Abstract
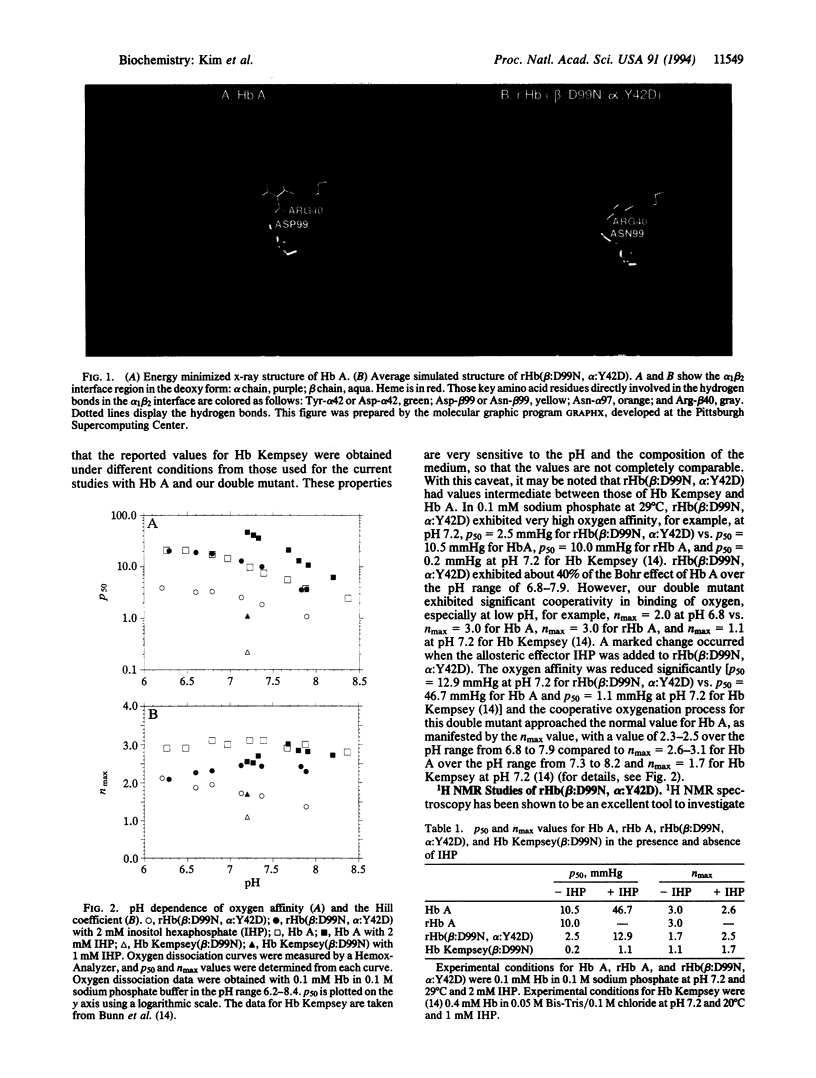
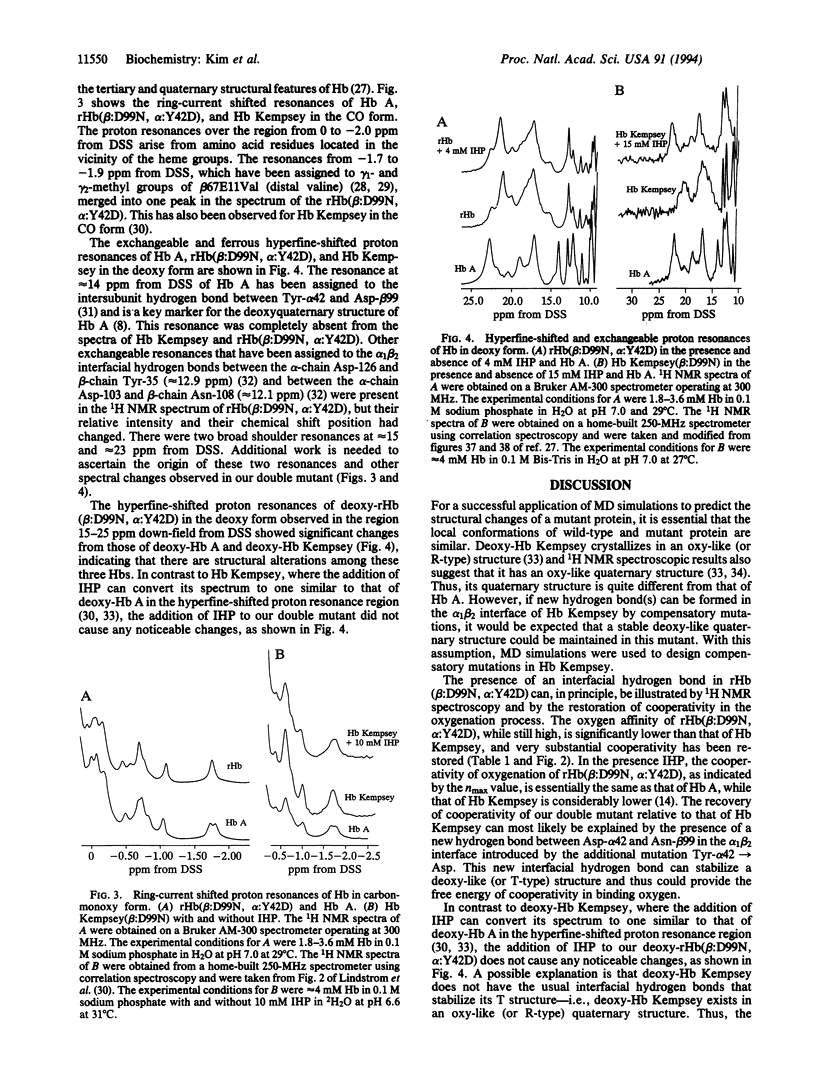
Abnormal human hemoglobins (HBs) with amino acid substitutions in the alpha 1 beta 2 interface have very high oxygen affinity and greatly reduced cooperativity in O2 binding compared to normal human Hb. In such abnormal Hbs with mutations at position beta 99, the intersubunit hydrogen bonds between Asp-beta 99 and Tyr-alpha 42 and between Asp-beta 99 and Asn-alpha 97 are broken, thus destabilizing the deoxyquaternary structure of these Hbs. A molecular dynamics method has been used to design compensatory amino acid substitutions in these Hbs that can restore their allosteric properties. We have designed a compensatory mutation in a naturally occurring mutant Hb, Hb Kempsey (Asp-beta 99-->Asn), and have produced it using our Escherichia coli expression plasmid pHE2. We have determined the O2 binding properties of this recombinant double mutant Hb, Hb(Asp-beta 99-->Asn and Tyr-alpha 42-->Asp) and have used 1H NMR spectroscopy to investigate the tertiary structures around the heme groups and the quaternary structure in the alpha 1 beta 2 subunit interface. Our results clearly show that the Tyr-alpha 42-->Asp replacement can substantially compensate for the functional defect of Hb Kempsey caused by the Asp-beta 99-->Asn substitution. The structural and functional information derived from this recombinant Hb provides insights into the structural basis of allosterism and the design of compensatory amino acid substitutions to restore the functional properties of other abnormal HBs associated with hemoglobinopathies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Bassat A., Bauer K., Chang S. Y., Myambo K., Boosman A., Chang S. Processing of the initiation methionine from proteins: properties of the Escherichia coli methionine aminopeptidase and its gene structure. J Bacteriol. 1987 Feb;169(2):751–757. doi: 10.1128/jb.169.2.751-757.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge D. L., DiCapua F. M. Free energy via molecular simulation: applications to chemical and biomolecular systems. Annu Rev Biophys Biophys Chem. 1989;18:431–492. doi: 10.1146/annurev.bb.18.060189.002243. [DOI] [PubMed] [Google Scholar]
- Brooks C. L., 3rd, Karplus M. Solvent effects on protein motion and protein effects on solvent motion. Dynamics of the active site region of lysozyme. J Mol Biol. 1989 Jul 5;208(1):159–181. doi: 10.1016/0022-2836(89)90093-4. [DOI] [PubMed] [Google Scholar]
- Bunn H. F., Wohl R. C., Bradley T. B., Cooley M., Gibson Q. H. Functional properties of hemoglobin Kempsey. J Biol Chem. 1974 Dec 10;249(23):7402–7409. [PubMed] [Google Scholar]
- Dalvit C., Ho C. Proton nuclear Overhauser effect investigation of the heme pockets in ligated hemoglobin: conformational differences between oxy and carbonmonoxy forms. Biochemistry. 1985 Jul 2;24(14):3398–3407. doi: 10.1021/bi00335a003. [DOI] [PubMed] [Google Scholar]
- Fermi G., Perutz M. F., Shaanan B., Fourme R. The crystal structure of human deoxyhaemoglobin at 1.74 A resolution. J Mol Biol. 1984 May 15;175(2):159–174. doi: 10.1016/0022-2836(84)90472-8. [DOI] [PubMed] [Google Scholar]
- Fung L. W., Ho C. A proton nuclear magnetic resonance study of the quaternary structure of human homoglobins in water. Biochemistry. 1975 Jun 3;14(11):2526–2535. doi: 10.1021/bi00682a036. [DOI] [PubMed] [Google Scholar]
- Gao J., Kuczera K., Tidor B., Karplus M. Hidden thermodynamics of mutant proteins: a molecular dynamics analysis. Science. 1989 Jun 2;244(4908):1069–1072. doi: 10.1126/science.2727695. [DOI] [PubMed] [Google Scholar]
- Glynn K. P., Penner J. A., Smith J. R., Rucknagel D. L. Familial erythrocytosis. A description of three families, one with hemoglobin Ypsilanti. Ann Intern Med. 1968 Oct;69(4):769–776. doi: 10.7326/0003-4819-69-4-769. [DOI] [PubMed] [Google Scholar]
- Hayashi A., Suzuki T., Shin M. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim Biophys Acta. 1973 Jun 15;310(2):309–316. doi: 10.1016/0005-2795(73)90110-4. [DOI] [PubMed] [Google Scholar]
- Ho C. Proton nuclear magnetic resonance studies on hemoglobin: cooperative interactions and partially ligated intermediates. Adv Protein Chem. 1992;43:153–312. doi: 10.1016/s0065-3233(08)60555-0. [DOI] [PubMed] [Google Scholar]
- Hoffman S. J., Looker D. L., Roehrich J. M., Cozart P. E., Durfee S. L., Tedesco J. L., Stetler G. L. Expression of fully functional tetrameric human hemoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8521–8525. doi: 10.1073/pnas.87.21.8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T., Osgood E. E., Brimhall B., Koler R. D. Hemoglobin Yakina. I. Clinical and biochemical studies. J Clin Invest. 1967 Nov;46(11):1840–1847. doi: 10.1172/JCI105674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom T. R., Baldassare J. J., Bunn H. F., Ho C. Nuclear magnetic resonance and spin-label studies of hemoglobin Kempsey. Biochemistry. 1973 Oct 9;12(21):4212–4217. doi: 10.1021/bi00745a027. [DOI] [PubMed] [Google Scholar]
- Lindstrom T. R., Norén I. B., Charache S., Lehmann H., Ho C. Nuclear magnetic resonance studies of hemoglobins. VII. Tertiary structure around ligand binding site in carbonmonoxyhemoglobin. Biochemistry. 1972 Apr 25;11(9):1677–1681. doi: 10.1021/bi00759a023. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Ponder J. W., Richards F. M. Tertiary templates for proteins. Use of packing criteria in the enumeration of allowed sequences for different structural classes. J Mol Biol. 1987 Feb 20;193(4):775–791. doi: 10.1016/0022-2836(87)90358-5. [DOI] [PubMed] [Google Scholar]
- Prevost M., Wodak S. J., Tidor B., Karplus M. Contribution of the hydrophobic effect to protein stability: analysis based on simulations of the Ile-96----Ala mutation in barnase. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10880–10884. doi: 10.1073/pnas.88.23.10880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed C. S., Hampson R., Gordon S., Jones R. T., Novy M. J., Brimhall B., Edwards M. J., Koler R. D. Erythrocytosis secondary to increased oxygen affinity of a mutant hemoglobin, hemoglobin Kempsey. Blood. 1968 May;31(5):623–632. [PubMed] [Google Scholar]
- Russu I. M., Lin A. K., Ferro-Dosch S., Ho C. A proton nuclear magnetic resonance investigation of human hemoglobin A2. Implications on the intermolecular contacts in sickle hemoglobin fibers and on the Bohr effect of human normal adult hemoglobin. Biochim Biophys Acta. 1984 Mar 29;785(3):123–131. doi: 10.1016/0167-4838(84)90136-5. [DOI] [PubMed] [Google Scholar]
- Shaanan B. Structure of human oxyhaemoglobin at 2.1 A resolution. J Mol Biol. 1983 Nov 25;171(1):31–59. doi: 10.1016/s0022-2836(83)80313-1. [DOI] [PubMed] [Google Scholar]
- Shen T. J., Ho N. T., Simplaceanu V., Zou M., Green B. N., Tam M. F., Ho C. Production of unmodified human adult hemoglobin in Escherichia coli. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):8108–8112. doi: 10.1073/pnas.90.17.8108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T. J., Zhu L. Q., Sun X. A marker-coupled method for site-directed mutagenesis. Gene. 1991 Jul 15;103(1):73–77. doi: 10.1016/0378-1119(91)90393-p. [DOI] [PubMed] [Google Scholar]
- Takahashi S., Lin A. K., Ho C. A proton nuclear magnetic resonance investigation of proximal histidyl residues in human normal and abnormal hemoglobins. A probe for the heme pocket. Biophys J. 1982 Jul;39(1):33–40. doi: 10.1016/S0006-3495(82)84487-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thillet J., Arous N., Rosa J. Functional studies of two new abnormal hemoglobins with their mutation located at intersubunit contacts: Hb Hotel Dieu beta99 (G1) Asp replaced by Gly and Hb Pitie Salpetriere beta34 (B16) Val replaced by Phe. Biochim Biophys Acta. 1981 Sep 29;670(2):260–264. doi: 10.1016/0005-2795(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Tidor B., Karplus M. Simulation analysis of the stability mutant R96H of T4 lysozyme. Biochemistry. 1991 Apr 2;30(13):3217–3228. doi: 10.1021/bi00227a009. [DOI] [PubMed] [Google Scholar]
- Weatherall D. J., Clegg J. B., Callender S. T., Wells R. M., Gale R. E., Huehns E. R., Perutz M. F., Viggiano G., Ho C. Haemoglobin Radcliffe (alpha2beta299(Gi)Ala): a high oxygen-affinity variant causing familial polycythaemia. Br J Haematol. 1977 Feb;35(2):177–191. doi: 10.1111/j.1365-2141.1977.tb00575.x. [DOI] [PubMed] [Google Scholar]




