Abstract
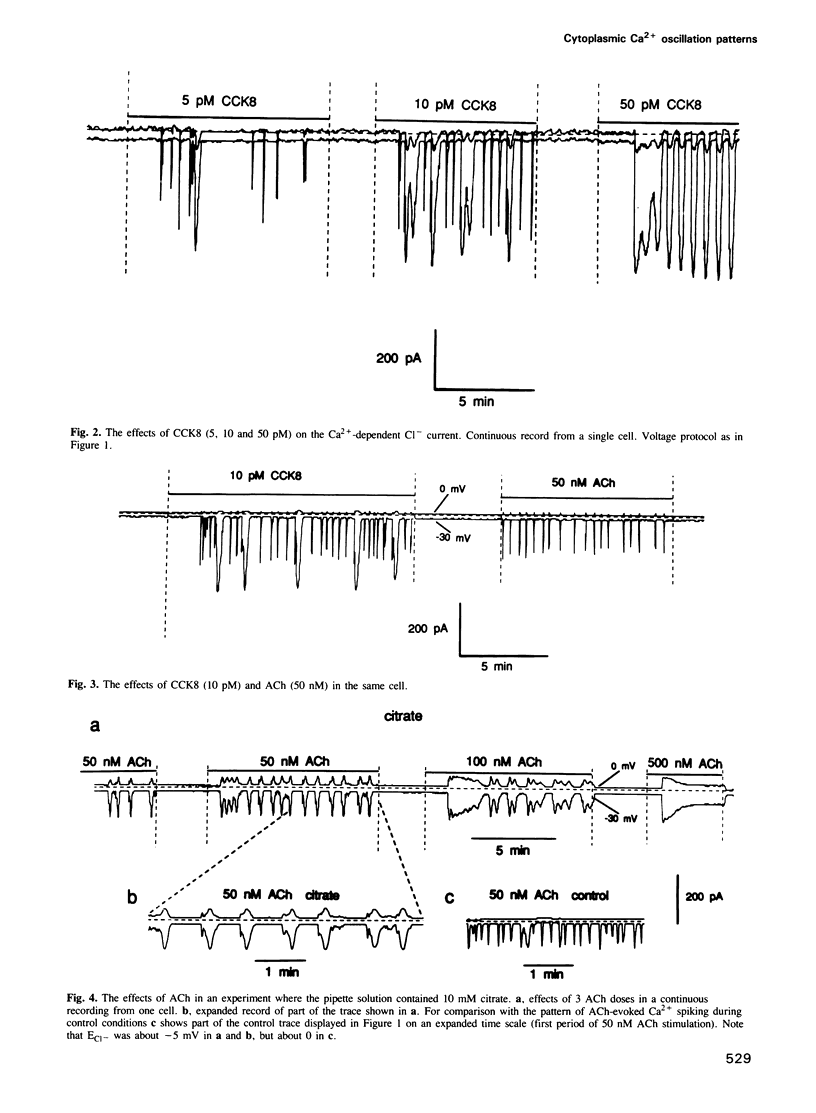
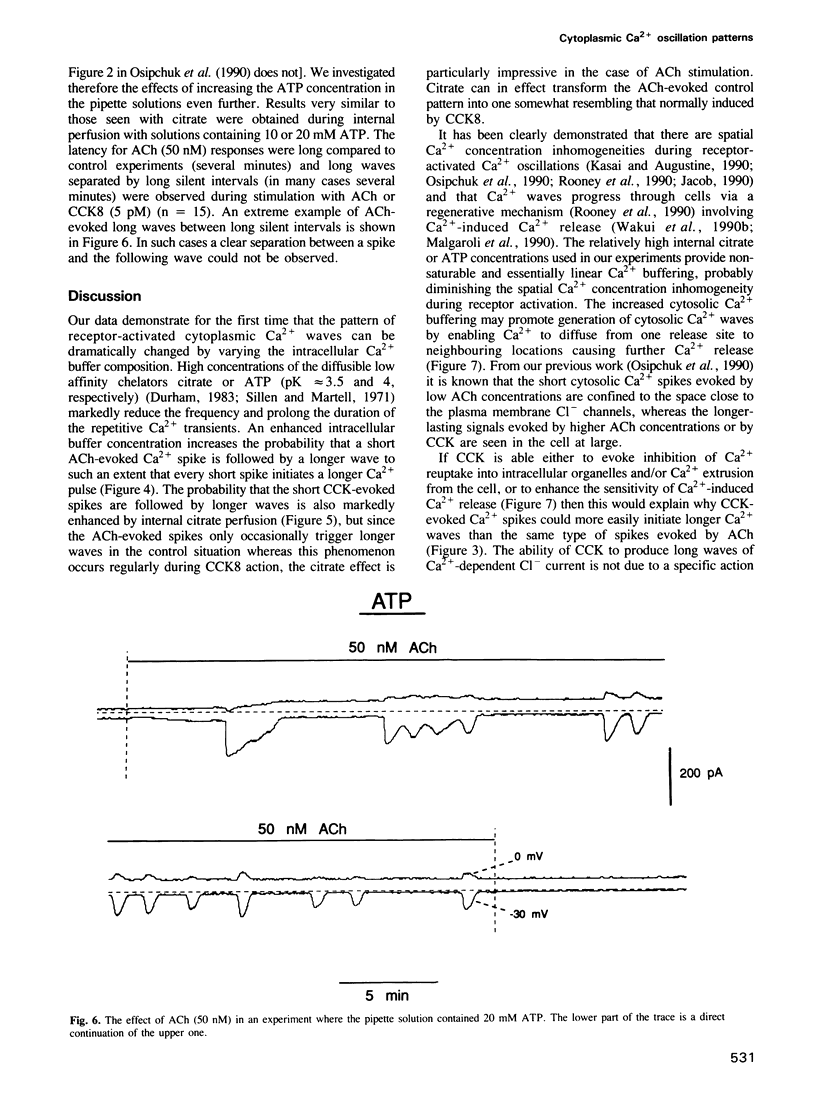
Agonist-specific cytosolic Ca2+ oscillation patterns can be observed in individual cells and these have been explained by the co-existence of separate oscillatory mechanisms. In pancreatic acinar cells activation of muscarinic receptors typically evokes sinusoidal oscillations whereas stimulation of cholecystokinin (CCK) receptors evokes transient oscillations consisting of Ca2+ waves with long intervals between them. We have monitored changes in the cytosolic Ca2+ concentration ([Ca2+]i) by measuring Ca2(+)-activated Cl- currents in single internally perfused mouse pancreatic acinar cells. With minimal intracellular Ca2+ buffering we found that low concentrations of both ACh (50 nM) and CCK (10 pM) evoked repetitive short-lasting Ca2+ spikes of the same duration and frequency, but the probability of a spike being followed by a longer and larger Ca2+ wave was low for ACh and high for CCK. The probability that the receptor-evoked shortlasting Ca2+ spikes would initiate more substantial Ca2+ waves was dramatically increased by intracellular perfusion with solutions containing high concentrations of the mobile low affinity Ca2+ buffers citrate (10-40 mM) or ATP (10-20 mM). The different Ca2+ oscillation patterns normally induced by ACh and CCK would therefore appear not to be caused by separate mechanisms. We propose that specific receptor-controlled modulation of Ca2+ signal spreading, either by regulation of Ca2+ uptake into organelles and/or cellular Ca2+ extrusion, or by changing the sensitivity of the Ca2(+)-induced Ca2+ release mechanism, can be mimicked experimentally by different degrees of cytosolic Ca2+ buffering and can account for the various cytosolic Ca2+ spike patterns.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Calcium oscillations. J Biol Chem. 1990 Jun 15;265(17):9583–9586. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durham A. C. A survey of readily available chelators for buffering calcium ion concentrations in physiological solutions. Cell Calcium. 1983 Feb;4(1):33–46. doi: 10.1016/0143-4160(83)90047-7. [DOI] [PubMed] [Google Scholar]
- Goldbeter A., Dupont G., Berridge M. J. Minimal model for signal-induced Ca2+ oscillations and for their frequency encoding through protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1461–1465. doi: 10.1073/pnas.87.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hosey M. M., Lazdunski M. Calcium channels: molecular pharmacology, structure and regulation. J Membr Biol. 1988 Sep;104(2):81–105. doi: 10.1007/BF01870922. [DOI] [PubMed] [Google Scholar]
- Jacob R. Imaging cytoplasmic free calcium in histamine stimulated endothelial cells and in fMet-Leu-Phe stimulated neutrophils. Cell Calcium. 1990 Feb-Mar;11(2-3):241–249. doi: 10.1016/0143-4160(90)90075-6. [DOI] [PubMed] [Google Scholar]
- Jauch P., Petersen O. H., Läuger P. Electrogenic properties of the sodium-alanine cotransporter in pancreatic acinar cells: I. Tight-seal whole-cell recordings. J Membr Biol. 1986;94(2):99–115. doi: 10.1007/BF01871191. [DOI] [PubMed] [Google Scholar]
- Kasai H., Augustine G. J. Cytosolic Ca2+ gradients triggering unidirectional fluid secretion from exocrine pancreas. Nature. 1990 Dec 20;348(6303):735–738. doi: 10.1038/348735a0. [DOI] [PubMed] [Google Scholar]
- Llano I., Marty A. Protein kinase C activators inhibit the inositol trisphosphate-mediated muscarinic current responses in rat lacrimal cells. J Physiol. 1987 Dec;394:239–248. doi: 10.1113/jphysiol.1987.sp016868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malgaroli A., Fesce R., Meldolesi J. Spontaneous [Ca2+]i fluctuations in rat chromaffin cells do not require inositol 1,4,5-trisphosphate elevations but are generated by a caffeine- and ryanodine-sensitive intracellular Ca2+ store. J Biol Chem. 1990 Feb 25;265(6):3005–3008. [PubMed] [Google Scholar]
- Maruyama Y. Activation and desensitization mechanisms of muscarinic current response in single pancreatic acinar cells of rats. J Physiol. 1989 Oct;417:343–359. doi: 10.1113/jphysiol.1989.sp017805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipchuk Y. V., Wakui M., Yule D. I., Gallacher D. V., Petersen O. H. Cytoplasmic Ca2+ oscillations evoked by receptor stimulation, G-protein activation, internal application of inositol trisphosphate or Ca2+: simultaneous microfluorimetry and Ca2+ dependent Cl- current recording in single pancreatic acinar cells. EMBO J. 1990 Mar;9(3):697–704. doi: 10.1002/j.1460-2075.1990.tb08162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen O. H., Wakui M. Oscillating intracellular Ca2+ signals evoked by activation of receptors linked to inositol lipid hydrolysis: mechanism of generation. J Membr Biol. 1990 Nov;118(2):93–105. doi: 10.1007/BF01868467. [DOI] [PubMed] [Google Scholar]
- Pralong W. F., Bartley C., Wollheim C. B. Single islet beta-cell stimulation by nutrients: relationship between pyridine nucleotides, cytosolic Ca2+ and secretion. EMBO J. 1990 Jan;9(1):53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Agonist-induced cytosolic calcium oscillations originate from a specific locus in single hepatocytes. J Biol Chem. 1990 Jun 25;265(18):10792–10796. [PubMed] [Google Scholar]
- Rooney T. A., Sass E. J., Thomas A. P. Characterization of cytosolic calcium oscillations induced by phenylephrine and vasopressin in single fura-2-loaded hepatocytes. J Biol Chem. 1989 Oct 15;264(29):17131–17141. [PubMed] [Google Scholar]
- Sanchez-Bueno A., Dixon C. J., Woods N. M., Cuthbertson K. S., Cobbold P. H. The hepatocyte calcium oscillator. Adv Second Messenger Phosphoprotein Res. 1990;24:115–121. [PubMed] [Google Scholar]
- Schnefel S., Pröfrock A., Hinsch K. D., Schulz I. Cholecystokinin activates Gi1-, Gi2-, Gi3- and several Gs-proteins in rat pancreatic acinar cells. Biochem J. 1990 Jul 15;269(2):483–488. doi: 10.1042/bj2690483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streb H., Irvine R. F., Berridge M. J., Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983 Nov 3;306(5938):67–69. doi: 10.1038/306067a0. [DOI] [PubMed] [Google Scholar]
- Swann K., Igusa Y., Miyazaki S. Evidence for an inhibitory effect of protein kinase C on G-protein-mediated repetitive calcium transients in hamster eggs. EMBO J. 1989 Dec 1;8(12):3711–3718. doi: 10.1002/j.1460-2075.1989.tb08546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakui M., Itaya K., Birchall D., Petersen O. H. Intracellular aluminium inhibits acetylcholine- and caffeine-evoked Ca2+ mobilization. FEBS Lett. 1990 Jul 16;267(2):301–304. doi: 10.1016/0014-5793(90)80949-j. [DOI] [PubMed] [Google Scholar]
- Wakui M., Osipchuk Y. V., Petersen O. H. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2(+)-induced Ca2+ release. Cell. 1990 Nov 30;63(5):1025–1032. doi: 10.1016/0092-8674(90)90505-9. [DOI] [PubMed] [Google Scholar]
- Wakui M., Potter B. V., Petersen O. H. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature. 1989 May 25;339(6222):317–320. doi: 10.1038/339317a0. [DOI] [PubMed] [Google Scholar]
- Woods N. M., Cuthbertson K. S., Cobbold P. H. Agonist-induced oscillations in cytoplasmic free calcium concentration in single rat hepatocytes. Cell Calcium. 1987 Feb;8(1):79–100. doi: 10.1016/0143-4160(87)90038-8. [DOI] [PubMed] [Google Scholar]
- Yu D. H., Huang S. C., Wank S. A., Mantey S., Gardner J. D., Jensen R. T. Pancreatic receptors for cholecystokinin: evidence for three receptor classes. Am J Physiol. 1990 Jan;258(1 Pt 1):G86–G95. doi: 10.1152/ajpgi.1990.258.1.G86. [DOI] [PubMed] [Google Scholar]
- Yule D. I., Gallacher D. V. Oscillations of cytosolic calcium in single pancreatic acinar cells stimulated by acetylcholine. FEBS Lett. 1988 Nov 7;239(2):358–362. doi: 10.1016/0014-5793(88)80951-7. [DOI] [PubMed] [Google Scholar]



