Abstract
Hyperkalemia contributes to significant mortality and limits the use of cardioprotective and renoprotective renin–angiotensin–aldosterone blockers. Current therapies are poorly tolerated and not always effective. Here we conducted a phase 2 randomized, double-blind, placebo-controlled dose-escalation study to assess safety and efficacy of ZS-9. This oral selective cation exchanger that preferentially entraps potassium in the gastrointestinal tract was given to patients with stable Stage 3 chronic kidney disease and hyperkalemia (5.0 to 6.0 mEq/l) during a 2-day period. Of 90 eligible patients with mean baseline serum potassium of 5.1 mEq/l, 30 were randomized to placebo, 12–0.3 g, 24–3 g, or 24 to 10 g of ZS-9 three times daily for 2 days with regular meals. None withdrew and ZS-9 dose-dependently reduced serum potassium. The primary efficacy end point (rate of serum potassium decline in the first 48 h) was met with significance in the 3- and 10-g cohorts. From baseline, mean serum potassium was significantly decreased by 0.92±0.52 mEq/l at 38 h. Urinary potassium excretion significantly decreased with 10-g ZS-9 as compared to placebo at day 2 (+15.8 +/− 21.8 vs. +8.9 +/− 22.9 mEq per 24h) from placebo at day 2. In this short-term study, no serious adverse events were reported; only mild constipation in the 3-g dose group was possibly related to treatment. Thus, ZS-9 was well-tolerated in patients with stable chronic kidney disease and hyperkalemia leading to a rapid, sustained reduction in serum potassium.
Keywords: chronic kidney disease, hyperkalemia, potassium, potassium trap, zirconium silicate, ZS-9
Hyperkalemia is an independent risk factor for mortality in patients with cardiovascular disease, chronic kidney disease (CKD), and end-stage renal disease,1, 2, 3, 4, 5 and it limits the use of renin–angiotensin–aldosterone system (RAAS) inhibitors in the populations expected to derive the most benefit from this drug class.6 Despite the need for safe, effective, and predictable hyperkalemia treatment, current treatment options remain limited.7
Sodium polystyrene sulfonate (SPS; Kayexalate®) is the only product approved in the United States to treat hyperkalemia.7, 8, 9 Kayexalate is an organic polymer resin that nonselectively binds potassium (K+) and other divalent cations (e.g., calcium (Ca2+), magnesium (Mg2+)), and subsequently removes them via defecation, often stimulated by the additive sorbitol (a laxative and cathartic).8, 10 Despite its long history of use, Kayexalate has never been evaluated in a randomized, prospective, placebo-controlled setting.11, 12, 13 Its association with poor gastrointestinal tolerability and other substantial adverse events (AEs; e.g., sodium loading, hypocalcemia, hypomagnesemia, colonic necrosis)8, 14, 15, 16 makes it unsuitable for chronic administration.
Zirconium is a biologically inert trace element found widely in nature; estimated human dietary intake is ∼4 mg daily.17 Partly owing to their inertness, zirconium-containing compounds have been used extensively in biomedical applications,18 including hemodialysis with sorbent regeneration.19, 20, 21 Sodium zirconium cyclosilicate (ZS-9), unlike traditional organic polymers such as Kayexalate and the investigational agent patiromer, was engineered with a highly selective, high-capacity inorganic crystalline lattice structure that preferentially entraps monovalent cations (specifically K+ and ammonium (NH4+)) over divalent cations (e.g., Ca2+ and Mg2+) in the gastrointestinal tract.22, 23 Thermodynamic modeling studies have estimated that K+-binding of ZS-9 is 20.4 kcal/mol more stable than its native sodium-bound form, thus enabling the exchange of K+ for Na+ (data on file, ZS Pharma). As a zirconium silicate crystal, ZS-9 is completely insoluble in aqueous solutions24 and is not systemically absorbed following oral ingestion, based on 99% recovery of zirconium in feces of ZS-9-treated rats (Supplementary Figure S1 online). ZS-9 also increases fecal excretion of K+ and reduction of urinary K+ in rats (Supplementary Figure S2 online).
Here we report results from the first-in-human study with ZS-9, a randomized, double-blind, placebo-controlled Phase 2 study to assess safety and efficacy of the oral sorbent ZS-9 in hyperkalemia patients with Stage 3 CKD.
RESULTS
Patient demographics and disposition
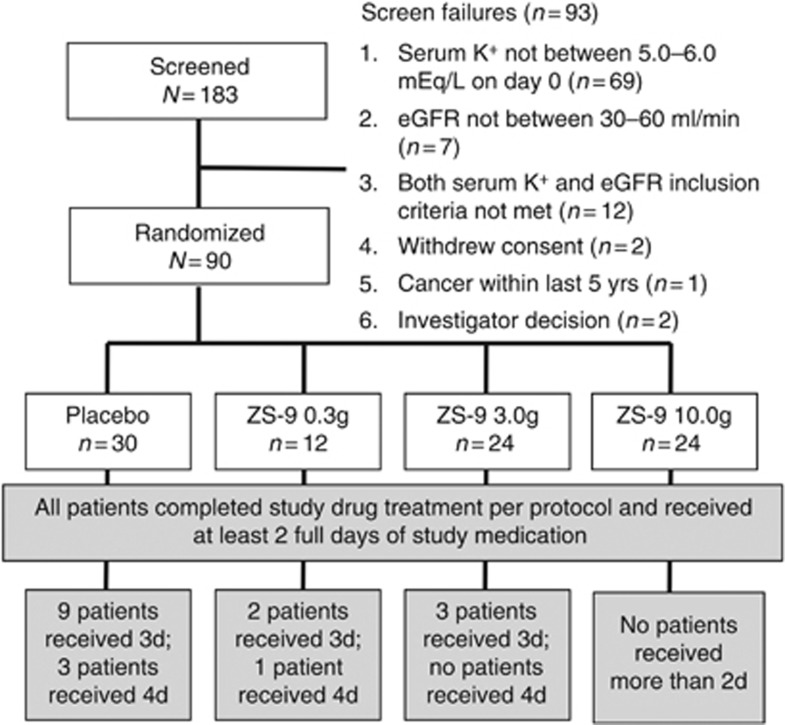
The study was conducted at nine US sites (November 2011–May 2012). Ninety patients met all eligibility criteria and were randomized 2:1 to receive ZS-9 (0.3 g (n=12), 3 g (n=24), and 10 g (n=24)) or placebo (n=30). All randomized patients completed the study as per protocol, and they are included in the primary efficacy and safety analyses (Figure 1).
Figure 1.
Patient disposition. eGFR, estimated glomerular filtration rate; ZS-9, sodium zirconium cyclosilicate.
Treatment groups were well-balanced with regard to baseline demographics, except that there were more men in the placebo group than in the ZS-9 groups (Table 1). Across groups, 56% of patients had a history of diabetes at baseline; 62% were receiving a RAAS inhibitor (Table 1).
Table 1. Baseline demographic and clinical characteristics.
|
ZS-9 dose group |
||||
|---|---|---|---|---|
| Parameter | Placebo (N=30) | 0.3 g (N=12) | 3 g (N=24) | 10 g (N=24) |
| Demographics | ||||
| Age, year | 69.7±11.0 | 70.3±6.9 | 72.0±6.3 | 72.3±11.7 |
| Male, n (%) | 23 (77) | 6 (50) | 14 (58) | 9 (38) |
| White, n (%) | 29 (96.7) | 12 (100.0) | 24 (100.0) | 23 (95.8) |
| Weight, kg | 95.2±22.1 | 84.8±18.0 | 89.0±22.0 | 86.6±26.3 |
| Eligibility criteria | ||||
| Serum potassium, mEq/la | 5.1±0.4 | 5.2±0.3 | 5.0±0.3 | 5.1±0.4 |
| eGFR, ml/min per 1.73 m2 | 58.1±26.5 | 56.5±24.0 | 57.1±22.1 | 51.6±22.3 |
| History of diabetes, n (%) | 19 (63.3) | 7 (58.3) | 13 (54.2) | 11 (45.8) |
| Serum glucose, mg/dl | 161.4±66.1 | 123.4±41.3 | 127.8±40.7 | 126.3±34.3 |
| Serum creatinine, mg/dl | 1.5±0.3 | 1.4±0.2 | 1.4±0.2 | 1.4±0.3 |
| 24-h urinary potassium, mEq/day | 56.5±34.0 | 62.4±31.5 | 58.9±20.4 | 46.9±23.7 |
| Serum sodium, mEq/l | 139.4±2.8 | 139.1±3.7 | 140.0±3.1 | 137.7±3.5 |
| Any concomitant RAAS blocker, n (%) | 15 (50) | 9 (75) | 16 (67) | 16 (67) |
| ACE-I only | 11 (37) | 6 (50) | 12 (50) | 13 (54) |
| ARB only | 2 (13) | 3 (33) | 1 (13) | 3 (29) |
| Spironolactone only | 2 (13) | 0 (0) | 3 (17) | 0 (0) |
| Dual therapy (ACE-I+ARB or ACE-I or ARB+spironolactone) | 3 (10) | 1 (8) | 2 (8) | 4 (17) |
| Diuretic use, n (%) | 8 (26.7) | 2 (16.7) | 11 (45.8) | 9 (37.5) |
Abbreviations: ACE-I, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; eGFR, estimated glomerular filtration rate; K+, potassium; ZS-9, sodium zirconium cyclosilicate.
Data are presented as mean±s.d. unless otherwise noted.
Mean baseline serum potassium level was calculated for each patient on the basis of an average of four samples: 3 different samples were collected at 30-min intervals on Study Day 1, and one sample was collected on Study Day 1 before the initial dose of the study drug.
Treatment exposure
All patients received the protocol-specified minimum dose (six doses) of the study drug in the initial 48-h treatment period. None in the 10-g ZS-9 group required treatment beyond 48 h. Continued treatment was required on day 3 (doses seven to nine) in placebo (30.0%), 0.3-g (16.7%), and 3-g (12.5%) patients, and on day 4 (doses 10–12) in placebo (10.0%) and 0.3-g (8.3%) patients.
Serum potassium
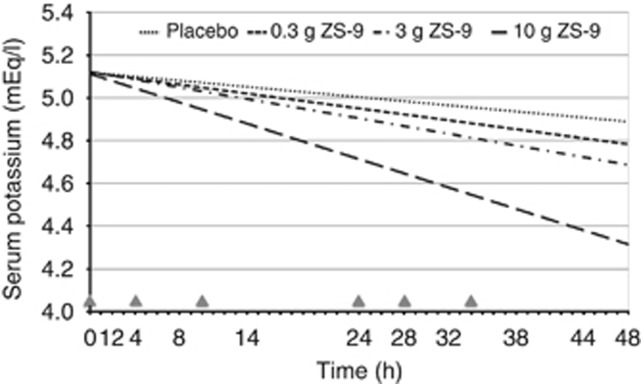
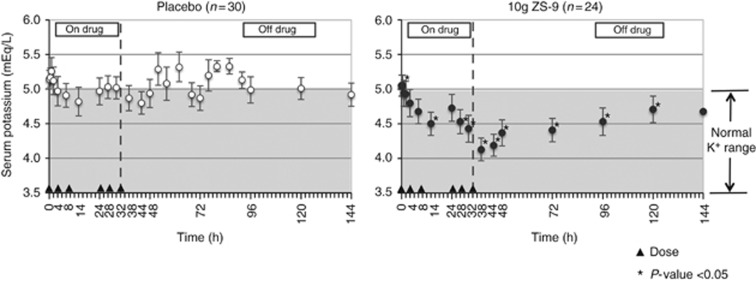
ZS-9 dose-dependently reduced in serum K+ (s-K+); the primary efficacy end point was met in the 3-g (P=0.048) and 10-g (P<0.0001) dose groups versus placebo (Figure 2). In the 10-g ZS-9 group, mean s-K+ decreased significantly from baseline by 0.11 mEq/l at 1 h after the first dose (P=0.02 vs. placebo). Greater mean reductions in s-K+ were seen on day 2 (Hour 28 to 48) with 10-g ZS-9 versus placebo (P⩽0.001), achieving a maximum reduction of 0.92 mEq/l at 38 h (P<0.001; Table 2, Figure 3). At Hour 38, 41.7% of patients on 10-g ZS-9 versus 3.4% on placebo had a >1.0 mEq/l drop in s-K+. The last dose in the 10-g ZS-9 group was administered on day 2, yet s-K+ remained significantly lower than placebo for 3.5 additional days (i.e., Hour 120; Figure 3).
Figure 2.
Rate of decline in serum potassium over the first 48 h of treatment with 0.3-g (n=12), 3-g (n=24), and 10-g ZS-9 (n=24) or placebo (n=30)—intent-to-treat population. This model prediction uses every serum K+ data point from each patient. Although the rate of decline is actually a curve, during the 48-h time frame of interest, it appears linear as presented here. The plotted rates of decline in serum K+ also visually illustrate the dose–response relationship. Triangles indicate study drug administration (six doses in 34 h). ZS-9, sodium zirconium cyclosilicate.
Table 2. Serum K+ at baseline and change from baseline during the on-drug evaluation periods on Day 1 and 2 (up to 4 h after the last dose on each day): ITT population (A) and subgroup on concomitant RAAS therapy (B).
|
ZS-9 dose group |
||||
|---|---|---|---|---|
| Serum K+ (mEq/l) | Placebo | 0.3 g | 3 g | 10 g |
| A. ITT population | n=30 | n=12 | n=24 | n=24 |
| Baseline | 5.14±0.36 | 5.22±0.26 | 5.02±0.32 | 5.05±0.36 |
| Day 1 | ||||
| Hour 0.5 | 0.03±0.31 | 0.05±0.32 | 0.06±0.42 | −0.02±0.24 |
| Hour 1 | 0.12±0.36 | 0.01±0.46 | 0.00±0.47 | −0.11±0.46 (P=0.04) |
| Hour 2 | −0.02±0.34 | 0.14±0.37 | 0.03±0.47 | −0.13±0.35 |
| Hour 4 | −0.16±0.54 | −0.16±0.36 | −0.09±0.47 | −0.25±0.41* |
| Hour 8 | −0.24±0.46* | −0.08±0.52 | −0.23±0.49* | −0.37±0.38* |
| Hour 14 | −0.32±0.50* | −0.31±0.41* | −0.35±0.47* | −0.55±0.47* |
| Day 2 | ||||
| Hour 0 | −0.17±0.43* | −0.32±0.37* | −0.36±0.36* | −0.32±0.48* |
| Hour 4 | −0.10±0.40 | −0.25±0.29* | −0.22±0.44* | −0.52±0.49 (P=0.001)* |
| Hour 8 | −0.11±0.43 | −0.18±0.39 | −0.29±0.48* | −0.62±0.45 (P<0.001)* |
| Hour 14 | −0.26±0.4* | −0.39±0.4* | −0.42±0.45* | −0.92±0.52 (P<0.001)* |
| B. RAAS subgroup | n=18 | n=10 | n=18 | n=20 |
| Baseline | 5.2±0.4 | 5.2±0.3 | 5.1±0.3 | 5.1±0.4 |
| Day 1 | ||||
| Hour 0.5 | −0.07±0.29 | 0.11±0.31 | 0.05±0.45 | −0.02±0.26 |
| Hour 1 | 0.19±0.31 | −0.01±0.51 | −0.04±0.51 | −0.12±0.50 (P<0.03) |
| Hour 2 | 0.01±0.39 | 0.11±0.39 | 0.00±0.44 | −0.19±0.26 |
| Hour 4 | −0.19±0.60 | −0.17±0.39 | −0.13±0.48 | −0.33±0.35 |
| Hour 8 | −0.21±0.54 | −0.03±0.57 | −0.16±0.52 | −0.34±0.41 |
| Hour 14 | −0.31±0.60 | −0.28±0.44 | −0.27±0.48 | −0.61±0.49 |
| Day 2 | ||||
| Hour 0 | −0.20±0.49 | −0.39±0.29 | −0.40±0.35 | −0.43±0.43 |
| Hour 4 | −0.12±0.45 | −0.26±0.32 | −0.17±0.46 | −0.60±0.44 (P=0.002) |
| Hour 8 | −0.13±0.48 | −0.19±0.43 | −0.23±0.48 | −0.74±0.35 (P<0.001) |
| Hour 14 | −0.25±0.46 | −0.49±0.38 | −0.32±0.48 | −1.05±0.47 (P<0.001) |
Abbreviation: ZS-9, sodium zirconium cyclosilicate.
Values are mean±s.d. P-values shown in the table were calculated using unpaired t-tests based on differences with the placebo group; P-values are provided only when the differences compared with placebo were significant (P<0.05). Asterisks denote P<0.05 for comparisons against baseline. The P-values listed in the table refer to comparisons against placebo.
Figure 3.
Mean serum potassium over 6 days in the placebo (left) and 10-g ZS-9 (right) groups. *Indicates significant difference compared with the respective placebo group (P<0.05). Triangles indicate study drug administration (six doses in 48 h). Shaded portion indicates range of normal serum potassium levels. Bars indicate 95% confidence interval. ZS-9, sodium zirconium cyclosilicate.
Mean s-K+ changes in the subgroup of patients on RAASi therapy were consistent with the changes in the overall population (Table 2). The primary efficacy end point was met in the 10-g ZS-9 dose group versus placebo (P<0.0001) in this subgroup.
Other serum and urine parameters
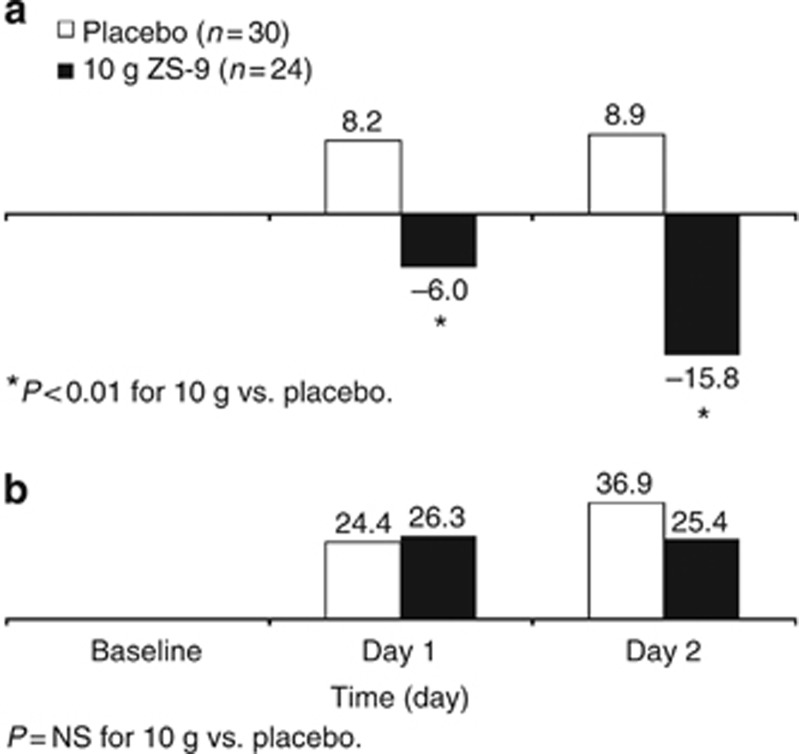
Twenty-four-hour urinary K+ excretion declined significantly in the 10-g ZS-9 group relative to placebo (−15.8 vs. +8.9 mEq/24-h; P<0.001) at day 2 (Figure 4a). There were no apparent differences in 24-h urinary sodium excretion between placebo and any ZS-9 dose (Figure 4b).
Figure 4.
Mean change from baseline in 24-h urinary excretion of potassium (a) and sodium (b). *Indicates significant difference compared with placebo. NS, not significant; ZS-9, sodium zirconium cyclosilicate.
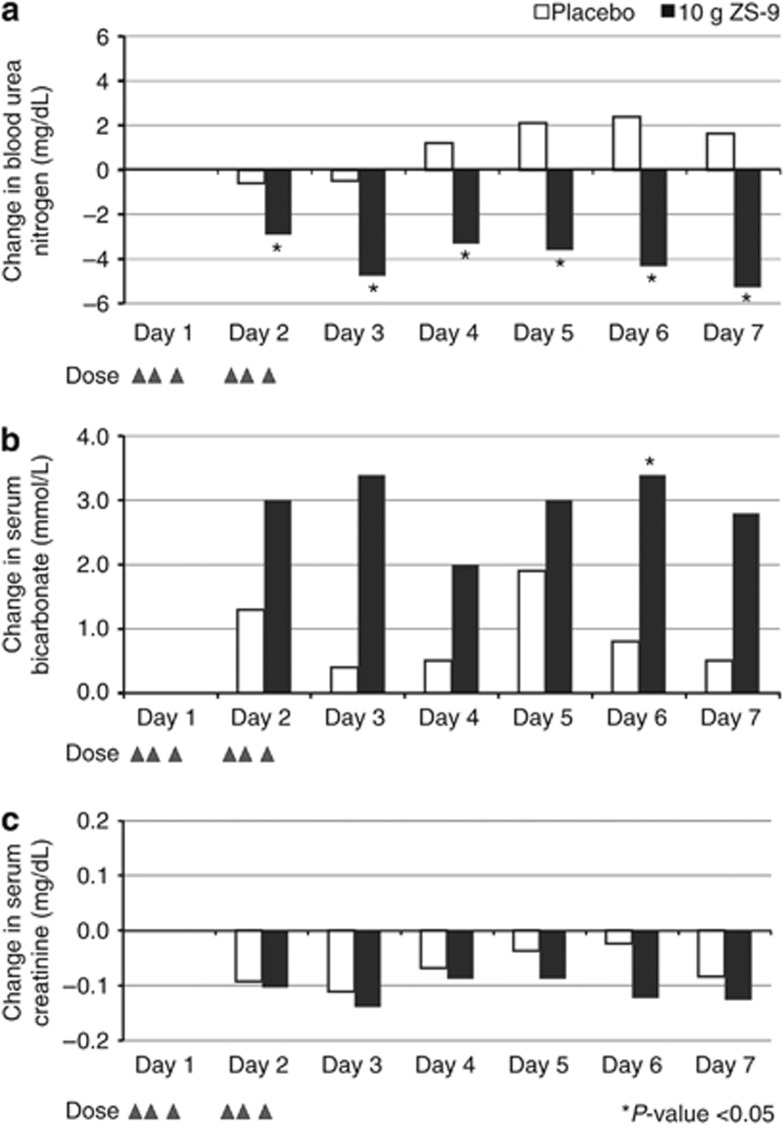
Baseline blood urea nitrogen was higher than placebo in the 10-g ZS-9 group (P=0.01) and similar in the other groups. There was evidence of a dose-related effect of ZS-9 on blood urea nitrogen; the greatest effect was in the 10-g group, with baseline decreases differing significantly from placebo at all measurements in days 2–7 (Figure 5a). Serum bicarbonate increased ∼10% with 10-g ZS-9, from 27.4 mmol/l at baseline to 30.1 mmol/l on days 2 (P=0.05) and 3 (P=0.067; Figure 5b).
Figure 5.
Serum parameters in 10-g ZS-9 (n=24) versus placebo (n=30) over 7 days. Mean change from baseline in (a) blood urea nitrogen, (b) serum bicarbonate, and (c) serum creatinine. Triangles indicate study drug administration (six doses in 48 h), and * indicates significant difference compared with placebo, P<0.05. ZS-9, sodium zirconium cyclosilicate.
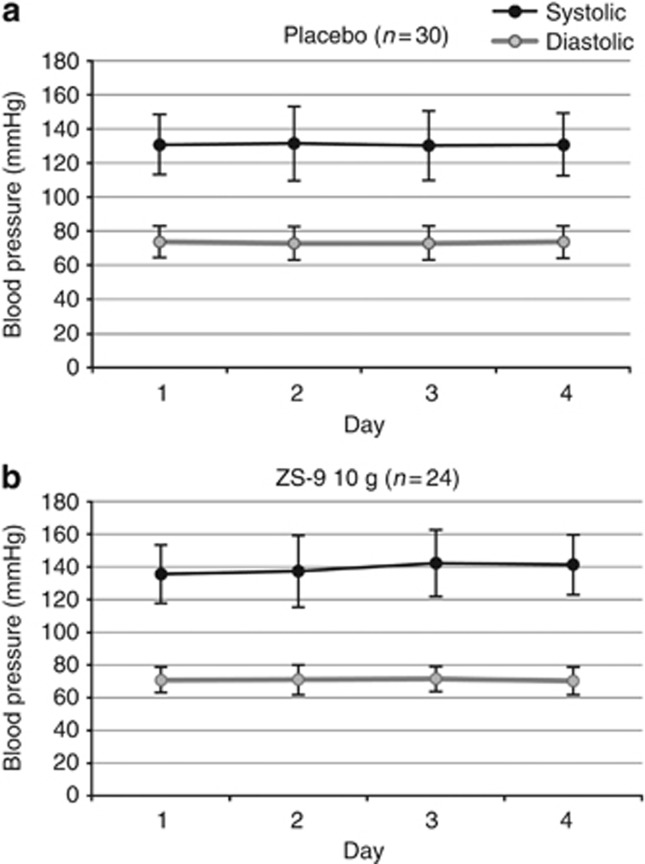
There were no clinically relevant changes in serum Ca2+, Mg2+, or Na+ or in kidney function parameters (serum creatinine (Figure 5c), 24-h urea nitrogen, urine creatinine, and urinary sediment), nor were there any significant changes in systolic and diastolic blood pressure across dose groups (Figure 6).
Figure 6.
Blood pressure over 4 days in 10-g ZS-9 (n=24) versus placebo (n=30). Systolic and diastolic blood pressure in patients in the (a) placebo group and (b) ZS-9 10-g dose group. ZS-9, sodium zirconium cyclosilicate.
Safety
ZS-9 was well-tolerated; across the placebo and the 0.3-, 3-, and 10-g ZS-9 groups, ⩾1 treatment-emergent AE occurred in 3 (10%), 1 (8%), 3 (13%), and 8 (33%) patients (Table 3). The investigators rated all treatment-emergent AEs mild or moderate; most were transient and did not require treatment. No serious AEs were reported. No AEs led to study discontinuation. Three treatment-emergent AEs were considered to be possibly related to the study treatment: mild constipation (3 g ZS-9), and mild nausea and mild vomiting (placebo). No patients had any notable changes in bowel movement frequency or stool appearance.
Table 3. Treatment-emergent adverse events.
|
ZS-9 dose group |
||||
|---|---|---|---|---|
| No. (%) of patients | Placebo (N=30) | 0.3 g (N=12) | 3 g (N=24) | 10 g (N=24) |
| At least one TEAE | 3 (10) | 1 (8) | 3 (13) | 8 (33) |
| Cardiac | ||||
| Hypertension | 0 | 0 | 0 | 1 (4) |
| Gastrointestinal disorders | ||||
| Constipation | 0 | 0 | 1 (4) | 0 |
| Diarrhea | 0 | 1 (8) | 0 | 1 (4) |
| Heartburn | 0 | 0 | 0 | 1 (4) |
| Nausea | 1 (3) | 0 | 1 (4) | 2 (8) |
| Vomiting | 1 (3) | 0 | 0 | 3 (13) |
| Investigations | ||||
| Aspartate aminotransferase increased | 0 | 0 | 1 (4) | 0 |
| Nervous system disorders | ||||
| Headache | 1 (3) | 0 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Abdominal cramp | 0 | 1 (8) | 0 | 0 |
| Epigastric tenderness | 1 (3) | 0 | 0 | 0 |
| Right upper quadrant tenderness | 1 (3) | 0 | 0 | 0 |
| Thigh pain | 0 | 0 | 0 | 1 (4) |
| Vascular disorders | ||||
| Anemia | 0 | 0 | 0 | 1 (4) |
| Renal and urinary disorders | ||||
| Urinary tract infection | 0 | 0 | 1 (4) | 2 (8) |
| TEAEs possibly related to study drug | ||||
| Constipation | — | — | 1 (mild) | — |
| Nausea | 1 (mild) | — | — | — |
| Vomiting | 1 (mild) | — | — | — |
Abbreviation: TEAE, treatment-emergent adverse events; ZS-9, sodium zirconium cyclosilicate.
Note: All TEAEs were mild or moderate in severity, as judged by the investigators, and the majority were transient in duration; none were considered definitely related to study drug; there were no serious TEAEs.
No cases of significant hypocalcemia (⩽8.0 mg/dl), hypomagnesemia (⩽1.2 mEq/l), or hypokalemia (<3.0 mEq/l) were reported. ZS-9 had no dose-related effects on serum glucose, blood pressure, heart rate, or other vital signs.
DISCUSSION
These results demonstrate that oral ingestion of ZS-9 with meals significantly lowered s-K+ in patients with stage 3 CKD. The 10-g dose of ZS-9 rapidly and substantially reduced mean s-K+: (1) levels were 0.92 mEq/l below baseline after 38 h of treatment (P<0.001), and (2) levels remained significantly lower than placebo for up to 3.5 days after the last dose. The 3-g dose also significantly reduced the mean s-K+ in the first 48 h of treatment, although the time to effect was longer and the overall magnitude of the change was smaller compared with the 10-g dose.
The rapid and significant fall in mean s-K+ by 0.92 mEq/l after 38 h of treatment with the 10-g dose of ZS-9 is clinically relevant in the context of hyperkalemia; a decline of ⩾1 mEq/l at 48 h in postcritical care may eliminate the significant association between high K+ levels and mortality.3 Although s-K+ levels remained lowered for up to 3.5 days after the last 10-g ZS-9 dose, they increased gradually after treatment discontinuation, emphasizing the need for maintenance treatment in CKD and hyperkalemia patients. Of note, mean s-K+ also decreased slightly with placebo at 8 and 14 h after the first dose (Table 1), after which s-K+ levels varied widely, but never decreased below 4.8 mEq/l (Figure 3).
A s-K+ reduction in patients entering an inpatient facility is not unexpected, given that their in-center diet, although unrestricted, likely differed from their usual home diet. In animal studies, ZS-9 was effective when given with food, and it did not affect food consumption or weight (data on file, ZS Pharma). ZS-9 was administered with meals in the present study to mimic the use of the compound in a real-world setting. It seems unlikely that changes in dietary K+ intake influenced the efficacy conclusions for the following reasons: first, the between-group difference was maintained at nearly all time points to Day 6 (Figure 3), and, second, any changes in dietary K+ intake would likely have occurred across treatment groups in this double-blind study. In line with these findings, 24-h urinary K+ excretion declined significantly by 24.7 mEq/day with 10-g ZS-9 at day 2 compared with placebo (Figure 4a). Over the 2-day trial, the cumulative difference in urinary potassium excretion between placebo and the 10-g-treated group was approximately 40 mEq.
ZS-9 binds both NH4+ and K+ in vitro, owing to compatible ionic diameters with ZS-9 pore size. Consistent with ZS-9 binding of NH4+, small increases in serum bicarbonate levels were observed in patients on ZS-9 (3 mEq/l with 10-g dose), as well as a significant decrease in blood urea nitrogen. Intravenous sodium bicarbonate has been used as a treatment for patients with marked hyperkalemia and acidosis, but most studies have shown that the effect of this therapy is small, yielding an ∼0.1 mEq/l decrease in s-K+ for each 1 mEq/l increase in serum bicarbonate—after accounting for extracellular fluid volume changes.25, 26, 27, 28 Other studies have demonstrated that changes in serum bicarbonate have no effect on s-K+ levels.29, 30, 31 Correction of acidosis has been shown to diminish the rate of progression of renal disease.24, 32 The ability of ZS-9 to elicit additional kidney-protective effects via persistent improvement of acid–base balance warrants further study.
Factors related to diabetes control or compliance with concomitant medications (e.g., loop or thiazide diuretics) may also have contributed to s-K+ decreases in the placebo group. The distribution of patients with diabetes and on diuretics was comparable across treatment groups, and there was no evidence of a dose-related effect of ZS-9 on blood glucose.
ZS-9 treatment was well-tolerated. AE rates were comparable to placebo at 0.3- and 3-g ZS-9. AE rates were higher with 10-g ZS-9, primarily related to a higher incidence of gastrointestinal-related events (seven events in four patients); however, most of the events were transient and mild. Screening urinalysis was positive for bacterial culture in three patients with urinary tract infection reported as AEs with ZS-9; the patients' clinical conditions did not worsen during the study. No sodium loading was observed with ZS-9. There was no evidence of worsening hypertension or fluid retention. The present study evaluated the effects of ZS-9 t.i.d. for 2 days (and up to two more days if s-K+ ⩾5.0 mEq/l), and thus it does not provide information on chronic treatment with less frequent daily dosing. A recently completed, two-stage (acute and extended) phase 3 study (n=753) evaluated ZS-9 once daily for up to 12 days in patients with hyperkalemia (with or without CKD) who achieved normokalemia in the acute phase; the results will provide data on the safety and efficacy profile of ZS-9 in the extended treatment setting.
The relatively small number of patients per treatment arm is a limitation to the current study; still, the consistency of the efficacy end points in the overall population and the RAAS subgroup, dose response, and compelling P-values suggest that a true treatment effect was observed. The K+ content of meals was not measured; the degree to which dietary K+ affects s-K+ levels during ZS-9 treatment remains to be determined. Few patients had s-K+ >5.5 mEq/l; meaningful conclusions cannot be drawn about ZS-9's effects in patients with more severe hyperkalemia. This will be evaluated in the Phase 3 trials of ZS-9. Similarly, only patients with stage 3 CKD were included in the present study, and thus results cannot be extrapolated to patients with more severe CKD. Although RAAS blockade use was not an entry criterion, more than half of the patients were receiving an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at baseline. This, combined with the lack of dietary restrictions, suggests that the study setting was consistent with what clinicians encounter in daily practice.
In conclusion, these results demonstrate that ZS-9 is well-tolerated and effective at acutely reducing s-K+ in hyperkalemic patients with stable stage 3 CKD. Further study of the long-term efficacy and safety of ZS-9 to decrease K+ and maintain normokalemia in a broader population of patients with more severe hyperkalemia is ongoing.
MATERIALS AND METHODS
This prospective, double-blind, randomized, placebo-controlled, multicenter Phase 2 study with three dose cohorts was conducted in compliance with local and national regulations, Good Clinical Practice guidelines, and the Declaration of Helsinki Principles (clinicaltrials.gov NCT01493024, 19 November 2012). All patients signed informed consent before enrollment. An independent data safety monitoring committee periodically evaluated accrued safety data.
Patients
Eligible patients, identified during routine or urgent visits to a nephrologist or other healthcare providers, were aged ⩾18 years, with stable Stage 3 CKD, estimated glomerular filtration rate of 30–60 ml/min per 1.73 m2 estimated by CKD Epidemiology Collaboration (CKD-EPI) equation, and mild-to-moderate hyperkalemia (s-K+ 5.0–6.0 mEq/l). Patients with diabetes, heart failure, and hypertension were allowed; patients were instructed to maintain RAAS inhibitors and other medications as prescribed during the study, with compliance monitored by study staff. Exclusion criteria included pseudohyperkalemia, treatment with oral Kayexalate or phosphate binders ⩽7 days before enrollment, severe acidosis, acute kidney injury, and/or hyperkalemia-related electrocardiogram changes.
Randomization and study conduct
Three placebo-controlled dose-escalating cohorts were treated sequentially, with ⩾1 week between cohorts to allow the data safety monitoring committee to evaluate safety/tolerability. A centralized computer-generated, block randomization scheme was used (random block size, three or six per site for each cohort). Patients were randomly allocated to ZS-9 or placebo, administered as a suspension in water t.i.d., in a 2:1 ratio per cohort (cohort 1: n=18 (12–0.3 g ZS-9, 6 to placebo); cohort 2: n=36 (24–3 g ZS-9, 12 to placebo); cohort 3: n=36 (24–10 g ZS-9, 12 to placebo)).
On Study Day -1 [within 24 h of randomization], patients with mean s-K+5.0-6.0 mEq/l (based on three samples collected 30 min apart) were instructed to return to the clinic the next day (Study Day 0) for baseline estimated glomerular filtration rate determination (CKD-EPI). If the mean of two estimated glomerular filtration rate determinations (at 0 and 60 min) was within the prespecified range (40–60 ml/min per 1.73 m2 for cohort 1; 30–60 ml/min per 1.73 m2 for 9 of 36 patients in cohort 2; and 30–60 ml/min per 1.73 m2 for all other patients in cohorts 2 and 3), the patient was randomized and returned to the clinic the next day (day 1). Mean baseline s-K+ levels were calculated on day 1, on the basis of a weighted average of the three assessments from Study Day −1 and an additional assessment on Study Day 1 before the first dose.
During the inpatient treatment phase, patients were treated with ZS-9 or matching placebo with meals for the first 48 h (days 1–2) to normalize s-K+ (3.5–4.9 mEq/l). After 48 h (i.e., six doses), patients with normalized s-K+ (3.5–4.9 mEq/l) were discharged; patients with elevated s-K+ (⩾5.0 mEq/l) received an additional day of treatment. This process was repeated on the morning of day 4. Patients could receive a minimum of 6 doses of study drug (over 2 days) or a maximum of 12 doses (over 4 days). Patients returned to the clinic on days 5–7 (the end-of-study visit). Diet was unrestricted; patients were instructed to maintain normal food intake during the study. During the inpatient phase, patients were offered the standard clinic diet or could order food from local restaurants.
Patients could be discontinued from treatment at any time owing to safety concerns. Safety stopping rules, including treatment discontinuation if s-K+ was >6.5 or <3.5 mEq/l, were prespecified and shared with the data safety monitoring committee.
Dosage and administration
The 0.3-, 3-, and 10-g doses were selected on the basis of animal oral toxicity studies and in vitro K+ binding capacity of ZS-9 (23, data on file, ZS Pharma). The 3- and 10-g ZS-9 doses were selected to determine a possible dose–response relationship; the 0.3-g dose was selected to evaluate a presumed subtherapeutic dose. Placebo was PROSOLV SMCC 90 (silicified microcrystalline cellulose; JRS Pharma, Rosenberg, Germany), a tasteless, odorless, water-insoluble, white powder that is not systemically absorbed and was indistinguishable from ZS-9 suspension.
An unblinded site pharmacist with no other involvement in the study dispensed placebo (3 g) or ZS-9 (0.3, 3, or 10 g) loaded approximately 50:50 with Na+ and H+ in powdered form into an opaque container; it was mixed with 180 ml of water by blinded clinic staff immediately before administration. Starting on Study Day 1, patients orally ingested study drug suspensions t.i.d. with meals (∼0800, ∼1200, and ∼1800 h ±30 min), except that on day 1 breakfast was provided at 0930 h (90 min after the first dose). The dosing bottle was sequentially rinsed twice with 30 ml of water, and the patients consumed the rinses. Clinic staff oversaw administration of the study drug and concomitant medications. No diarrhea-inducing medications were used.
Assessments
Blood was collected daily, predose, to determine serum chemistry and hematology parameters; s-K+ samples were collected at 0.5, 1, 2, and 4 h after the initial dose on day 1 and every 4 h post dose thereafter. Serum samples were analyzed locally and by the central laboratory; study eligibility used local laboratory values. A baseline 24-h urine sample was collected starting on day 0 (after randomization patients were sent home with a urine container and instructed to collect and store their urine until they returned to the clinic on day 1). On-treatment 24-h urine samples were collected on day 1 and on each subsequent study day until the patient was discharged from the clinic.
Outcomes
The primary efficacy end point was the rate of s-K+ decline in the first 48 h, using all post-baseline s-K+ data. Rate of s-K+ decline was chosen as a more clinically relevant end point than absolute change from baseline. Secondary efficacy end points included changes in s-K+ at various time points after start of treatment; secondary pharmacodynamic variables included urine excretion (K+, Na+), serum electrolytes (Ca2+ and Mg2+, Na+), and kidney function parameters (i.e., blood urea nitrogen, serum creatinine, 24-h urea nitrogen and urine creatinine excretion, urinary sediment).
Safety was evaluated by AEs, vital signs, ECGs, and concomitant medications, and evaluation of clinically relevant chemistry, hematology, and urinalysis parameters.
Statistical methods
The efficacy data included all randomized patients who received study drug and had s-K+ determined at least at the 48-h time point after the start of treatment. The safety population included all treated patients with any post-baseline safety data. Central laboratory values were used for all statistical analyses.
The sample size was determined to provide 90% power to detect a 0.105 log difference between a ZS-9 dose group and placebo, with an overall two-sided 5% Type 1 error. For the primary efficacy end point, treatment groups were compared with placebo using a prespecified longitudinal model (SAS PROC MIXED, SAS Institute, Cary, NC) that included all post-baseline s-K+ during the initial 48 h of treatment. Data were log-transformed and fitted using the baseline, time, and the dose–time interaction. A closed testing procedure was prespecified to compare ZS-9 doses with placebo from the highest to the lowest dose to preserve the Type 1 error. Treatment groups were also compared using an unpaired t-test for efficacy and pharmacodynamic end points. Safety data were summarized descriptively by treatment group.
Acknowledgments
This study was funded by ZS Pharma. The authors thank the principal investigators who contributed to this study, the patients who participated in this study, and the contributing staff at the study sites. Primary results of this study were previously reported during an oral presentation at the annual meeting of the American Society of Nephrology, 7–10 November 2013, Atlanta, GA. Medical writing assistance was provided by Xelay Acumen, and was funded by ZS Pharma.
SRA owns a minority interest in HemoCleanse, which owns a minority interest in ZS Pharma. BS was an employee of Apex Research of Riverside at the time of the study, has served as a consultant for Amgen and Keryx, and is on the speakers' bureau for Questcor. PTL is an employee of Boston Biostatistics Research Foundation, which conducted the statistical analyses for the study. BS, HSR, and FS are employees of, and hold stock options in, ZS Pharma.
Footnotes
SUPPLEMENTARY MATERIAL
Figure S1. ZS-9 recovery in feces of Sprague–Dawley rats.
Figure S2. Potassium excretion in Sprague–Dawley rats (n=20) administered escalating doses of 2, 4, and 6g ZS-9 (human dose equivalent of 21, 42, and 62 g/day) for 5 days with a 5-day washout between each dose escalation.
Supplementary material is linked to the online version of the paper at http://www.nature.com/ki
Supplementary Material
References
- Jain N, Kotla S, Little BB, et al. Predictors of hyperkalemia and death in patients with cardiac and renal disease. Am J Cardiol. 2012;109:1510–1513. doi: 10.1016/j.amjcard.2012.01.367. [DOI] [PubMed] [Google Scholar]
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169:1156–1162. doi: 10.1001/archinternmed.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon GM, Mendu ML, Gibbons FK, et al. Association between hyperkalemia at critical care initiation and mortality. Intensive Care Med. 2012;38:1834–1842. doi: 10.1007/s00134-012-2636-7. [DOI] [PubMed] [Google Scholar]
- Goyal A, Spertus JA, Gosch K, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- Torlen K, Kalantar-Zadeh K, Molnar MZ, et al. Serum potassium and cause-specific mortality in a large peritoneal dialysis cohort. Clin J Am Soc Nephrol. 2012;7:1272–1284. doi: 10.2215/CJN.00960112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AJ, Foley RN, Herzog C, et al. US Renal Data System 2012 Annual Data Report. Am J Kidney Dis. 2013;61 (1 Suppl 1:e1–e480. doi: 10.1053/j.ajkd.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Fordjour KN, Walton T, Doran JJ. Management of hyperkalemia in hospitalized patients. Am J Med Sci. 2014;347:93–100. doi: 10.1097/MAJ.0b013e318279b105. [DOI] [PubMed] [Google Scholar]
- Product Label, Kayexalate®. December 2010 (Sanofi-Aventis US, LLC).
- Watson MA, Baker TP, Nguyen A, et al. Association of prescription of oral sodium polystyrene sulfonate with sorbitol in an inpatient setting with colonic necrosis: a retrospective cohort study. Am J Kidney Dis. 2012;60:409–416. doi: 10.1053/j.ajkd.2012.04.023. [DOI] [PubMed] [Google Scholar]
- Gruy-Kapral C, Emmett M, Santa Ana CA, et al. Effect of single dose resin-cathartic therapy on serum potassium concentration in patients with end-stage renal disease. J Am Soc Nephrol. 1998;9:1924–1930. doi: 10.1681/ASN.V9101924. [DOI] [PubMed] [Google Scholar]
- Kessler C, Ng J, Valdez K, et al. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J Hosp Med. 2011;6:136–140. doi: 10.1002/jhm.834. [DOI] [PubMed] [Google Scholar]
- Scherr L, Ogden DA, Mead AW, et al. Management of hyperkalemia with a cation-exchange resin. N Engl J Med. 1961;264:115–119. doi: 10.1056/NEJM196101192640303. [DOI] [PubMed] [Google Scholar]
- Flinn RB, Merrill JP, Welzant WR. Treatment of the oliguric patient with a new sodium-exchange resin and sorbitol; a preliminary report. N Engl J Med. 1961;264:111–115. doi: 10.1056/NEJM196101192640302. [DOI] [PubMed] [Google Scholar]
- Castillo-Cejas MD, De-Torres-Ramirez I, Alonso-Cotoner C. Colonic necrosis due to calcium polystyrene sulfonate (Kalimate) not suspended in sorbitol. Rev Esp Enferm Dig. 2013;105:232–234. doi: 10.4321/s1130-01082013000400010. [DOI] [PubMed] [Google Scholar]
- Harel Z, Harel S, Shah PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (Kayexalate) use: a systematic review Am J Med 2013126264e269–224. [DOI] [PubMed] [Google Scholar]
- Sterns RH, Rojas M, Bernstein P, et al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective. J Am Soc Nephrol. 2010;21:733–735. doi: 10.1681/ASN.2010010079. [DOI] [PubMed] [Google Scholar]
- Schroeder HA, Balassa JJ. Abnormal trace metals in man: zirconium. J Chronic Dis. 1966;19:573–586. doi: 10.1016/0021-9681(66)90095-6. [DOI] [PubMed] [Google Scholar]
- Lee DBN, Roberts M, Bluchel CG, et al. Zirconium: biomedical and nephrological applications. ASAIO J. 2010;56:550–556. doi: 10.1097/MAT.0b013e3181e73f20. [DOI] [PubMed] [Google Scholar]
- Ash SR. Sorbents in treatment of uremia: a short history and a great future. Semin Dialysis. 2009;22:615–622. doi: 10.1111/j.1525-139X.2009.00657.x. [DOI] [PubMed] [Google Scholar]
- Agar JW. Review: understanding sorbent dialysis systems. Nephrology (Carlton) 2010;15:406–411. doi: 10.1111/j.1440-1797.2010.01321.x. [DOI] [PubMed] [Google Scholar]
- Bern DS, Sherman JD, Ash SA, et al. Ammonium removal with a novel zirconium silicate. ASAIO J. 2001;47:151. [Google Scholar]
- Berlyne GM, Janabi K, Shaw AB, et al. Dangers of resonium A in the treatment of hyperkalemia in renal failure. Lancet. 1966;287:169–172. doi: 10.1016/s0140-6736(66)90697-0. [DOI] [PubMed] [Google Scholar]
- Stavros F.Submitted manuscript.
- Goraya N, Wesson DE. Does correction of metabolic acidosis slow chronic kidney disease progression. Curr Opin Nephrol Hypertens. 2013;22:193–197. doi: 10.1097/MNH.0b013e32835dcbbe. [DOI] [PubMed] [Google Scholar]
- Blumberg A, Weidmann P, Ferrari P. Effect of prolonged bicarbonate administration on plasma potassium in terminal renal failure. Kidney Int. 1992;41:369–374. doi: 10.1038/ki.1992.51. [DOI] [PubMed] [Google Scholar]
- Fraley DS, Adler S. Correction of hyperkalemisa by bicarbonate despite constant blood pH. Kidney Int. 1977;12:354–360. doi: 10.1038/ki.1977.122. [DOI] [PubMed] [Google Scholar]
- Gutierrez R, Schlessinger F, Oster JR, et al. Effect of hypertonic versus isotonic sodium bicarbonate on plasma potassium concentration in patients with end-stage renal disease. Miner Electrolyte Metab. 1991;17:297–302. [PubMed] [Google Scholar]
- Abramowitz MK, Melamed ML, Bauer C, et al. Effects of oral sodium bicarbonate in patients with CKD. Clin J Am Soc Nephrol. 2013;8:714–720. doi: 10.2215/CJN.08340812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney BA, Smith WA, Lo DS, et al. Emergency interventions for hyperkalaemia. Cochrane Database Syst Rev. 2005. p. CD003235. [DOI] [PMC free article] [PubMed]
- Allon M, Shanklin N. Effect of bicarbonate administration on plasma potassium in dialysis patients: interactions with insulin and albuterol. Am J Kidney Dis. 1996;28:508–514. doi: 10.1016/s0272-6386(96)90460-6. [DOI] [PubMed] [Google Scholar]
- Blumberg A, Weidmann P, Shaw S, et al. Effect of various therapeutic approaches on plasma potassium and major regulating factors in terminal renal failure. Am J Med. 1988;85:507–512. doi: 10.1016/s0002-9343(88)80086-x. [DOI] [PubMed] [Google Scholar]
- Koniewski I, Wesson DE. Bicarbonate therapy for prevention of chronic kidney disease progression. Kidney Int. 2014;85:529–535. doi: 10.1038/ki.2013.401. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.