Abstract
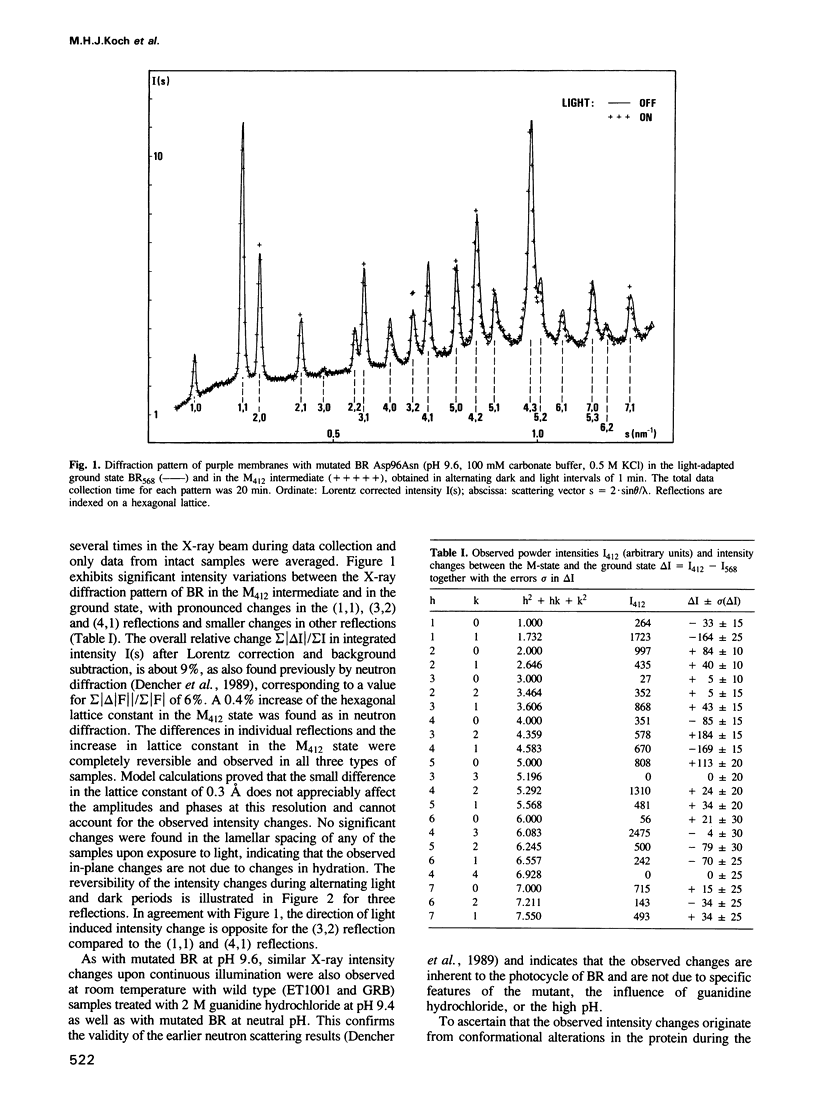
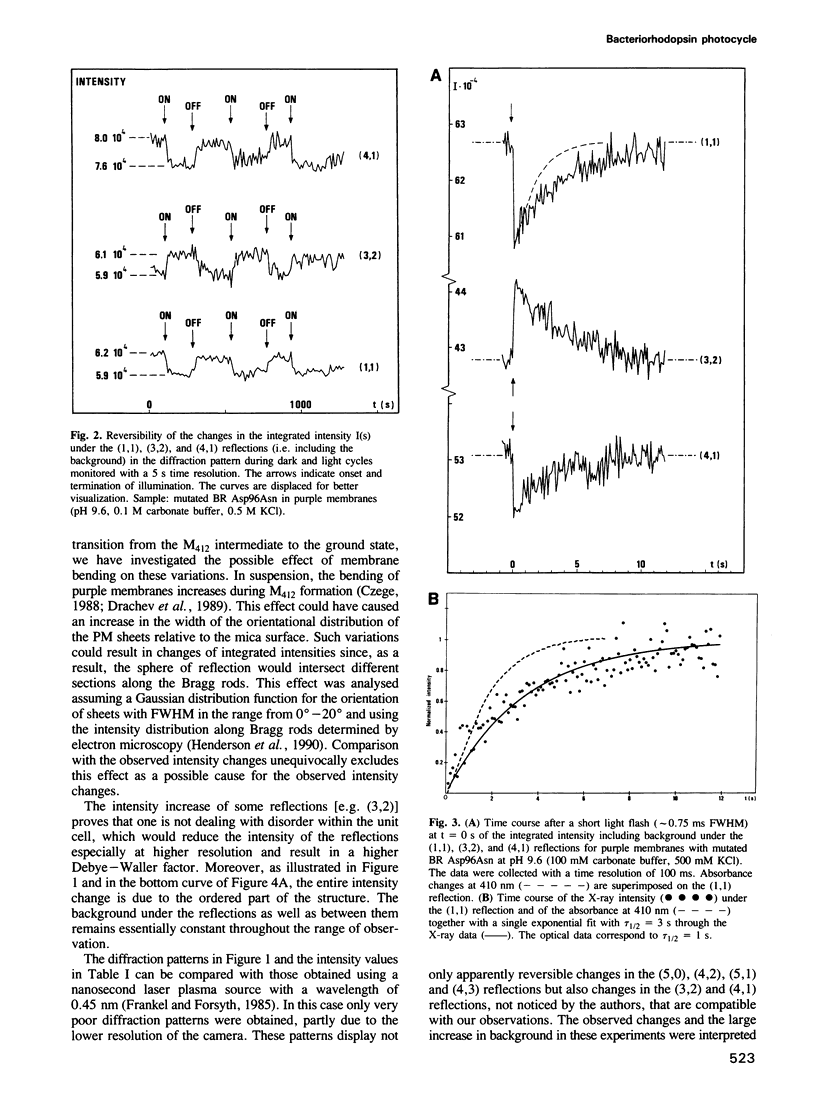
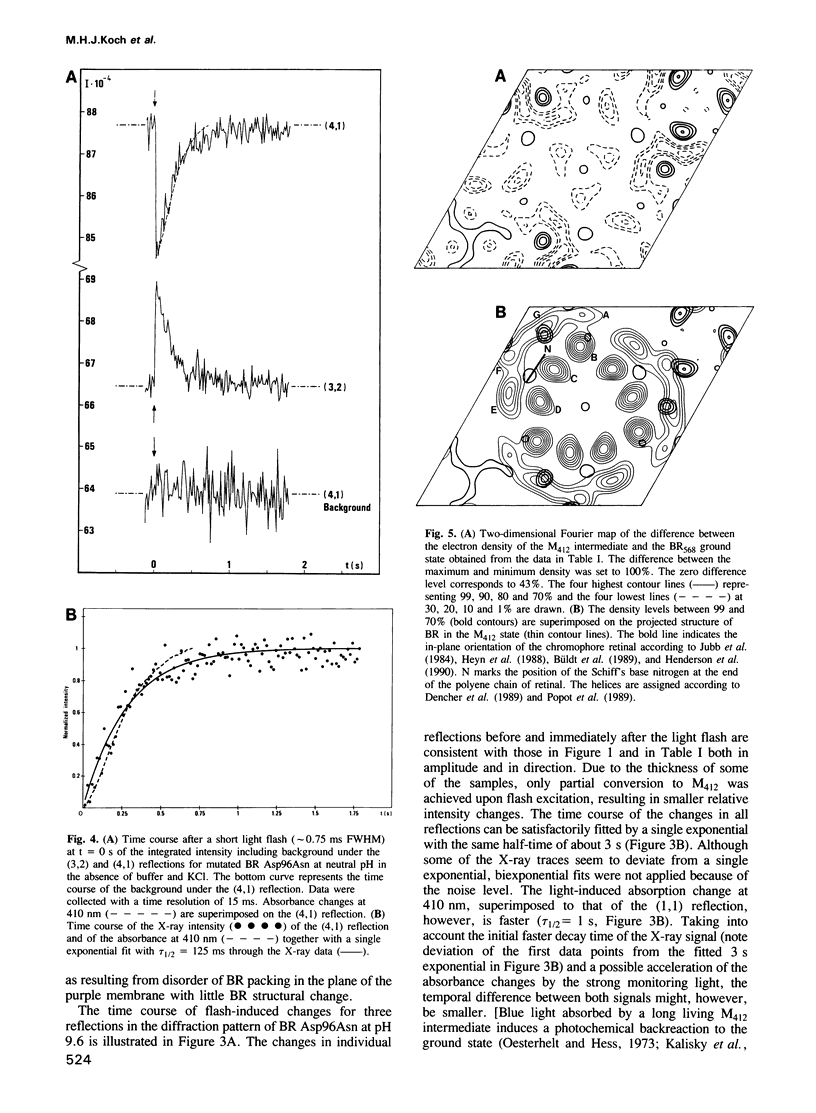
The time course of structural changes accompanying the transition from the M412 intermediate to the BR568 ground state in the photocycle of bacteriorhodopsin (BR) from Halobacterium halobium was studied at room temperature with a time resolution of 15 ms using synchrotron radiation X-ray diffraction. The M412 decay rate was slowed down by employing mutated BR Asp96Asn in purple membranes at two different pH-values. The observed light-induced intensity changes of in-plane X-ray reflections were fully reversible. For the mutated BR at neutral pH the kinetics of the structural alterations (tau 1/2 = 125 ms) were very similar to those of the optical changes characterizing the M412 decay, whereas at pH 9.6 the structural relaxation (tau 1/2 = 3 s) slightly lagged behind the absorbance changes at 410 nm. The overall X-ray intensity change between the M412 intermediate and the ground state was about 9% for the different samples investigated and is associated with electron density changes close to helix G, B and E. Similar changes (tau 1/2 = 1.3-3.6 s), which also confirm earlier neutron scattering results on the BR568 and M412 intermediates trapped at -180 degrees C, were observed with wild type BR retarded by 2 M guanidine hydrochloride (pH 9.4). The results unequivocally prove that the tertiary structure of BR changes during the photocycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahl P. L., Cone R. A. Light activates rotations of bacteriorhodopsin in the purple membrane. Biophys J. 1984 Jun;45(6):1039–1049. doi: 10.1016/S0006-3495(84)84251-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Butt H. J., Fendler K., Bamberg E., Tittor J., Oesterhelt D. Aspartic acids 96 and 85 play a central role in the function of bacteriorhodopsin as a proton pump. EMBO J. 1989 Jun;8(6):1657–1663. doi: 10.1002/j.1460-2075.1989.tb03556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dencher N. A., Dresselhaus D., Zaccai G., Büldt G. Structural changes in bacteriorhodopsin during proton translocation revealed by neutron diffraction. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7876–7879. doi: 10.1073/pnas.86.20.7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draheim J. E., Cassim J. Y. Large Scale Global Structural Changes of the Purple Membrane during the Photocycle. Biophys J. 1985 Apr;47(4):497–507. doi: 10.1016/S0006-3495(85)83943-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor S. P., Ames J. B., Gebhard R., van den Berg E. M., Stoeckenius W., Lugtenburg J., Mathies R. A. Chromophore structure in bacteriorhodopsin's N intermediate: implications for the proton-pumping mechanism. Biochemistry. 1988 Sep 6;27(18):7097–7101. doi: 10.1021/bi00418a064. [DOI] [PubMed] [Google Scholar]
- Frankel R. D., Forsyth J. M. Time-resolved x-ray diffraction study of photostimulated purple membrane. Biophys J. 1985 Mar;47(3):387–393. doi: 10.1016/S0006-3495(85)83930-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaeser R. M., Baldwin J., Ceska T. A., Henderson R. Electron diffraction analysis of the M412 intermediate of bacteriorhodopsin. Biophys J. 1986 Nov;50(5):913–920. doi: 10.1016/S0006-3495(86)83532-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Heyn M. P., Westerhausen J., Wallat I., Seiff F. High-sensitivity neutron diffraction of membranes: Location of the Schiff base end of the chromophore of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2146–2150. doi: 10.1073/pnas.85.7.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jubb J. S., Worcester D. L., Crespi H. L., Zaccaï G. Retinal location in purple membrane of Halobacterium halobium: a neutron diffraction study of membranes labelled in vivo with deuterated retinal. EMBO J. 1984 Jul;3(7):1455–1461. doi: 10.1002/j.1460-2075.1984.tb01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Hess B. Reversible photolysis of the purple complex in the purple membrane of Halobacterium halobium. Eur J Biochem. 1973 Aug 17;37(2):316–326. doi: 10.1111/j.1432-1033.1973.tb02990.x. [DOI] [PubMed] [Google Scholar]
- Popot J. L., Engelman D. M., Gurel O., Zaccaï G. Tertiary structure of bacteriorhodopsin. Positions and orientations of helices A and B in the structural map determined by neutron diffraction. J Mol Biol. 1989 Dec 20;210(4):829–847. doi: 10.1016/0022-2836(89)90111-3. [DOI] [PubMed] [Google Scholar]
- Rapp G., Güth K. A low cost high intensity flash device for photolysis experiments. Pflugers Arch. 1988 Feb;411(2):200–203. doi: 10.1007/BF00582315. [DOI] [PubMed] [Google Scholar]
- Soppa J., Oesterhelt D. Bacteriorhodopsin mutants of Halobacterium sp. GRB. I. The 5-bromo-2'-deoxyuridine selection as a method to isolate point mutants in halobacteria. J Biol Chem. 1989 Aug 5;264(22):13043–13048. [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


