011.568786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin DB, Thomas N. Combining propensity-score matching with additional adjustments for prognostic covariates. J Am Stat Assoc. 2000;95:573–585. [Google Scholar]
- Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdani M, Sykora K, Li P, et al. Reader's guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. Bmj. 2005;330:960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. Assessing balance in measured baseline covariates when using many-to-one matching on the propensity-score. Pharmacoepidemiol Drug Saf. 2008;17:1218–1225. doi: 10.1002/pds.1674. [DOI] [PubMed] [Google Scholar]
- Andersen PKGR. Cox's regression model for counting processes: a large sample study. Ann Stat. 1982;10:1100–1120. [Google Scholar]
- Satagopan JM, Ben-Porat L, Berwick M, et al. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. doi: 10.1038/sj.bjc.6602102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Latouche A, Rocha V, et al. Competing risks regression for stratified data. Biometrics. 2011;67:661–670. doi: 10.1111/j.1541-0420.2010.01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming RG, Sherrington C, Lord SR, et al. Cluster randomised trial of a targeted multifactorial intervention to prevent falls among older people in hospital. BMJ. 2008;336:758–760. doi: 10.1136/bmj.39499.546030.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin PC. Type I error rates, coverage of confidence intervals, and variance estimation in propensity-score matched analyses. Int J Biostat. Article 13. 2009;5 doi: 10.2202/1557-4679.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]