Abstract
The TP53 mutation is frequently detected in acute myeloid leukemia (AML) patients with complex karyotype (CK), but the stability of this mutation during the clinical course remains unclear. In this study, TP53 mutations were identified in 7% of 500 patients with de novo AML and 58.8% of patients with CK. TP53 mutations were closely associated with older age, lower white blood cell (WBC) and platelet counts, FAB M6 subtype, unfavorable-risk cytogenetics and CK, but negatively associated with NPM1 mutation, FLT3/ITD and DNMT3A mutation. Multivariate analysis demonstrated that TP53 mutation was an independent poor prognostic factor for overall survival and disease-free survival among the total cohort and the subgroup of patients with CK. A scoring system incorporating TP53 mutation and nine other prognostic factors, including age, WBC counts, cytogenetics and gene mutations, into survival analysis proved to be very useful to stratify AML patients. Sequential study of 420 samples showed that TP53 mutations were stable during AML evolution, whereas the mutation was acquired only in 1 of the 126 TP53 wild-type patients when therapy-related AML originated from different clone emerged. In conclusion, TP53 mutations are associated with distinct clinic-biological features and poor prognosis in de novo AML patients and are rather stable during disease progression.
Introduction
Somatic mutation of the tumor suppressor gene, TP53, located in 17p13 is one of the most frequent alterations in cancer.1, 2 The TP53 protein exerts its tight regulation of apoptosis and cell cycle integrity, and inactivation of TP53 may lead to uncontrolled cell proliferation and promote cancer development.3, 4 The frequency of TP53 mutation is usually increased in the patients with advanced stages or aggressive types of cancers.5, 6
Several studies have shown TP53 mutations are frequently detected in patients with therapy-related acute myeloid leukemia (AML)7 or AML with complex karyotype (AML-CK).8, 9, 10 The incidences of this mutation in AML-CK varied from 53% in a British series,10 to 60–69% in two German studies.8, 9 In contrast, TP53 mutations rarely occurred in patients without CK (2.1%)8 or 17p chromosomal abnormality (2.8%).11 The reports regarding the prognostic relevance of TP53 mutations in patients with AML-CK showed controversial results. Rucker et al.9 showed that TP53 mutation was an independent poor risk factor for overall survival (OS) in AML patients with CK; however, the same finding could not be shown by Bowen et al.10
Whether there is geographic difference in the incidence of TP53 mutations in AML between Western and Asian people remains to be determined. In addition, the interaction of TP53 mutations with other genetic alterations in AML was largely unknown. Furthermore, to the best of our knowledge, there has been no report in literature concerning the stability of TP53 mutations during the clinical course. In this study, we investigated TP53 mutation in 500 patients with de novo AML and analyzed its interactions with 17 other genetic alterations. Longitudinal follow-ups of the status of TP53 mutation during the clinical course were also performed in 131 patients to investigate the stability and pathogenic role of this mutation in AML. Furthermore, to better stratify AML patients into different risk groups, a scoring system integrating TP53 mutations with nine other prognostic factors, including age, white blood cell (WBC) count, cytogenetics, NPM1/FLT3-ITD, CEBPA, RUNX1, WT1, DNMT3A and IDH2 mutations, into survival analysis was proposed.
Materials and methods
Subjects
From March 1995 to December 2008, a total of 500 adult patients who were newly diagnosed as having de novo AML at the National Taiwan University Hospital and had enough cryopreserved cells for analysis were enrolled consecutively. Patients with antecedent hematological diseases or therapy-related AML were excluded. Diagnosis and classification of AML were made according to the FAB (French–American–British) Cooperative Group Criteria. Among them, 363 (72.6%) patients received standard induction chemotherapy (Idarubicin 12 mg/m2 per day on days 1–3 and Cytarabine 100 mg/m2 per day on days 1–7) and then consolidation chemotherapy with 2–4 courses of high-dose Cytarabine (2000 mg/m2 q.12 h, days 1–4, total 8 doses), with or without an anthracycline (Idarubicin or Novatrone), after achieving complete remission (CR).12, 13 The patients with acute promyelocytic leukemia (M3 subtype) received concurrent all-trans retinoic acid and chemotherapy. The remaining 137 patients received palliative therapy with supportive care and/or low-dose chemotherapy because of underlying comorbidity or based on the decision of the patients. A total of 45 patients received allogeneic hematopoietic stem cell transplantation in first CR. This study was approved by the institutional review board of the National Taiwan University Hospital; and written informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Cytogenetics
Bone marrow (BM) cells were harvested directly or after 1–3 days of unstimulated culture as described previously.14 Metaphase chromosomes were banded by trypsin-Giemsa technique and karyotyped according to the International System for Human Cytogenetic Nomenclature.
Immunophenotype analysis
A panel of monoclonal antibodies to myeloid-associated antigens, including CD13, CD33, CD11b, CD15, CD14 and CD41a, as well as lymphoid-associated antigens, including CD2, CD5, CD7, CD19, CD10 and CD20, and lineage nonspecific antigens HLA-DR, CD34 and CD56 were used to characterize the phenotypes of the leukemia cells as previously described.12
Mutation analysis
Mutation analysis of TP53 exons 3–9 was performed by PCR and direct sequencing according to previous reports with mild modification.8, 9 The primer sequences are shown in Supplementary Table 1. Abnormal sequencing results were confirmed by at least two repeated analyses. Sequential analysis of TP53 mutation during the clinical course was performed in 420 samples from 131 patients. Mutation analyses of 17 other relevant molecular marker genes, including class I mutations such as FLT3/ITD and FLT3/TKD,15 NRAS,16 KRAS,16 JAK2,16 KIT17 and PTPN11 mutations,18 and class II mutations such as CEBPA19 and RUNX1 mutations,20 as well as NPM1,21 WT1,22 and those genes related to epigenetic modification such as MLL/PTD,23 ASXL1,24 IDH1,25 IDH2,26 TET2,27 and DNMT3A mutations12 were performed as previously described. To detect TP53 mutation at diagnosis, we used DNA amplified in vitro from patients' BM cells by Illustra GenomiPhi V2 DNA amplification kit as described by the manufacturer (GE Healthcare, Buckinghamshire, UK). All the mutations detected in such samples were verified in the original nonamplified samples.
TA cloning analysis
For the patients with discrepancy of the mutation status of the TP53 in paired samples, Taq polymerase-amplified (TA) cloning was performed in the samples without detectable mutant by direct sequencing. The DNA spanning the mutation spots of TP53 detected at either diagnosis or during subsequent follow-ups was amplified and the PCR products were then cloned into the TA cloning vector pGEM-T Easy (Promega, Madison, WI, USA). Direct sequencing was then performed on the selected clones. More than 40 clones were selected for sequencing as previously described.28
Statistical analysis
The discrete variables of patients with and without TP53 mutation were compared using the χ2 tests, but if the expected values of contingency tables were <5, Fisher's exact test was used. If the continuous data were not normally distributed, Mann–Whitney U-tests were used to compare continuous variables and medians of distributions. To evaluate the impact of TP53 mutation on clinical outcome, only the patients who received conventional standard chemotherapy, as mentioned above, were included in the analysis.12, 13 OS was measured from the date of first diagnosis to the date of last follow-up or death from any cause, whereas relapse was defined as a reappearance of at least 5% leukemic blasts in a BM aspirate or new extramedullary leukemia in patients with a previously documented CR.29 Disease-free status indicated that the patient achieved CR and did not relapse by the end of this study. Cox regression survival estimation was used to plot survival curves and to test the difference between groups. Multivariate Cox proportional hazard regression analysis was used to investigate independent prognostic factors for OS and disease-free survival (DFS). The proportional hazards assumption (constant hazards assumption) was examined by using time-dependent covariate Cox regression before conducting multivariate Cox proportional hazard regression. The variables including age, WBC counts, karyotype, NPM1/FLT3-ITD, CEBPA, IDH2, WT1, RUNX1, ASXL1, DNMT3A and TP53 mutations were used as covariates. Those patients who received hematopoietic stem cell transplantation were censored at the time of hematopoietic stem cell transplantation in survival analysis to ameliorate the influence of the treatment.12, 13 A P-value of <0.05 was considered statistically significant. All statistical analyses were performed with the SPSS 19 (SPSS Inc., Chicago, IL, USA) and Statsdirect (Cheshire, UK).
Results
TP53 mutations in patients with de novo AML
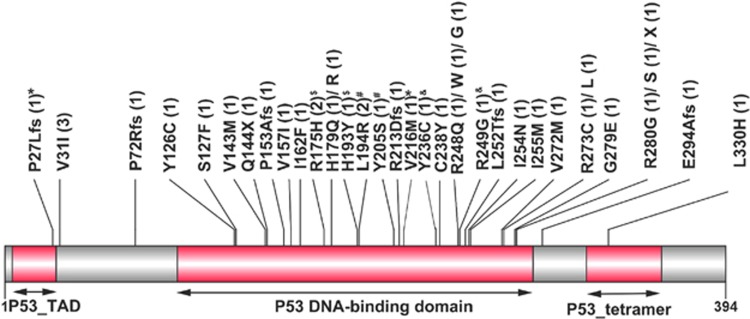
A total of 36 different TP53 mutations were identified in 35 patients (Table 1 and Figure 1). Of these, 28 were missense mutations, 2 were nonsense mutations, 5 were frame-shift mutations and 1 was in-frame mutation. V31I occurred in three patients, R175H and L194R in two each and all other mutations in only one each. Five patients had double heterozygous mutations (patients 1, 4, 14, 21 and 23). The remaining 30 patients showed only one mutation; all were heterozygous.
Table 1. The mutation patterns in 35 patients with TP53 mutations at diagnosis.
| UPN | Age/sex | FAB |
TP53 mutation |
Other accompanied gene mutations | ||
|---|---|---|---|---|---|---|
| Location | DNA change | Protein change | ||||
| 1 | 60/M | M1 | Exon 6 | c.581T>G | L194R | PTPN11, RUNX1 |
| Exon 6 | c.614A>C | Y205S | ||||
| 2 | 70/M | M4 | Exon 4 | c.91G>A | V31I | IDH1 |
| 3 | 60/M | M2 | Exon 6 | c.636del | R213DfsX34 | ─ |
| 4 | 58/F | M8 | Exon 4 | c.80delC | P27LfsX17 | ─ |
| Exon 6 | c.646G>A | V216M | ||||
| 5 | 78/M | M1 | Exon 8 | c.814G>A | V272M | ─ |
| 6 | 41/F | M1 | Exon 7 | c.743G>A | R248Q | ─ |
| 7 | 79/F | M2 | Exon 7 | c.752_754del | L252TfsX142 | ─ |
| 8 | 47/F | M6 | Exon 8 | c.989T>A | L330H | ─ |
| 9 | 66/F | M2 | Exon 8 | c.817C>T | R273C | ─ |
| 10 | 67/M | M0 | Exon 8 | c.840A>T | R280S | ASXL1 |
| 11 | 51/F | M6 | Exon 6 | c.581T>G | L194R | ─ |
| 12 | 37/F | M1 | Exon 7 | c.742C>G | R248G | ─ |
| 13 | 67/M | M2 | Exon 7 | c.761T>A | I254N | NRAS |
| 14 | 43/F | M8 | Exon 7 | c.707A>G | Y236C | WT1 |
| Exon 7 | c.745A>G | R249G | ||||
| 15 | 66/F | M4 | Exon 8 | c.836G>A | G279E | TET2 |
| 16 | 58/M | M1 | Exon 5 | c.430C>T | Q144X | ─ |
| 17 | 72/M | M4 | Exon 5 | c.484A>T | I162F | CEBPA, TET2 |
| 18 | 81/F | M1 | Exon 7 | c.742C>T | R248W | ─ |
| 19 | 36/F | M2 | Exon 5 | c.469G>A | V157I | ASXL1 |
| 20 | 74/F | M4 | Exon 5 | c.450_451insC | P153AfsX28 | NRAS |
| 21 | 72/M | M2 | Exon 6 | c.524G>A | R175H | ─ |
| Exon 6 | c.577C>T | H193Y | ||||
| 22 | 37/F | M6 | Exon 7 | c.713G>A | C238Y | ─ |
| 23 | 72/M | M2 | Exon 5 | c.536A>G | H179R | ─ |
| Exon 8 | c.838A>T | R280X | ||||
| 24 | 68/F | M2 | Exon 8 | c.879_880del | E294AfsX11 | ─ |
| 25 | 74/M | M8 | Exon 4 | c.215_225del | P72RfsX73 | ─ |
| 26 | 54/M | M2 | Exon 5 | c.524G>A | R175H | ─ |
| 27 | 43/F | M1 | Exon 5 | c.427G>A | V143M | ─ |
| 28 | 83/M | M2 | Exon 5 | c.380C>T | S127F | ─ |
| 29 | 80/M | M1 | Exon 8 | c.818G>T | R273L | RUNX1 |
| 30 | 71/F | M1 | Exon 5 | c.377A>G | Y126C | ─ |
| 31 | 79/F | M4 | Exon 7 | c.764T>A | I255N | DNMT3A |
| 32 | 30/F | M1 | Exon 4 | c.91G>A | V31I | CEBPA, IDH2 |
| 33 | 66/F | M1 | Exon 8 | c.838A>G | R280G | ─ |
| 34 | 72/M | M2 | Exon 4 | c.91G>A | V31I | NPM1, PTPN11 |
| 35 | 66/F | M2 | Exon 5 | c.537T>G | H179Q | ─ |
Abbreviations: F, female; FAB, French–American–British; M, male; UPN, unique patient number.
Figure 1.
Patterns and locations of the TP53 mutations. The positions and predicted translational consequences of TP53 mutations detected in 500 AML samples are shown. The number of patients with the mutation is indicated in the parenthesis behind each mutation. The symbols ‘#', ‘*', ‘&', and ‘$' indicate that patients have two mutations.
Correlation of TP53 mutations with clinical and laboratory features
In total, 500 de novo AML patients, including 35 (7%) TP53-mutated and 465 TP53 wild-type patients, were enrolled into the analysis. The comparison of clinical characteristics of patients with and without TP53 mutations is shown in Table 2.
Table 2. Comparison of clinical and laboratory features between AML patients with and without TP53 mutation.
| Variables | Total (n=500) | TP53 mutated (n=35, 7%) | TP53 wild-type (n=465, 93%) | P-value |
|---|---|---|---|---|
| Sexa | 0.3763 | |||
| Male | 285 | 17 (6) | 268 (94) | |
| Female | 215 | 18 (8.4) | 197 (91.6) | |
| Age (years)b | 51 (15–90) | 67 (30–83) | 50 (15–90) | 0.0003 |
| Lab datab | ||||
| WBC (/μl) | 19 075 (120–627 800) | 3690 (720–178 400) | 22 510 (120–627 800) | <0.0001 |
| Hb (g/dl) | 8 (2.9–16.2) | 7.4 (4.5–12.7) | 8 (2.9–16.2) | 0.1772 |
| Platelet ( × 1000 /μl) | 42 (2–802) | 24 (3–802) | 44 (2–712) | 0.0267 |
| Blast (/μl) | 7401 (0–456 725) | 1145 (0–100 974) | 9744 (0–456 725) | <0.0001 |
| LDH (U/l) | 889 (206–15 000) | 751 (274–15 000) | 860 (206–13 130) | 0.3508 |
| FABa | ||||
| M0 | 10 | 1 (10) | 9 (90) | 0.5193 |
| M1 | 112 | 11 (9.8) | 101 (90.2) | 0.2067 |
| M2 | 171 | 12 (7) | 159 (93) | >0.9999 |
| M3 | 38 | 0 (0) | 38 (100) | 0.0976 |
| M4 | 124 | 5 (4) | 119 (96) | 0.1584 |
| M5 | 24 | 0 (0) | 24 (100) | 0.3994 |
| M6 | 12 | 3 (25) | 9 (75) | 0.0447 |
| Undetermined | 9 | 3 (33.3) | 6 (66.7) | 0.0198 |
| Induction responsec | 363 | 14 | 349 | |
| CR | 284 | 4 (28.6) | 280 (80.2) | <0.0001 |
| PR/refractory | 54 | 7 (50) | 47 (13.5) | 0.0017 |
| Induction death | 25 | 3 (21.4) | 22 (6.3) | 0.0634 |
| Relapsec | 144 | 3 (75) | 141 (50.4) | 0.6225 |
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; FAB, French–American–British; Hb, hemoglobin; LDH, lactate dehydrogenase; PR, partial remission; WBC, white blood cell.
Number of patients (%).
Median (range).
Only 363 patients, including 14 with TP53 mutation and 349 without, who received conventional intensive induction chemotherapy and then consolidation chemotherapy if CR was achieved, as mentioned in the text, were included in the analysis.
TP53-mutated patients were older (median, 67 vs 50 years, P=0.0003) and had lower WBC, blast and platelet counts than TP53 wild-type patients (P<0.0001, <0.0001 and 0.0267, respectively). Patients with FAB M6 subtype of AML had the highest incidence of TP53 mutation than those with other subtypes. The mutations were positively associated with the expression of CD34 on the leukemic cells (Supplementary Table 2). There was no difference in the expression of other antigens between the patients with and without TP53 mutation.
Association of TP53 mutations with cytogenetic abnormalities
Chromosome data were available in 482 patients at diagnosis, including 35 TP53-mutated and 447 TP53 wild-type patients (Supplementary Table 3). TP53 mutations occurred more frequently in patients with unfavorable-risk cytogenetics (46.2%) than in those with favorable- or intermediate-risk cytogenetics (1.2%, P<0.0001). There was also a significant difference in the incidence of the TP53 mutation among patients with normal karyotype (1.8%), simple chromosomal abnormalities with one or two changes (0.5%) and CK with three or more abnormalities (58.8%, P<0.0001). Besides, TP53-mutated patients had a higher degree of karyotypic complexity, as defined by five or more chromosomal changes, than TP53 wild-type patients in the subgroup of patients with CK (90% vs 42.9%, P=0.0005). None of the patients with t(15;17), inv(16), t(7;11) or 11q23 translocations showed TP53 mutation, but one patient with t(8;21) harbored this mutation concurrently. There was no association of TP53 mutation with other chromosomal abnormalities, including +8, +11, +13, +21, −5/del(5q) and −7/del(7q).
Association of TP53 mutation with other molecular abnormalities
To investigate the interaction of gene mutations in the pathogenesis of adult AML, a complete mutational screening of 17 other genes was performed in all 500 patients (Table 3). Among the 35 patients with TP53 mutations, 13 (37.1%) showed additional molecular abnormalities at diagnosis (Tables 1 and 4). Nine had one additional change and four had two. The associated molecular events included NRAS, PTPN11, CEBPA, RUNX1, ASXL1 and TET2 mutations that each occurred in two patients. Patients with TP53 mutations had significantly lower incidences of NPM1 mutation, FLT3/ITD and DNMT3A mutations than those with TP53 wild-type (2.9% vs 21.9%, P=0.0041; 0% vs 24.3%, P=0.0002; and 2.9% vs 14.8% P=0.045, respectively). There was no difference in the incidence of other molecular mutations between patients with and without TP53 mutation. Interestingly, TP53-mutated patients with complex cytogenetics had lower probability of concurrent other molecular alterations than those without (26.7% vs 100%, P=0.004).
Table 3. Association of TP53 mutation with other gene mutations.
| Variables |
No. of patients with alteration (%) |
P-value | ||
|---|---|---|---|---|
| Whole cohort (n=500) | TP53-mutated patients (n=35) | TP53 wild-type patients (n=465) | ||
| FLT3/ITD | 113 (22.6) | 0 (0) | 113 (24.3) | 0.0002 |
| FLT3/TKD | 38 (7.6) | 0 (0) | 38 (8.2) | 0.097 |
| NRAS | 61 (12.2) | 2 (5.7) | 59 (12.7) | 0.2918 |
| KRAS | 16 (3.2) | 0 (0) | 16 ((3.4) | 0.6175 |
| PTPN11 | 18 (3.6) | 2 (5.7) | 16 (3.4) | 0.3635 |
| KIT | 15 (3.0) | 0 (0) | 15 (3.2) | 0.6143 |
| JAK2 | 3 (0.6) | 0 (0) | 3 (0.6) | >0.9999 |
| WTI | 33 (6.6) | 1 (2.9) | 32 (6.9) | 0.7195 |
| NPM1 | 103 (20.6) | 1 (2.9) | 102 (21.9) | 0.0041 |
| CEBPA | 66 (13.2) | 2 (5.7) | 64 (13.8) | 0.2957 |
| RUNX1 | 62 (12.4) | 2 (5.7) | 60 (12.9) | 0.2912 |
| MLL/PTD | 27 (5.4) | 0 (0) | 27 (5.8) | 0.2444 |
| ASXL1 | 50 (10.0) | 2 (5.7) | 48 (10.3) | 0.5613 |
| IDH1 | 27 (5.4) | 1 (2.9) | 26 (5.6) | 0.7115 |
| IDH2 | 55 (11) | 1 (2.9) | 54 (11.6) | 0.1584 |
| TET2 | 66 (13.2) | 2 (5.7) | 64 (13.8) | 0.2957 |
| DNMT3A | 70 (14.0) | 1 (2.9) | 69 (14.8) | 0.045 |
Table 4. Multivariate analysis (Cox regression) on the disease-free survival and overall survival.
| Variables |
Overall survival |
Disease-free survival |
||||||
|---|---|---|---|---|---|---|---|---|
|
95% CI |
95% CI |
|||||||
| RR | Lower | Upper | P | RR | Lower | Upper | P | |
| Agea | 2.426 | 1.736 | 3.391 | <0.001b | 1.431 | 1.084 | 1.888 | 0.011b |
| WBCc | 2.127 | 1.481 | 3.056 | <0.001b | 1.762 | 1.309 | 2.370 | <0.001b |
| Karyotyped | 1.971 | 1.035 | 3.751 | 0.039b | 1.935 | 1.181 | 3.168 | 0.009b |
| NPM1/FLT3-ITDe | 0.304 | 0.147 | 0.631 | 0.001b | 0.304 | 0.162 | 0.567 | <0.001b |
| CEBPAf | 0.423 | 0.211 | 0.848 | 0.015b | 0.596 | 0.367 | 0.970 | 0.037b |
| IDH2g | 0.563 | 0.292 | 1.086 | 0.087 | 0.937 | 0.595 | 1.475 | 0.778 |
| WT1 | 2.387 | 1.387 | 4.109 | 0.002b | 2.315 | 1.505 | 3.561 | <0.001b |
| RUNX1 | 2.103 | 1.210 | 3.656 | 0.008b | 1.985 | 1.271 | 3.100 | 0.003b |
| ASXL1 | 0.726 | 0.403 | 1.306 | 0.285 | 0.941 | 0.544 | 1.628 | 0.828 |
| DNMT3A | 2.204 | 1.336 | 3.637 | 0.002b | 2.134 | 1.398 | 3.256 | <0.001b |
| TP53 | 4.684 | 2.073 | 10.584 | <0.001b | 2.547 | 1.244 | 5.214 | 0.011b |
Abbreviations: CI, confidence interval; RR, relative risk.
Age >50 years relative to age ≤50 years (the reference).
Statistically significant (P<0.05).
White blood cell (WBC) count >50 000/μl vs ≤50 000/μl.
Unfavorable cytogenetics vs others.
NPM1mut/FLT3-ITDneg vs other subtypes.
CEBPAdouble-mutation vs others.
IDH2 mutations included R140 and R172 mutations.
Impact of TP53 mutation on response to therapy and clinical outcome
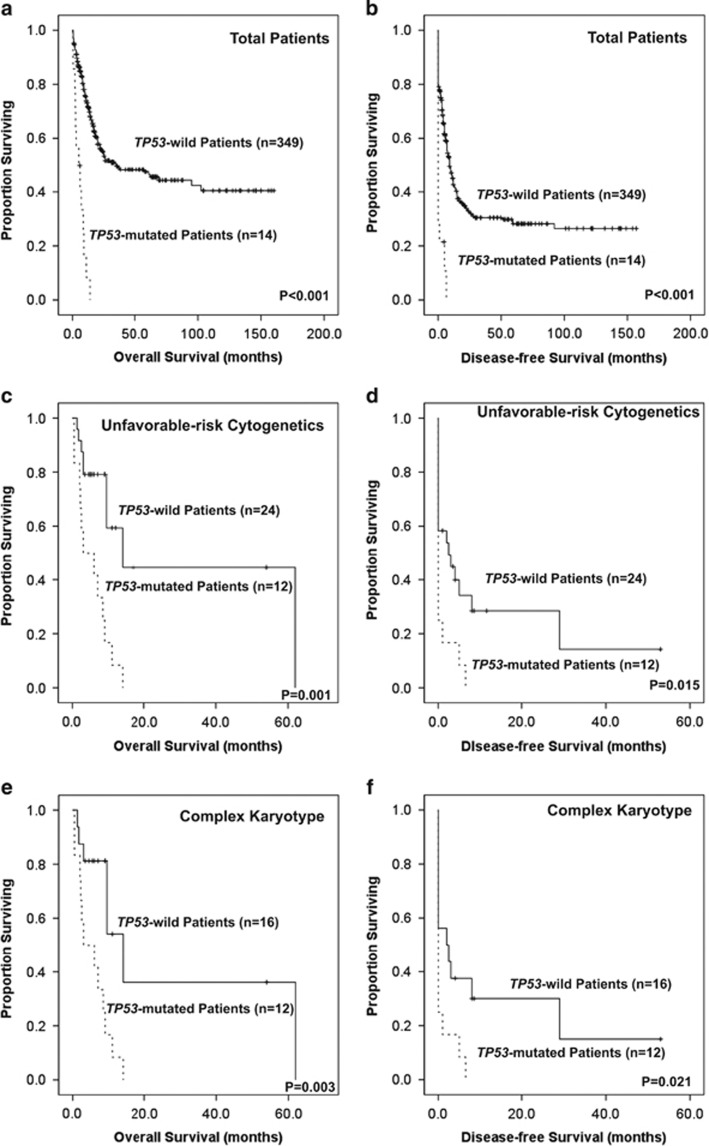
Of the 363 AML patients undergoing conventional intensive induction chemotherapy, 284 (78.5%) patients achieved a CR. TP53 mutation was associated with an inferior response rate (CR rate 28.6% vs 80.2%, P<0.0001) and higher probability to be refractory to treatment (50% vs 13.5%, P=0.0017). With a median follow-up of 55 months (range, 1.0–160), patients with TP53 mutation had significantly poorer OS and DFS than those without TP53 mutation (median, 5 vs 35 months, P<0.001, and median, 0 vs 9 months, P<0.001, respectively, Figures 2a and b). In the subgroups of 36 patients with unfavorable-risk cytogenetics, the differences in OS and DFS were still significant between patients with and without TP53 mutation (median, 9.5 vs 14 months, P=0.001, Figure 2c and median, 0 vs 2.5 months, P= 0.015, Figure 2d, respectively). The same was also true for the subgroup of 28 patients with CK (median, 9 vs 14 months, P=0.003, Figure 2e and median, 0 vs 2 months, P=0.021, Figure 2f, respectively). Intriguingly, among the patients without TP53 mutation, the OS is similar between those with intermediate-risk cytogenetics and unfavorable-risk cytogenetics (P=0.304).
Figure 2.
Kaplan–Meier survival curves for OS and DFS in a total of 363 AML patients (a, b), 36 patients with unfavorable-risk cytogenetics (c, d) and 28 patients with complex karyotype (e, f) who received standard intensive chemotherapy.
In multivariate analysis (Table 4), the independent poor risk factors for OS were older age >50 years, high WBC counts >50 000/μl, unfavorable-risk cytogenetics and TP53, RUNX1, WT1 and DNMT3A mutations. On the other hand, CEBPAdouble mutation and NPM1 mutation in the absence of FLT3-ITD (NPM1+/FLT3-ITD-) were independent favorable prognostic factors. There was a trend of better OS in patients with IDH2 mutation (hazard ratio 0.563, 95% confidence interval 0.292–1.086, P=0.087). Similarly, the independent poor risk factors for DFS included older age >50 years, high WBC counts >50 000/μl, unfavorable-risk cytogenetics and TP53, RUNX1, WT1 and DNMT3A mutations. On the other hand, CEBPAdouble mutation and NPM1+/FLT3-ITD- were independent favorable prognostic factors.
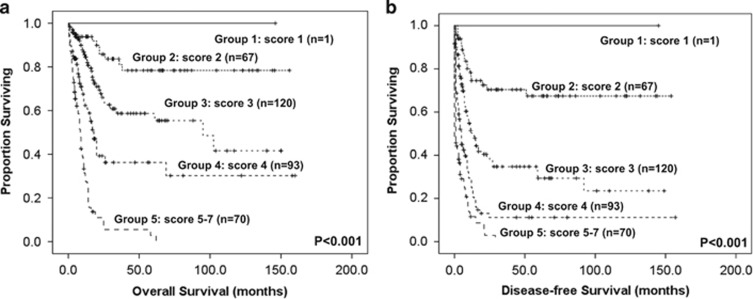
To better stratify the AML patients into different risk groups, a scoring system incorporating 10 prognostic markers, including age, WBC counts, cytogenetics at diagnosis, NPM1/FLT3-ITD and mutations of CEBPA, IDH2, TP53, DNMT3A, RUNX1 and WT1, into survival analysis was formulated based on the results of our Cox proportional hazards model. A score of −1 was assigned for each parameter associated with a favorable outcome (CEBPAdouble mutation, IDH2 mutation and NPM1+/FLT3-ITD-), whereas a score of +1 for each factor associated with an adverse outcome (TP53, DNMT3A, WT1 and RUNX1 mutations, older age and higher WBC counts at diagnosis). The karyotypes were stratified into three groups (unfavorable: +2, intermediate: +1 and favorable: 0). The algebraic summation of these scores of each patient was the final score. This score system divided the AML patients into five groups with different clinical outcomes (P<0.001 for both OS and DFS, Figure 3).
Figure 3.
Kaplan–Meier survival curves for OS (a) and DFS (b) in AML patients based on scoring system (P<0.001 for both OS and DFS). AML patients were grouped according to scoring system based on TP53 mutation and 9 other prognostic markers (CEBPAdouble-mutation, NPM1/FLT3-ITD, IDH2, TP53, WT1, RUNX1 and DNMT3A mutations, age and WBC counts at diagnosis). A score of −1 was assigned for each parameter associated with a favorable outcome (CEBPAdouble mutation, IDH2 mutation and NPM1+/FLT3-ITD-), whereas a score of +1 was assigned for each factor associated with an adverse outcome (TP53, WT1, RUNX1 and DNMT3A mutations, older age and higher WBC counts at diagnosis). The karyotypes were stratified into three groups (unfavorable: +2, intermediate: +1 and favorable: 0). The algebraic summation of these scores of each patient was the final score. The 12 patients without chromosome data were not included in the analysis.
Sequential studies of TP53 mutations
TP53 mutations were serially studied in 420 samples from 131 patients, including 5 patients with TP53 mutations and 126 patients without this mutation at diagnosis (Table 5). Among the five patients with TP53 mutations who had ever obtained a CR and had available samples for study, three lost the original mutation at remission status, but two (patients 11 and 32) retained it (Table 5). These two patients relapsed soon and died of uncontrolled disease. In the three patients who had available samples for serial study at relapse, the original mutation could be detected at relapse in two patients (patients 11 and 12), but was lost in one (patient 30). Because direct sequencing might not be sensitive enough to detect low level of TP53 mutation signal, we therefore sequenced TA clones of the PCR product from patient 30 at relapse. Two mutant clones out of 45 were detected. Among the 126 patients who had no TP53 mutation at diagnosis, one (patient 36) acquired TP53 mutation at second relapse. Patient 36 was diagnosed as having acute promyelocytic leukemia with karyotypic change of t(15;17) and trisomy 8 (Table 5). He acquired TP53 mutation at second relapse, 106 months after the initial study at diagnosis and 39.5 months after CR2. At that time, karyotypic evolution with complex chromosomal change but loss of the original t(15;17) and trisomy 8 was found. Because cytogenetic analysis might not be sensitive to detect minor clones, we performed both fluorescence in situ hybridization and real-time quantitative PCR for the PML-RARA fusion transcript. No PML-RARA mutant was detected at second relapse. Intriguingly, we could identify TP53 mutation in 1 of 39 clones by a sensitive cloning technique at first relapse when t(15;17) was still present. However, we did not find TP53 mutant in the BM cells from the patients at diagnosis even after using a sensitive technique.
Table 5. Sequential studies in the AML patients with TP53 mutationsa.
| UPN | Date | Status | Karyotype | TP53 mutation | Other mutations |
|---|---|---|---|---|---|
| 10 | 8/1/2002 | Initial | +der(1)t(1;12)(p34;q21),+2,-5,+del(6)(p21p23),+8,+del(9)(p12),-12,-13,add(17)(p13),-19,+20, +21,+mar1,+mar2 | R280S | ASXL1 |
| 9/23/2002 | CR | ND | — | — | |
| 11 | 2003/5/9 | Initial | 45-46,XY,add(1)(q21),add(6)(q27),-14,-15,add(16)(p12),der(19)add(19)(p13)add(19)(q13),add(22)(q12)[cp8] | L194R | — |
| 2003/7/10 | CR1 | NK | L194R | — | |
| 2003/12/18 | Relapse 1 | del(4)(q2?1),+der(4)t(1;4)(p13;q23),del(6)(q23q27),-10,-14,der(19)add(19)(p13)add(19)(q13),+mar1 | L194R | — | |
| 12 | 2003/4/15 | Initial | +X,add(1)(p11),-2,dup(3)(p12p13),-5,del(5)(p13p15),der(7)(7pter->7qter::?::12q13->12qter),+8,+10,der(11)dup(11)(q13q25)hsr(11)(q25),-12,+13,-15,-17,+der(?)t(1;?)(p22;?)x2,+der(?)t(?;15)(?;q13),+mar | R248G | — |
| 2003/7/8 | CR1 | NK | — | — | |
| 2005/3/1 | Relapse 1 | ND | R248G | — | |
| 2005/3/25 | CR2 | NK | — | — | |
| 30 | 2005/1/13 | Initial | del(5)(q13q33),-7,+r(16)(p13q24),-17,-17,-18,+der(?)t(?;17)(?;q11),+mar1,+mar2 | Y126C | — |
| 2005/2/14 | CR1 | NK | — | — | |
| 2005/8/18 | Relapse 1 | NK | Y126Cb | — | |
| 32 | 2006/1/4 | Initial | NK | V31I | CEBPA, IDH2 |
| 2006/5/15 | CR | NK | V31I | — | |
| 36 | 1995/5/27 | New | del(16)(?q21), +8,t(15;17)(q22;q21) | — | — |
| 1995/8/10 | CR1 | NK | ND | — | |
| 2000/10/16 | Relapse 1 | +8,t(15;17)(q22;q21) | E286Kb | — | |
| 2000/11/24 | CR2 | NK | — | — | |
| 2004/3/9 | Relapse 2c | add(X)(p22),del(1)(q21q44),add(2)(p24),del(3)(p12),-5,add(10)(p13),-16,add(16)(p11),-18,add(18)(q23),add(19)(p13),-22,-22,+mar1,+mar2 | E286K | — |
Abbreviations: AML, acute myeloid leukemia; CR, complete remission; NK, normal karyotype; ND, not done; UPN, unique patient number.
The results of serial studies in 125 patients without TP53 mutation at both diagnosis and relapse were not shown in this table.
No mutation was detected by direct sequencing, but by more sensitive TA cloning technique, TP53 mutation could be found in two of the 45 clones in patient 30 and in one of the 40 clones in patient 36.
More accurately, this was therapy-related leukemia rather than relapsed leukemia, as the original t(15;17) and other chromosomal changes were no more detected, but complex cytogenetic abnormalities and TP53 mutation emerged instead.
Discussion
In this study, we found that the TP53 mutation was associated with distinct clinic-biological features and was a poor prognostic factor in AML patients, independent of age, WBC counts, karyotype and other genetic markers.
A total of 36 TP53 mutations, most commonly in the DNA-binding domain, were detected in 7% of patients (Figure 1). The majority were missense mutations that were suggested to abolish the DNA-binding activity and transactivation capacity.3 Overall, three involved exon 4, nine exon 5, six exon 6, nine exon 7, nine exon 8 and none involved exon 9. Most TP53 mutations were found in exons 5–8,30, 31 and few mutations occurred outside exons 5–8.9 We analyzed exons 3–9 in this study to avoid missing some mutations outside exons 5–8. The probability that the TP53 wild-type patients in this study would have mutations outside the area we screened was low, though it could not be totally excluded.
Most studies on TP53 mutations were focused on patients with CK, 17p abnormalities or older population.9, 32, 33 In this study, we analyzed 500 consecutive patients, both cytogenetically normal and abnormal, so the frequency and clinical characteristics of TP53 mutations in unselected de novo AML patients could be known. TP53 mutations were found in 7%, 1%, 1.3% and 46.2%, respectively, in whole cohort and in patients with favorable-, intermediate- and unfavorable-risk cytogenetics. The patients with CK had the highest incidence (58.8%) of TP53 mutations, an incidence similar to two previous reports (53–60%),9, 10 but lower than that of Haferlach et al.8 (69% in 149 patients). The reason of the variability in the incidence of TP53 mutations in different studies is unknown but may be because of the differences in ethnic background, patient populations recruited and methods used. We also found that TP53-mutated patients had a higher degree of karyotypic complexity than TP53 wild-type patients in the subgroup of patients with CK (90% vs 42.9%, P=0.0005).
Although a close association was observed between TP53 mutations and a complex karyotype, little is known about the interaction between TP53 mutations and other molecular genetic alterations in AML patients. In a study of mutational status of TP53, NPM1, MLL/PTD and FLT3 in 235 patients, including 214 with de novo AML, 13 therapy-related AML and 8 AML evolving from myelodysplastic syndrome, 1 of the 33 TP53-mutated patients had concurrent MLL/PTD and another 1 patient had FLT3 length mutation.8 NPM1 mutation was not observed in patients with TP53 mutation. Similarly, only 2 patients had concurrent TP53 and FLT3 or RAS mutations in 140 elderly patients studied.32 In the comprehensive analyses of the 17 gene mutations in 500 patients, we found that 13 (37.1%) of 35 patients with TP53 mutations showed additional molecular abnormalities at diagnosis, including NRAS, PTPN11, CEBPA, RUNX1, ASXL1 and TET2 mutations that occurred in 2 patients each. Patients with TP53 mutations had significantly lower incidences of NPM1 mutation, FLT3/ITD and DNMT3A mutations than those with TP53 wild-type. Interestingly, TP53-mutated patients with CK had lower probability of concurrent other molecular alterations than those without.
To the best of our knowledge, this study recruited the largest number of AML patients for sequential analysis of TP53 mutations during the clinical course. In contrast to the instability of FLT3-ITD during disease evolution,34 we found that the TP53 mutation seemed rather stable, analogous to DNMT3A mutations.12 At relapse, the original TP53 mutations in all three TP53-mutated patients studied were retained, but the mutant level in one of them was much reduced at the time of AML relapse, as it could only be detected by a sensitive cloning technique but not by direct sequencing (patient 30, Table 5). On the other hand, among the 126 patients who had no TP53 mutation at diagnosis, 1 acquired a novel TP53 mutation at second relapse. This patient was diagnosed as having acute promyelocytic leukemia with t(15;17). She acquired TP53 mutation at second relapse, 106 months after the initial study at diagnosis; the leukemic cells showed complex chromosomal changes with loss of the original t(15;17) and trisomy 8 at that time (Table 5). It is most likely that the patient developed therapy-related leukemia. We did not find TP53 mutant in the BM cells from the patients at diagnosis even after using a sensitive cloning technique; intriguingly, we could identify TP53 mutation in 1 of 39 clones at first relapse of the original leukemia 65 months after diagnosis when t(15;17) was still present. In other words, the minor clone with TP53 mutant already emerged 41 months before the development of therapy-related leukemia; the minor clone of cells escaped subsequent treatments, expanded and finally transformed to AML accompanied by complex cytogenetic abnormalities.35 Taken together, TP53 mutations are quite stable during AML progression. The acquisition of novel TP53 mutations in TP53 wild-type patients may be an indicator of the emergence of therapy-related AML and warrants intervention treatment.
TP53 mutations within the DNA-binding domain have been associated with poor treatment response and shorter survival in solid tumors.3 Regarding the prognostic relevance of TP53 mutations in AML-CK, Rucker et al.9 showed that TP53 mutation was the most important prognostic factor, outweighing all other variables, but another study demonstrated that there was no significant difference in CR and OS between TP53-mutant and TP53 wild-type patients in this group.10 In this study, we distinctly identified that patients with TP53 mutations had poor prognosis in both total cohort and AML-CK. To better stratify AML patients into different risk groups, a survival scoring system incorporating TP53 mutation and nine other prognostic factors, including age, WBC counts, cytogenetics, NPM1/FLT3-ITD, CEBPA, IDH2, RUNX1, WT1 and DNMT3A mutations, into survival analysis was formulated. Indeed, this scoring system was more powerful than single marker to separate patients into different prognostic groups. Further studies in independent cohorts are needed to validate the clinical implication of the proposed scoring system.
In summary, this study demonstrated that TP53-mutated patients had specific clinic-biologic features and cytogenetic changes. TP53 mutations were mutually exclusive with NPM1 mutation, FLT3/ITD and DNMT3A mutations. Furthermore, the TP53 mutation was an independent poor-risk factor for OS and DFS among total cohort and AML-CK patients. Incorporation of TP53 mutation with nine other prognostic factors into survival analyses can better stratify AML patients into different risk groups. Sequential study during the clinical course showed that TP53 mutation was quite stable during AML evolution. The acquisition of TP53 mutation in TP53 wild-type patients during clinical follow-ups may be an indicator of the emergence of therapy-related leukemia.
Acknowledgments
This work was partially sponsored by Grants MOST 100-2628-B-002-003-MY3,103-2628-B-002-008-MY3 and 103-2923-B-002-001 from the Ministry of Science and Technology (Taiwan), MOHW103-TD-B-111-04 from the Ministry of Health and Welfare (Taiwan) and NTUH 102P06 and UN 102-015 from the Department of Medical Research, National Taiwan University Hospital.
Author Contributions
H-AH was responsible for study design and plan, literature collection, data management and interpretation, statistical analysis and manuscript writing; C-YL was responsible for statistical analysis and interpretation of the statistical findings; Y-YK and L-IL were responsible for mutation analysis and interpretation; C-YC, W-CC, MY, S-YH, J-LT, C-CL, B-SK, S-CH, C-TL, S-JW, WT and Y-CC contributed patient samples and clinical data; M-CL, M-HT, C-FH, Y-CC and C-WL performed gene mutation and chromosomal studies; H-FT planned, designed, wrote manuscript and coordinated the study over the entire period.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Blood Cancer Journal website (http://www.nature.com/bcj)
Supplementary Material
References
- Isobe M, Emanuel BS, Givol D, Oren M, Croce CM. Localization of gene for human p53 tumour antigen to band 17p13. Nature. 1986;320:84–85. doi: 10.1038/320084a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991;253:49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–310. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- Olivier M, Petitjean A, Marcel V, Petre A, Mounawar M, Plymoth A, et al. Recent advances in p53 research: an interdisciplinary perspective. Cancer Gene Ther. 2009;16:1–12. doi: 10.1038/cgt.2008.69. [DOI] [PubMed] [Google Scholar]
- Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- Haferlach C, Dicker F, Herholz H, Schnittger S, Kern W, Haferlach T. Mutations of the TP53 gene in acute myeloid leukemia are strongly associated with a complex aberrant karyotype. Leukemia. 2008;22:1539–1541. doi: 10.1038/leu.2008.143. [DOI] [PubMed] [Google Scholar]
- Rucker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- Bowen D, Groves MJ, Burnett AK, Patel Y, Allen C, Green C, et al. TP53 gene mutation is frequent in patients with acute myeloid leukemia and complex karyotype, and is associated with very poor prognosis. Leukemia. 2009;23:203–206. doi: 10.1038/leu.2008.173. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Preudhomme C, Quiquandon I, Jonveaux P, Lai JL, Vanrumbeke M, et al. Mutations of the P53 gene in acute myeloid leukaemia. Br J Haematol. 1992;80:178–183. doi: 10.1111/j.1365-2141.1992.tb08897.x. [DOI] [PubMed] [Google Scholar]
- Hou HA, Kuo YY, Liu CY, Chou WC, Lee MC, Chen CY, et al. DNMT3A mutations in acute myeloid leukemia: stability during disease evolution and clinical implications. Blood. 2012;119:559–568. doi: 10.1182/blood-2011-07-369934. [DOI] [PubMed] [Google Scholar]
- Hou HA, Lin CC, Chou WC, Liu CY, Chen CY, Tang JL, et al. Integration of cytogenetic and molecular alterations in risk stratification of 318 patients with de novo non-M3 acute myeloid leukemia. Leukemia. 2014;28:50–58. doi: 10.1038/leu.2013.236. [DOI] [PubMed] [Google Scholar]
- Tien HF, Wang CH, Lin MT, Lee FY, Liu MC, Chuang SM, et al. Correlation of cytogenetic results with immunophenotype, genotype, clinical features, and ras mutation in acute myeloid leukemia. A study of 235 Chinese patients in Taiwan. Cancer Genet Cytogenet. 1995;84:60–68. doi: 10.1016/0165-4608(95)00084-4. [DOI] [PubMed] [Google Scholar]
- Chou WC, Tang JL, Lin LI, Yao M, Tsay W, Chen CY, et al. Nucleophosmin mutations in de novo acute myeloid leukemia: the age-dependent incidences and the stability during disease evolution. Cancer Res. 2006;66:3310–3316. doi: 10.1158/0008-5472.CAN-05-4316. [DOI] [PubMed] [Google Scholar]
- Chen CY, Lin LI, Tang JL, Tsay W, Chang HH, Yeh YC, et al. Acquisition of JAK2, PTPN11, and RAS mutations during disease progression in primary myelodysplastic syndrome. Leukemia. 2006;20:1155–1158. doi: 10.1038/sj.leu.2404190. [DOI] [PubMed] [Google Scholar]
- Chen CY, Lin LI, Tang JL, Ko BS, Tsay W, Chou WC, et al. RUNX1 gene mutation in primary myelodysplastic syndrome—the mutation can be detected early at diagnosis or acquired during disease progression and is associated with poor outcome. Br J Haematol. 2007;139:405–414. doi: 10.1111/j.1365-2141.2007.06811.x. [DOI] [PubMed] [Google Scholar]
- Hou HA, Chou WC, Lin LI, Chen CY, Tang JL, Tseng MH, et al. Characterization of acute myeloid leukemia with PTPN11 mutation: the mutation is closely associated with NPM1 mutation but inversely related to FLT3/ITD. Leukemia. 2008;22:1075–1078. doi: 10.1038/sj.leu.2405005. [DOI] [PubMed] [Google Scholar]
- Lin LI, Chen CY, Lin DT, Tsay W, Tang JL, Yeh YC, et al. Characterization of CEBPA mutations in acute myeloid leukemia: most patients with CEBPA mutations have biallelic mutations and show a distinct immunophenotype of the leukemic cells. Clin Cancer Res. 2005;11:1372–1379. doi: 10.1158/1078-0432.CCR-04-1816. [DOI] [PubMed] [Google Scholar]
- Tang JL, Hou HA, Chen CY, Liu CY, Chou WC, Tseng MH, et al. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114:5352–5361. doi: 10.1182/blood-2009-05-223784. [DOI] [PubMed] [Google Scholar]
- Falini B, Mecucci C, Tiacci E, Alcalay M, Rosati R, Pasqualucci L, et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N Engl J Med. 2005;352:254–266. doi: 10.1056/NEJMoa041974. [DOI] [PubMed] [Google Scholar]
- Hou HA, Huang TC, Lin LI, Liu CY, Chen CY, Chou WC, et al. WT1 mutation in 470 adult patients with acute myeloid leukemia: stability during disease evolution and implication of its incorporation into a survival scoring system. Blood. 2010;115:5222–5231. doi: 10.1182/blood-2009-12-259390. [DOI] [PubMed] [Google Scholar]
- Shiah HS, Kuo YY, Tang JL, Huang SY, Yao M, Tsay W, et al. Clinical and biological implications of partial tandem duplication of the MLL gene in acute myeloid leukemia without chromosomal abnormalities at 11q23. Leukemia. 2002;16:196–202. doi: 10.1038/sj.leu.2402352. [DOI] [PubMed] [Google Scholar]
- Chen TC, Hou HA, Chou WC, Tang JL, Kuo YY, Chen CY, et al. Dynamics of ASXL1 mutation and other associated genetic alterations during disease progression in patients with primary myelodysplastic syndrome. Blood Cancer J. 2014;4:e177. doi: 10.1038/bcj.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou WC, Hou HA, Chen CY, Tang JL, Yao M, Tsay W, et al. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood. 2010;115:2749–2754. doi: 10.1182/blood-2009-11-253070. [DOI] [PubMed] [Google Scholar]
- Lin CC, Hou HA, Chou WC, Kuo YY, Liu CY, Chen CY, et al. IDH mutations are closely associated with mutations of DNMT3A, ASXL1 and SRSF2 in patients with myelodysplastic syndromes and are stable during disease evolution. Am J Hematol. 2014;89:137–144. doi: 10.1002/ajh.23596. [DOI] [PubMed] [Google Scholar]
- Chou WC, Chou SC, Liu CY, Chen CY, Hou HA, Kuo YY, et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood. 2011;118:3803–3810. doi: 10.1182/blood-2011-02-339747. [DOI] [PubMed] [Google Scholar]
- Hou HA, Kuo YY, Tang JL, Chou WC, Yao M, Lai YJ, et al. Clinical implications of the SETBP1 mutation in patients with primary myelodysplastic syndrome and its stability during disease progression. Am J Hematol. 2013;89:181–186. doi: 10.1002/ajh.23611. [DOI] [PubMed] [Google Scholar]
- Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- Nigro JM, Baker SJ, Preisinger AC, Jessup JM, Hostetter R, Cleary K, et al. Mutations in the p53 gene occur in diverse human tumour types. Nature. 1989;342:705–708. doi: 10.1038/342705a0. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nau MM, Chiba I, Birrer MJ, Rosenberg RK, Vinocour M, et al. p53: a frequent target for genetic abnormalities in lung cancer. Science. 1989;246:491–494. doi: 10.1126/science.2554494. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- Fenaux P, Jonveaux P, Quiquandon I, Lai JL, Pignon JM, Loucheux-Lefebvre MH, et al. P53 gene mutations in acute myeloid leukemia with 17p monosomy. Blood. 1991;78:1652–1657. [PubMed] [Google Scholar]
- Shih LY, Huang CF, Wu JH, Lin TL, Dunn P, Wang PN, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: a comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2002;100:2387–2392. doi: 10.1182/blood-2002-01-0195. [DOI] [PubMed] [Google Scholar]
- Wong TN, Ramsingh G, Young AL, Miller CA, Touma W, Welch JS, et al. The role of early TP53 mutations on the evolution of therapy-related AML. Nature. 2015;518:552–555. doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.