Abstract
Liver diseases are a worldwide medical problem because the liver is the principal detoxifying organ and maintains metabolic homeostasis. The liver metabolizes various compounds that produce free radicals (FR). However, antioxidants scavenge FR and maintain the oxidative/antioxidative balance in the liver. When the liver oxidative/antioxidative balance is disrupted, the state is termed oxidative stress. Oxidative stress leads to deleterious processes in the liver and produces liver diseases. Therefore, restoring antioxidants is essential to maintain homeostasis. One method of restoring antioxidants is to consume natural compounds with antioxidant capacity. The objective of this review is to provide information pertaining to various antioxidants found in food that have demonstrated utility in improving liver diseases.
Keywords: Antioxidant, Oxidative stress, Naringenin, Quercetin, Curcumin, Resveratrol, Silymarin, Coffee, Liver diseases
Core tip: The objective of this review is to provide an evidence-based update of antioxidants present in food and to describe their benefits on liver diseases.
INTRODUCTION
The liver is the main organ that metabolizes xenobiotics and endogenous molecules to maintain metabolic homeostasis in the organism. Therefore, the liver is a target of many insults that result in dysregulated hepatic homeostasis and lead to hepatic diseases[1,2]. The liver is composed of the following cells types: hepatocytes, Kupffer cells, liver sinusoidal endothelial cells, pit cells, and hepatic stellate cells (HSC)[3]. Cirrhosis is caused by liver injury from a variety of etiological factors and is the end stage of progressive fibrosis[4]. Oxidative stress plays an important role in the establishment of fibrosis and subsequently in cirrhosis[5]. Therefore, the use of molecules with antioxidant properties has been proposed as a treatment for fibrosis and cirrhosis caused by oxidative stress.
LIVER DISEASES
Liver diseases are a major medical problem worldwide. There are numerous liver diseases caused by different insults, and the disease type depends on the location of development. The main causes of liver disease are viral and parasitic infections in regions such as Africa and Asia. Alcohol abuse is the most important cause of liver diseases in Europe and America. However, viral hepatitis has increased recently[6]. Cirrhosis is likely the most important liver disease, and it is characterized by the accumulation of extracellular matrix proteins (including collagens I, III and IV) and distortion of the hepatic architecture[7].
OXIDATIVE STRESS
Oxidative stress is defined as an imbalance between the production of free radicals (FR) and the antioxidant defenses[8]. The overproduction of pro-oxidants causes deleterious events in the cell such as lipid peroxidation, oxidative DNA damage, and protein damage[9]. FR are defined as atoms or molecules with one or more unpaired electrons. FR can exist as radical cations or radical anions[10] and are usually unstable and highly reactive because they can react with nearby molecules and abstract electrons. Oxygen can reduce and generate reactive oxygen species (ROS) by the interaction with transition metals or by the excitation of electrons secondary to the addition of energy[9]. Oxidative stress contributes to fibrogenesis by increasing harmful cytokines such as transforming grown factor-β (TGF-β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α)[2]. Furthermore, TGF-β increases ROS production in endothelial cells, epithelial cells, smooth muscle cells, and fibroblasts[3].
MAIN SOURCES OF FR IN BIOLOGICAL SYSTEMS
There are several pathways to produce FR, the principal source in the body is own metabolism into the cell; however, this is not the only mechanism to induce oxidative stress. The environment plays an important role in the production of FR, ROS and reactive nitrogen species (RNS), for example, air pollution, UV irradiation, X-rays and gamma-rays[10]. The production of ROS can be induced by endogenous or exogenous substances. The most important endogenous sources are cytochrome P450 metabolism, peroxisomes, microsomes, metal-catalyzed reactions, neutrophils, eosinophils and macrophages during inflammation, and mitochondria-catalyzed electron transport reactions in the complexes I and III[11,12]. Ubisemiquinone has been proposed as the main reductant of oxygen in mitochondrial membranes, consequently, mitochondria generates approximately 2-3 nmol of superoxide/min per mg of protein, indicating that this organelle is the most important physiological producer of ROS and hydrogen peroxide (H2O2)[12]. However, there are other sources of superoxide anion (O2-) like xanthine oxidase, an enzyme that belongs to molybdenum iron-sulphur flavin hydroxylases, which is widely distributed among species and is present in several tissues in mammals. This enzyme plays an important role in the hydroxylation of purines, particularly, by the oxidation of hypoxanthine to xanthine, then from xanthine to uric acid. In both reactions, molecular oxygen is reduced, forming O2- in the first reaction and H2O2 in the second[11]. Another endogenous source of ROS generation is during inflammation, by macrophages and neutrophils. Activated macrophages trigger an increase in oxygen uptake, resulting in the formation of O2-, nitric oxide (NO) and H2O2[13]. In neutrophils, nicotine adenine dinucleotide phosphate [NAD(P)H] oxidase generates O2- that is required for the respiratory burst necessary for bacterial destruction, also nonphagocytic NAD(P)H oxidases produce O2- in a range of 1%-10%[14]. Cytochrome P450 enzymes are another pathway of ROS production during the breakdown or uncoupling of the P450 catalytic cycle. Microsomes generate 80% of the H2O2 at hyperoxia sites, and peroxisomes produce H2O2 but not O2- under physiological conditions, the liver is the major organ where peroxisomes contribute with the overall H2O2 production[11]. Meanwhile, RNS like NO are synthetized by nitric oxide synthases (NOSs), which metabolizes arginine to citruline in a five-electron oxidative reaction, resulting in the formation of NO[15]. Cells from the immune system can produce also NO in the oxidative burst triggered during inflammation processes. In the extracellular environment, NO can react with oxygen and water then to form nitrate and nitrite anions, also the NO and O2- can react together and lead to a more reactive FR called peroxynitrite anion (ONOO-) that can cause lipid peroxidation and DNA fragmentation[16].
ANTIOXIDANTS
Antioxidants are molecules that in low concentrations can prevent or delay the oxidation of an oxidizable substrate[17]. Antioxidants are present in our body and exist in several foods. Antioxidants have a high affinity for FR and scavenge these molecules to protect our health. Compounds with antioxidant properties donate electrons to FR to reduce their reactivity and maintain the cellular pro-oxidant/antioxidant balance. There are many types of molecules with antioxidant activity. Natural compounds have been studied extensively and are relevant to many illnesses including liver diseases (Table 1).
Table 1.
Different antioxidants found in food (clinical effects and relevance)
| Antioxidant | Main clinical effects | Clinical relevance |
| Curcumin | Antioxidant | No studies available in human hepatic disorders |
| Anti-fibrotic | ||
| Anti-inflammatory | ||
| Anti-microbial | ||
| Wound healing | ||
| Anti-carcinogenic | ||
| Resveratrol | Antioxidant | Current data are contradictory, more clinical studies are needed |
| Anti-inflammatory | ||
| Anti-carcinogenic | ||
| Lipid modulation | ||
| Antifibrotic | ||
| Coffee | Antinecrotic | Inverse relationship between coffee-cirrhosis has been demonstrated, but it is |
| Antifibrotic | necessary to do more basic research and prospective clinical trials | |
| Antioxidant | ||
| Anticholestatic | ||
| Chemoprotective | ||
| Quercetin | Chelation of transition metal ions | No studies available in human hepatic disorders |
| Anti-carcinogenic | ||
| Cardioprotective | ||
| Bacteriostatic | ||
| Antioxidant | ||
| Antifibrotic | ||
| Anti-inflammatory | ||
| Anti-apoptotic | ||
| Anti-aggregatory | ||
| Vasodilating | ||
| Silymarin | Antioxidant | Silymarin has been shown to be effective, but it is necessary to do more clinical |
| Antifibrotic | trials focused on survival rates of patients with cirrhosis | |
| Anti-inflammatory | ||
| Anti-carcinogenic | ||
| Immunomodulation | ||
| Naringenin | Antioxidant | No studies available in human hepatic disorders |
| Hypocholesterolemic | ||
| Anti-estrogenic | ||
| Hypolipidemic | ||
| Antihypertensive | ||
| Anti-inflammatory | ||
| Antifibrotic | ||
| Anti-carcinogenic | ||
| Anti-atherogenic | ||
| Green tea | Anti-inflammatory | |
| Anti-arthritic | More clinical studies are needed | |
| Antimicrobial | ||
| Antioxidant | ||
| Neuroprotective | ||
| Antidiabetic | ||
| Antiangiogenesis | ||
| Anti-carcinogenic |
CURCUMIN
Curcumin is also known as diferuloylmethane or 1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hepadieno-3,5-Dione. It is obtained from the rhizomes of Curcuma longa and has several pharmacological properties including strong antioxidant, anti-fibrogenic, anti-inflammatory, anti-microbial, and anti-carcinogenic actions in addition to wound healing effects[18,19]. Approximately, intake of turmeric in the Indian diet is of 2-2.5 g in a 60-kg individual, this is equal to 60-100 mg of curcumin daily. The Food and Drug Administration classified turmeric as a generally recognized as safe (GRAS). Toxicity assays on animals proved that curcumin is safe even at high doses. However, some species like mice and rats with prolonged high-dose intake of turmeric are susceptible to hepatotoxicity[20]. 3H-curcumin was found to be poorly absorbed in the rat intestine[21]. It is metabolized into curcumin glucuronide and curcumin sulfonate[22]. When curcumin is administrated intraperitoneally, it is metabolized to tetrahydrocurcumin, hexahydrocurcumin and hexahydrocurcuminol[23]. Despite curcumin has low bioavailability when is administered orally, Arcaro et al[24] (2014) used piperine, an inhibitor of hepatic and intestinal concomitantly with curcumin. They had shown that both 90 mg/kg of curcumin and 20 mg/kg of piperine had antidiabetic and antioxidant effects. Nevertheless, coadministration of curcumin and piperine did not change the antidiabetic and antioxidant activity of curcumin. Additionally, when the dose of piperine was increased to 40 mg/kg this abrogated the beneficial effects of curcumin. Contrary, Sehgal et al[25] proved the effect of piperine in curcumin in mitigating benzo(a)pyrene toxicity in liver. They found that pretreatment of 100 mg/kg of body weight of curcumin protects against a single dose of benzo(a)pyrene; however, the coadministration with piperine produced a better effect than curcumin alone, suggesting an enhancer activity by piperine. Curcumin has demonstrated hepatoprotective actions on acute and chronic liver injury[26]. Both of types of liver injuries necrosis, oxidative stress, and an inflammatory state[27]. In 2007, Reyes-Gordillo et al[28] demonstrated that curcumin inhibits the increase in cytokines such as TNF-α, IL-1β and IL-6. Additionally, curcumin reduces oxidative stress induced via carbon tetrachloride (CCl4) metabolism by inactivating the nuclear factor-κB (NF-κB) pathway. Moreover, curcumin can elicit its hepatoprotective effect interacting with Fe3+ and Cu2+. In a study performed by Jiao et al[29], they suggest that curcumin could be an iron chelator because they found that transferrin receptor 1 and iron regulatory proteins, indicators of iron depletion, increased in response of curcumin. In agreement, Bernabé-Pineda et al[30], reported that when cyclic voltammograms are in basic media, that a chemical reaction has taken place between curcumin and specially Fe3+. On the other hand, curcumin has been tested in liver[31], but its chelating Cu2+ behavior has not been investigated; however, Baum et al[32] in 2004 tested the interaction of curcumin with Cu2+ and Fe2+, they reported that two molecules of curcumin bind to ion Cu2+ or Fe2+. In a study performed by Li et al[33], it was found that curcumin increases the levels of glutathione (GSH) and heme oxygenase-1 (HO-1), as well as, nuclear factor-erythroid 2-related factor 2 (Nrf2) proteins, suggesting another way to prevent oxidative stress by curcumin. In agreement, Charoensuk et al[34] have shown that curcumin increased the levels of mRNA and protein of Nrf2 and HO-1 and gene expression of NAD(P)H quinone oxidoreductase 1 (NQO1), glutamate cysteine ligase (GCL), activating transcription factor-3, peroxiredoxin 3 (Prdx3) and Prdx6, so increasing the antioxidant system in the cell. Curcumin also has demonstrated to increase the activity of superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST)[35,36] activity. Another mechanism of action of curcumin is by interacting with enzymes or genes implicated in liver cirrhosis. Hassan et al[37] proved effect of curcumin by modulating miRNA 199 and 200 that are the main miRNA associated to liver fibrosis. They showed that miRNA 199 and 200 were increased by the administration of CCl4. However, curcumin restored these miRNAS to their basal levels. Finally, curcumin has shown that in low concentrations, it inhibits the activity of CYP2E1 and its protein levels in alcohol-induced liver damage, thus inhibiting the metabolism of alcohol for this pathway[38]. However, other studies have shown that curcumin does not have an effect on CYP2E1 activity in the liver[39-41].
RESVERATROL
The phytoalexin resveratrol (3,5,4’-trans-trihydroxystilbene) is a polyphenol found in the skin of red grapes, red wine, peanuts and berries[42]. This compound possesses several beneficial activities including antioxidant, anti-inflammatory, anti-carcinogenic, and anti-fibrogenic properties, in addition to affecting lipid modulation[43]. The rate of absorption of resveratrol is approximately 75% after an oral administration[44]. Resveratrol is metabolized to resveratrol sulfate and in low concentrations to resveratrol glucuronide[45] via enzymes of phase II through UDP-glucuronosyltransferase (UGT) or sulfotransferase (ST)[46]. These results were based on in vitro experiments with hepatocytes treated with resveratrol. Conversely, in vivo experiments performed in rats demonstrated that the enterohepatic recirculation plays an important role in the overall systematic exposure to resveratrol when it was administered in aglycone or glucuronide form[47]. Resveratrol has been reported as a compound well tolerated in clinical trials[48]. Nevertheless, in a study performed by Crowell et al[49] in an animal model, resveratrol at the highest dose used (3000 mg/kg body weight/day for 4 wk) produced renal toxicity and reduced final body weights and food consumption as well as other markers of tissue lesions. However, no histological effects in the liver were observed, despite of the clinical chemistry changes and increased liver weight. On the other hand, Williams et al[50] reported not toxicity caused by high-purity trans-resveratrol at different times of exposure and doses. They used 700 mg/kg body weight/day for 90 d as the higher dose and time of exposure, not finding any adverse effect. In 2007, Chávez et al[43] demonstrated that resveratrol decreased the cytokine TGF-β and prevented hepatic fibrosis and NF-κB translocation to the nucleus following chronic use of CCl4. Resveratrol has antioxidant capacity and protects against ethanol-induced lipid peroxidation[51], toxicity by acetaminophen (APAP)[52], and oxidative stress in animal models of cholestasis[53]. It has been suggested that the OH groups play an important role in the antioxidant activity of resveratrol[6]. A study performed by blocking OH group methylation showed that resveratrol and trimethylated resveratrol afford some degree of protection, but the latter possesses the best protective effects[54]. One explanation of this phenomenon considers that the half-life of resveratrol is very short[55] and that trimethylated resveratrol may act as a prodrug and increase the protective effect of resveratrol. However, resveratrol could be less potent than trimethylated resveratrol[6]. Another hepatoprotection mechanism of resveratrol is by activating genes related to antioxidant system or inhibiting enzymes. A study performed by Cheng et al[56] suggest that resveratrol can activate extracellular signal-regulated kinase (ERK) signaling pathway, which in turn can enhance the activation and translocation of Nrf2 to the nucleus, therefore, elevating the expression of HO-1 and glyoxalase. According to the previous study, Bagul et al[57] have shown that resveratrol was able to elevate the translocation of Nrf2 the nucleus, thus suggesting an alternative pathway to protect from oxidative stress. Resveratrol has been reported to decrease acetylation of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC1-α) and increasing its activity by the activation of the protein deacetylase sirtuin 1 (SIRT1), thus improving mitochondrial function and protecting against metabolic disease[58]. Price et al[59] found that resveratrol activates AMP-activated protein kinase (AMPK) in mice treated with moderate dosage and increase nicotinamide adenine dinucleotide (NAD+) levels, also increasing the levels of PGC1-α. AMPK has been shown to augment SIRT1 by increasing NAD+ levels in an indirect way, while this protein deacetylates the AMPK liver kinase B1, leading to phosphorylation and activation of AMPK. Zhu et al[60] have shown that, after administration of resveratrol in mice, the antioxidant system was increased (SOD, GPx, and GSH) in liver tissue as well as the levels of SIRT1 and p-AMPK were upregulated. Resveratrol has also shown to inhibit the activity of CYP2E1 in microsomes of rat liver[61], and to significantly inhibit the activity of this P450 isoform in a model of APAP-induced liver injury[62] and in DEN-induced hepatocarcinogenesis model[63]. The only clinical double-blind study performed to determine the resveratrol hepatoprotective effect demonstrated that a 500 mg resveratrol dose for 12 wk caused a significant reduction in inflammatory cytokines, serum cytokeratin-18, NF-κB activation, liver alanine aminotransferase (ALT), and hepatic steatosis grade compared to the placebo group in patients with non-alcoholic fatty liver disease (NAFLD)[64]. Contrary to this study, a clinical trial performed by Chachay et al[65] in 2014 showed that resveratrol did not significantly improve any characteristics of NAFLD compared to the placebo group. Interestingly, the results showed increased hepatic stress and elevated levels of ALT and aspartate aminotransferase (AST) in the liver. Based on these data, additional clinical trials are needed to determine the actual hepatoprotective effect of resveratrol[66].
COFFEE
Coffee is a mixture of several different molecules including carbohydrates, lipids, vitamins, alkaloids, nitrogenous molecules, and phenolic compounds[67]. Coffee is possibly the most popular drink worldwide[68]. However, the amount of antioxidants in different bean roasts can vary[69]. The three major compounds present in coffee are caffeine, diterpene alcohols (cafestol and kahweol), and chlorogenic acid[70]. Coffee consumption has been associated with the reduction of several chronic diseases[71]. This result may be due to the pharmacological properties that have anti-necrotic, anti-fibrotic, anti-cholestatic, chemoprotective, and antioxidant functions[72]. Caffeine is the best-known active component of coffee, and it is absorbed rapidly after an oral ingestion (5 min) and reaches its peak blood levels after 30 min. When caffeine is consumed in high amounts produced side effects. Recommendations from Health Canada in 2013, stipulated that the caffeine intake per day for children should not exceed 2.5 mg/kg of body weight. Additionally, tachycardia and arrhythmia typically arise when more than 200 mg of caffeine are ingested[73]. Worthley et al[74] have given 250 mL of a sugar-free energy drink to 50 young people, this drink contained about 80 mg of caffeine, they have observed that caffeine increased the blood pressure compared with controls. Moreover, other kind of sickness have been reported for caffeine consumption such as cardiovascular diseases, a negatively impact in cognition, perpetual memory and learning[73]. Smith et al[75] in 2002, reported that the intake of 300 mg of caffeine increased anxiety and tension. Also, caffeine triggered hallucinatory experiences in people who drink 300 mg of coffee (about 7 cups per day). Patients with panic disorders were more sensitive to caffeine[73]. The half-life of caffeine is approximately 5-6 h[76,77]. Caffeine is almost completely metabolized in the liver. The principal metabolite is paraxanthine, which results from the activity of CYP1A2. However, this enzyme produces other metabolites such as theobromine, theophylline, and 1,3,7-trimethyluric acid. Other enzymes such as CYPs 1A1 and 2E1 participate in caffeine metabolism[78]. An important property of caffeine is that it easily passes through the blood-brain barrier[79]. The absorption rate of chlorogenic acid is 33% of the dose in both, rats and humans. Several metabolites have been identified in human plasma and urine, and these metabolites include polyphenolic acids, glycine conjugates, sulfates and glucuronides of hydroxycinnamic acid, and hydrogenated hydroxycinnamic acid[80]. Many studies have shown an inverse relationship among coffee consumption and liver cirrhosis[71]. In the prospective study performed by Klatsky et al[81], the authors demonstrated an inverse coffee-cirrhosis relationship for the first time. In 1994, Corrao et al[82] performed a study and identified a dose-response relationship between coffee intake and cirrhosis. The data show that the odds ratio for liver cirrhosis decreases from 1.0 for people who do not drink coffee to 0.47, 0.23, 0.21, and 0.16 for 1, 2, 3 or 4 cups of coffee daily, respectively. Furthermore, the study showed that the caffeine per se did not show any relationship with cirrhosis by testing other drinks with caffeine. Although coffee is beneficial to liver health, the study failed to demonstrate a causative role of coffee in preventing liver injury. Thus, additional basic research and controlled prospective studies are needed. Arauz et al[72] in 2013 demonstrated that coffee has a beneficial effect on liver injury caused by chronic administration of thioacetamide (TAA). Coffee prevented cholestasis and necrosis measured by the enzymes γ-glutamyl transpeptidase (γ-GTP), alkaline phosphatase, and ALT. Human trials demonstrated the same results[83]. This inverse relationship was particularly high in heavy alcohol drinkers[84]. Conversely, liver injury inhibits caffeine metabolism, and people with liver diseases may experience adverse consequences after drinking coffee. Furthermore, it is important to distinguish between former coffee drinkers and nondrinkers in future epidemiological studies[71,85]. Moreno et al[86] in 2011 and Arauz et al[72] in 2013 demonstrated in murine models that coffee prevents experimental liver cirrhosis in two models of liver injury using carbon tetrachloride and thioacetamide. Both studies showed that coffee reduced the expression of the profibrogenic cytokine TGF-β. The study by Arauz et al[72] measured the expression of connective tissue growth factor (CTGF), which has been suggested as an important downstream modulator of TGF-β that increases its profibrogenic response. This finding is consistent with the significant upregulation of extracellular matrix in fibrotic liver[72]. Cavin et al[87] have reported coffee as an inductor of GST, aldo-keto reductase, GSH, HO-1, glutathione-S-transferase P1, that are enzymes involved in the detoxification process. Also, they suggest that a possible mechanism of chemoprotection of coffee is by stimulation of Nrf2 pathway. In another study, coffee was able to elevate mRNA levels of NQO1 and glutathione-S-transferase A1 in liver and small intestine also, UDP-glucuronosyltransferase 1A6 and GCL catalytic (GCLC) were increased in small intestine. Further, the same group reported that this induction was bigger in mice possessing Nrf2 in contrast with Nrf2 knockout mice[88].
QUERCETIN
Quercetin is also known as 3,3’,4’,5,7-penta-hydroxyflavone. It is a flavonol found in natural products, especially in apples and onions[89]. Quercetin is known to have biological effects including chelation of heavy metals, anti-carcinogenic, cardioprotective, bacteriostatic, anti-inflammatory, and antioxidant properties[90], in addition to functioning as a hepatoprotective agent[91]. The normal intake of quercetin is less than 5-40 mg/d. However, people who eat the peel of food with high amounts of quercetin may consume 200-500 mg/d[90]. In 2004, high purity quercetin used in foods was GRAS in the range of 0.008%-0.5% or 10-125 mg/serving[90]. Bors et al[92] in 1990 showed the characteristics that an antioxidant must have to exert an effective activity. These characteristics include the presence of ortho-dihydroxy or catechol groups in the B-ring, a 2,3-double bond of the C-ring, and OH substitution on positions 3 and 5 of the C-ring and A-ring, respectively[92]. The quercetin ring presents all of these features. The structure of flavonoids can interact with both FR and metal ions like Fe3+ and Cu2+, therefore showing chelating properties. In a study performed by Mira et al[93], it was shown that quercetin was capable of reducing Fe3+ and Cu2+, due to its 2, 3-double bond and the catechol group in the β-ring. Furthermore, its ability to reduce the Cu2+ seems to be dependent of hydroxyl groups. After oral intake, quercetin is rapidly absorbed and peaks at approximately 30 min[94] before it is metabolized by glucuronidation and sulfation by the UGT and ST, respectively. Furthermore, the addition of O-methylation in the position 3’ or 4’ of the catechol group in the B-ring results in isorhamnetin (3’-O-methylquercetin) and tamaraxetin (4’-O-methylquercetin) by catechol-O-methyl transferase. These processes begin in the intestine, and the compounds are released into the lumen before conjugation in the liver by the same enzymes. However, other tissues such as the kidneys can also metabolize quercetin[94-97]. Quercetin has shown hepatoprotective properties in rats treated chronically with CCl4 for 8 wk by preventing the expression of profibrogenic genes including TGF-β, CTGF, and collagen-1α (Col-1α). Therefore, quercetin reduces the fibrogenic process and liver enzymes associated with a significant reduction of activated HSC and inhibition of NF-κB. Conversely, quercetin increased the gene expression and improved the activity of SOD and CAT, in addition to activating metalloproteinases 2 and 9 (MMP2 and MMP9)[91]. Pavanato et al[98] in 2003 used the same hepatotoxin for 16 wk and observed that quercetin improves the hepatic liver enzymes AST, ALT, inducible NOS (iNOS) expression, and collagen content and reduces lipid peroxidation. de David et al[99] showed similar results using TAA hepatotoxin and found that quercetin inhibited the change in the p-ERK 1/2 pathway and significantly prevented the increase in apoptosis by regulating the Bax/Bcl-2 ratio[99]. In a study performed by Granado-Serrano et al[100] in HepG2 cells, they found that quercetin modulated Nrf2 and p38, it was dependent on the concentration used and the time of exposure, quercetin rapidly activated Nrf2 by up-regulating its phosphorylation, consequently, translocation to the nucleus and binding to antioxidant response element (ARE), also increased GSH content and expression of GPx. However, when the time of exposure is larger, this effect was blocked by quercetin which, in turn activated p38-MAPK via. Therefore suggesting that Nef2-ARE acts as a sensor and responds to a chemical. However, Tanigawa et al[101] reported that quercetin possesses an enhanced effect in the ARE binding activity and Nrf2-mediated transcription activity in HepG2 cells. Moreover, quercetin apart from up-regulating expression of Nrf2 mRNA and protein, also stabilized Nrf2 protein inhibiting its proteasomal degradation and reduced the levels of kelch-like ECH-associated protein 1 (Keap1) through the formation of a modified Keap1. On the other hand, a study performed by Ji et al[102] showed that quercetin does not possesses an enhanced activity in mRNA expression of Nrf2 or Keap1. However, they suggested that quercetin could interact with Keap1 and fill the binding site of Nrf2 in Keap1, thus inhibiting its interaction and inducing the transcriptional activation of Nrf2. Quercetin has shown to suppress the activity of CYP2E1 when ethanol over activated it and induces HO-1 in hepatocytes[103]. According with this findings, in a non-alcoholic steatohepatitis (NASH) model, quercetin was able to decrease by 2-fold CYP2E1 activity compared with NASH group[104]. On the other hand, quercetin effect was inhibited by CYP2E1 compared with a control measuring by HPLC in rat liver microsomes[105]. Currently, there are no clinical studies available on quercetin hepatoprotection[106].
SILYMARIN
Silymarin is a natural substance derived from Silybum marianum, also known as Milk thistle or Saint Mary’s thistle[6]. Silymarin has been reported as a safe compound in acute doses in animal models due to its lack of side effects. In contrast, in a clinical trial, thousands of patients suffered mainly mild gastrointestinal disorders by silymarin consumption[107]. In other clinical trial, El-Kamary et al[108] (2009) no side effects were reported in 105 patients using 140 mg of silymarin. The range of doses used in literature is from 280 to 800 mg/kg of body weight/day. After oral administration, the silymarin peak plasma concentration is reached at approximately 6-8 h. Silymarin has poor bioavailability (23%-47%). The metabolites of silymarin are conjugated in the liver by UGT and ST (phase II reactions)[109]. Among the hepatoprotective effects of silymarin, it is known that silybin, the major constituent of silymarin, has iron-chelating properties[110,111]. Silymarin has also been probed as iron chelator in children with β-thalassemia with iron overload[112]. In a study performed by Najafzadeh et al[113], they suggest that hepatoprotective effect of silymarin in iron-overload induced hepatotoxicity was due to an iron-chelator activity but no studies have been made proving the chelating properties per se of silymarin in liver diseases. Silymarin has hepatoprotective properties against several hepatotoxins such as CCl4. Silymarin can prevent oxidative stress, fibrosis, cirrhosis, and lipid peroxidation by modulating the content of phosphatidylethanolamine[114]. Thus, it improves liver enzyme activities and protects against the harmful increases in cholesterol/phospholipids and sphingomyelin/phosphatidylcholine ratios in the membrane. This effect was associated with a decrease in Na+/K+ and Ca2+-ATPase activities induced by CCl4. However, silymarin does not reverse well-established cirrhosis[115-119]. Kim et al[120] showed that silymarin increases nuclear translocation of Nrf2 in activated HSC, however, expression of other molecules related to a detoxifying effect have not been measured. Also, silymarin has been reported to increase the activity of antioxidant enzymes like SOD, GPx[121] and CAT[122]. A clinical trial examining silymarin in a complex with phosphatidylcholine found reduced levels of the liver enzymes, ALT and γ-GTP, and serum bilirubin levels in a dose-dependent manner in patients with hepatitis caused by virus infection or alcohol abuse[123]. Another clinical study showed similar results when silymarin was administered alone[124]. In patients with cirrhosis, silymarin administration for 41 mo significantly increased the survival rate compared to a placebo group[125]. However, in the study performed by Parés et al[126], silymarin showed no effect on survival rate in the clinical course in alcoholic patients with liver cirrhosis.
NARINGENIN
Naringenin is also known as 5,7,4’-thihydroxyflavanone. Naringenin is a flavanone found in citrus fruits and tomatoes[127]. In a study performed recently, Yang et al[128] reported that naringenin does not cause deleterious effects in beagle dogs, the maximum time of exposure was 180 d and with doses varying of 20, 100, or 500 mg/kg body weight/day. Also, Surampalli et al[129], showed that naringenin was harmless upon exposure to rat gastrointestinal epithelium in doses ranging from 1 mmol/L to 100 mmol/L, thus suggesting naringenin as a safe compound. Naringenin has many pharmacological properties including hypolipidemic, anti-hypertensive, anti-inflammatory, antioxidant and anti-fibrotic functions[127]. Flavonoids are absorbed in the aglycone form rather than in the glycoside form like quercetin. The glycoside form of naringenin is cleaved in the small intestine before absorption, which results in sulfate and glucuronide metabolites in the small intestine wall and liver[127,130] by UGT and ST. Mira et al[93] showed that naringenin has shown capacity of reduction of the Fe3+ and Cu2+ but in less potential than quercetin. Chtourou et al[131] found that naringenin prevents the depletion of SOD, CAT, GPx and GSH. Conversely, naringenin also prevented the increase in lipid peroxidation, ALT and AST. Additionally, expression of the following genes was also affected in an NAFLD rat model induced by a high cholesterol diet: pro-inflammatory cytokines TNF-α, IL-6, and IL-1β, EGF-like module-containing mucin-like hormone receptor-like 1, iNOS, NF-κB, MMP2 and MMP9[131]. Similar results were obtained by Yen et al[132] using naringenin alone and a naringenin-loaded nanoparticle system (NARN). Both treatments exhibited antioxidant and hepatoprotective activities. The treatments also inhibited the activation of caspases 3, and 8. However, NARN was more effective as a hepatoprotector and antioxidant than free naringenin because it also inhibits caspase 9 during CCl4-induced hepatotoxicity in rats[132]. In a study performed by Goldwasser et al[133] it was found that naringenin activates peroxisome proliferator-activated receptor alpha (PPARα), then decreasing the levels of very low density lipoprotein production without causing lipid accumulation in hepatocytes, in a hepatitis C virus (HCV) model. Similar results were found by Cho et al[134], who have shown that naringenin intake causes a significant depletion in the amount of total triglycerides and cholesterol in plasma and liver of rats. Also, naringenin-fed animals showed an increment in PPARα protein expression in liver. Goldwasser et al[133] found that the flavonoid regulates the activity of PPARγ and liver X receptor alpha (LXRα), by activating the ligand-binding domain of PPARα and PPARγ, while inhibiting LXRα, thus modulating different genes related to fatty acid oxidation and lipogenesis. Han et al[135], found that a pretreatment with naringenin-7-O-glucoside increased NQO1, ERK and phosphorylation and translocation of Nrf2 to the nucleus in H9c2 cardiomyocytes, as well as, upregulating the mRNA expression of GCLC and GCL modifier[135], thus inducting endogenous antioxidant enzymes. Similar findings was reported by Esmaeili et al[136], they showed that naringenin was capable of attenuating CCl4-induced liver injury by downregulating TNF-α, iNOS and cyclo-oxigenase-2, both protein and mRNA, as well as by increasing Nfr2 and HO-1 expression. Motawi et al[137] suggested that naringenin could be another example of CYP2E1 inhibitor, they probed it, in rat liver microsomal assay in co-administration with simvastin, and such inhibition of CYP2E1 is another via to improve antioxidant defenses[137]. There are currently no studies available in human hepatic disorders[138].
GREEN TEA
Camellia sinensis, also known as green tea, is a worldwide consumed beverage. Its beneficial effects on health are due in part to its antioxidant, anti-inflammatory, anti-arthritic and anti-angiogenic effects. Moreover, green tea is a mixture of polyphenols (the major class of active compounds) including catechins (also known as flavan-3-ols) which constitute about 30% (mass fraction) of green tea leaves; the major catechins in green tea are (+)-catechin, (-)-epicatechin, (-)-epigallocatechin, (-)-epicatechin-3-gallate, (-)-gallocatechin, (-)-gallocatechin gallate and (-)-epigallocatechin-3-gallate (EGCG). Flavonoids like quercetin, kaempferol and myricetin; methylxanthine alkaloids such as caffeine, theophylline and theobromine, and phenolic acids (gallic acid, chlorogenic acid and caffeic acid)[139,140]. EGCG is the most abundant catechin and represents up to 50% of total polyphenols and possesses the strongest antioxidant capacity, therefore, it is considered the main biological active compound[140]. On the other hand, green tea does not only exert its antioxidant properties by polyphenols, L-theanine is the primary amino acid in green tea and represents 1%-2% of the leaf dry weight, it is synthetized in the roots of green tea and is concentrated in the leaves. L-theanine chemical structure is similar to glutamic acid, the latest is a precursor of GSH. Studies have shown that L-theanine protects the cell maintaining the levels of GSH in cancer and neurotixicity diseases[141]. The intake of green tea can be considered safe when its consumption does not exceed 1-2 cups/d. Nevertheless, hepatotoxicity has been attributed to the intake of green tea when it is used for weight control; furthermore[140]. Pérez-Vargas et al[141] found that L-theanine prevented the increased expression of NF-κB and down-regulated IL-1β and IL-6 and the cytokines TGF-β and CTGF induced by carbon tetrachloride. Moreover, the expression of the corresponding mRNAs decreased accordingly. On the other hand, L-theanine promoted the expression of IL-10 and the fibrolytic enzyme metalloproteinase 13 (MMP13). In a study performed by Yu et al[142] they have shown that EGCG ameliorates liver inflammation, necrosis and fibrosis and suppressed the expression of TNF-α, IL-1β, TGF-β, MMP9, α-SMA, and Col-1α1. Similar results were obtained in HSC cell line LX-2, where EGCG was capable of suppressing TGF-β1, Col-1α1, MMP2, MMP9, TIMP1, and α-SMA. Moreover, Bin Dajem et al[143] used the aqueous extract of green tea in a Schistosoma mansoni-infected mice model to investigate its effect on the oxidative stress, antioxidant system and liver pathology induced by the parasite. They found that green tea extract suppressed the oxidative stress by decreasing the lipid peroxides. However, failed to enhance the antioxidant system and to reverse alterations in the liver such as necrosis. In a study performed by Higashi et al[144] they found that EGCG modulates the growth of HSC activated cells by Rho-signaling pathways and induces the phosphorylation of ERK 1/2, c-Jun kinase and p38, suggesting a mechanism of its anti-fibrotic capacity. In a cisplatin-induced nephrotoxicity model in rats, EGCG increased the levels of Nfr2, HO-1, SOD, CAT, GPx and GSH[145]. In clinical trials, green tea has shown protective effects against various kinds of cancers, including premalignant prostate, esophageal, colon, rectum and pancreatic cancers[146]. Nevertheless, in hepatocellular carcinoma, green tea did not have any protective effect[147]. In a study performed by Halegoua-De Marzio et al[148] they have shown, after a single oral dose of green tea (400 mg), in patients with cirrhosis induced by HCV, that it is safe and well tolerable by all patients, therefore suggesting the use of green tea in the treatment of cirrhosis in the future. However, more clinical studies related to the beneficial effects on liver diseases are needed.
The information shown above represents some of the antioxidants uses in different kind of experiments in animals and clinical trials. However, it is difficult to say which of these antioxidants possess the best hepatoprotective properties since they have different chemical structures and antioxidant potency, then its scavenger capacity is not the same. Moreover, other parameters need to be considered, such as the bioavailability, and pharmacokinetics. We focus our hepatoprotective ranking mainly based on the chemical structure showed in Figure 1. We suggest that silymarin has the best hepatoprotective effect because is a mixture of flavonolignans including silybin, isosilybin, silydianin, silychristin, isosilychristin and the flavonoid taxifolin. In addition, silybinin is composed of 2 diasteroisomeric compounds (silybin A and silybin B) in a 1:1 ratio[149]. Flavonoids in its structure have different forms to stabilize FR including hydroxyl phenolic groups, double bonds and sometimes a catechol group[92]. Therefore, silymarin seems to be the best choice referred to hepatoprotective effect. Green tea is another mixture of polyphenols, as mentioned earlier, containing catechins, flavonoids and methylxanthine alkaloids. Nevertheless its data referred to hepatoprotection is lower than silymarin, for these reason we decided ranked green tea in the second place. The antioxidant property of EGCG is related of its hydroxyl phenolic groups, that maybe acts mainly from hydrogen atoms transfer or single electron transfer reactions. This groups are presented in the B- and D-rings of EGCG[150]. Quercetin, as mentioned above, is a flavonoid that have all the elements to exert a magnificent hepatoprotective effect related to its structure showing a catechol group in the B ring, substitution of hydroxyl phenolic groups in the A and C ring and a double bond in the position 2-3 of the C ring[92]. Curcumin has been used in the treatment of experimental liver diseases since 1970 and shows a powerful antioxidant capacity and immunomodulatory properties. However, it does not have the same structure of flavonoids, showing two hydroxyl phenolic groups and a heptadiene linkage two methoxyphenol rings. Ak et al[151] suggest that keto form of curcumin, the heptadienone linkage between the two methoxyphenol rings, contain a carbon atom that can donate a hydrogen, therefore, stabilizing FR. We considered that its capacity of stabilize FR is lower than quercetin. Resveratrol possesses hydroxyl phenolic groups and a system of conjugated double bonds that can donate electrons to FR. Resveratrol has two phenolic rings: monophenol and diphenol. Gülçin[152] suggests that subtraction of hydrogen atom is easily in the monofenol ring. Naringenin is another flavonoid with lower antioxidant capacity than quercetin, shows a hydroxyl phenolic group in its structure in the A ring. However, it does not have the catechol group or the double bond[92]. Also, Cao et al[153] suggest that in flavonoids the hydroxyl substitution is relevant in the ORACOH. activity. Caffeine has double bonds in its structure. Chu et al[154] reported that pure caffeine had very low ORACOH. values, whereas, crude caffeine had higher values than pure caffeine. We considered that caffeine has the lowest antioxidant activity of all the compounds showed; therefore coffee has the lowest antioxidant capacity.
Figure 1.
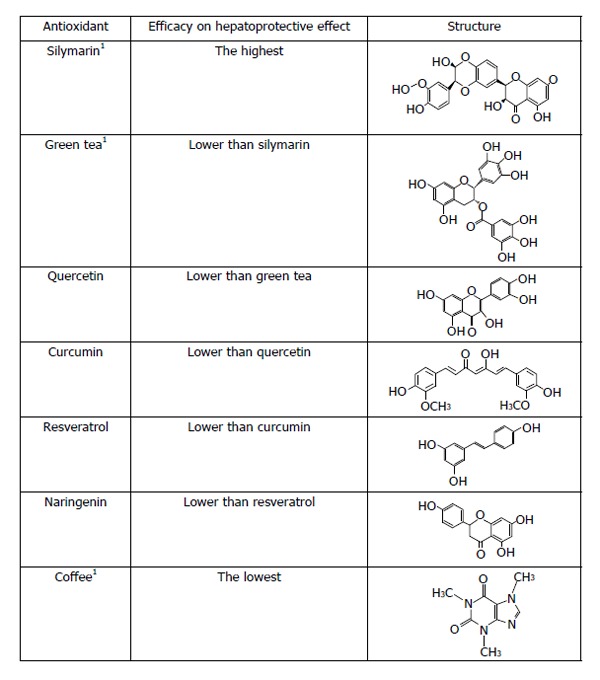
Comparison between hepatoprotective effect-related antioxidant capacities. 1The structure presented is based on the most studied compound. For silymarin we show the structure of silybin, in the case of green tea the structure of (-)-epigallocatechin-3-gallate, and in the case of coffee the structure of caffeine.
CONCLUSION
Investigations of antioxidants show that compounds in food are candidates for the treatment of several diseases because they improve the antioxidant system in the body, especially when the disease involves oxidative stress. This review describes antioxidants that can be investigated for experimental and clinical trials and will be used for the treatment of liver diseases such as liver cirrhosis. Curcumin, quercetin, and naringenin are effective in the treatment of experimental liver injury, and they can be studied in clinical trials. Green tea have been shown to protect against different kinds of cancer in clinical trials, except in hepatocellular carcinoma. Conversely, there are no clinical trials investigating resveratrol, coffee, and silymarin. However, the data are poor or contradictory, and it is necessary to perform more clinical trials to use these antioxidants for the treatment of liver diseases in patients.
ACKNOWLEDGMENTS
The authors express their gratitude to Martha Noyola and Maria Teresa García for their careful review of the manuscript. Sael Casas-Grajales was a fellow of Conacyt 288926.
Footnotes
P- Reviewer: Acuna-Castroviejo D S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Conflict-of-interest statement: The authors declare that there are no conflicts of interest in the present work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: February 6, 2015
First decision: April 10, 2015
Article in press: June 19, 2015
References
- 1.Muriel P. Some experimental models of liver damage. In: Sahu SC, editor. Hepatotoxicity: From genomics to in vitro and in vivo models. West Sussex, England: Wiley; 2007. pp. 119–137. [Google Scholar]
- 2.Muriel P. Cytokines in liver diseases. In: Sahu SC, editor. Hepatotoxicity: From genomics to in vitro and in vivo models. West Sussex, England: Wiley; 2007. pp. 371–389. [Google Scholar]
- 3.Muriel P, Arauz J. Coffee and liver health. In: Chu Y, editor. Coffee emerging health effects and disease prevention. West Sussex, UK: IFT Press/Wiley-Blackwell; 2012. pp. 123–139. [Google Scholar]
- 4.Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Curcumin prevents and reverses cirrhosis induced by bile duct obstruction or CCl4 in rats: role of TGF-beta modulation and oxidative stress. Fundam Clin Pharmacol. 2008;22:417–427. doi: 10.1111/j.1472-8206.2008.00611.x. [DOI] [PubMed] [Google Scholar]
- 5.Muriel P. Peroxidation of lipids and liver damage. In: Baskin SI, Salem H, editors. Oxidants, antioxidants and free radicals. Washington, D. C. USA: Taylor & Francis; 1997. pp. 237–357. [Google Scholar]
- 6.Muriel P, Rivera-Espinoza Y. Beneficial drugs for liver diseases. J Appl Toxicol. 2008;28:93–103. doi: 10.1002/jat.1310. [DOI] [PubMed] [Google Scholar]
- 7.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betteridge DJ. What is oxidative stress? Metabolism. 2000;49:3–8. doi: 10.1016/s0026-0495(00)80077-3. [DOI] [PubMed] [Google Scholar]
- 9.Yoshikawa T, Naito Y. What is oxidative stress? JMAJ. 2002;45:271–276. [Google Scholar]
- 10.Ternay JAL, Sorokin V. Redox, radicals, and antioxidants. In: Baskin SI, Salem H, editors. Oxidants, antioxidants, and free radicals. Washington, DC, USA: Taylor and Francis; 1997. pp. 1–21. [Google Scholar]
- 11.Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- 12.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M, Sato EF, Nishikawa M, Park AM, Kira Y, Imada I, Utsumi K. Mitochondrial generation of reactive oxygen species and its role in aerobic life. Curr Med Chem. 2003;10:2495–2505. doi: 10.2174/0929867033456477. [DOI] [PubMed] [Google Scholar]
- 14.Conner EM, Grisham MB. Inflammation, free radicals, and antioxidants. Nutrition. 1996;12:274–277. doi: 10.1016/s0899-9007(96)00000-8. [DOI] [PubMed] [Google Scholar]
- 15.Ghafourifar P, Cadenas E. Mitochondrial nitric oxide synthase. Trends Pharmacol Sci. 2005;26:190–195. doi: 10.1016/j.tips.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Carr AC, McCall MR, Frei B. Oxidation of LDL by myeloperoxidase and reactive nitrogen species: reaction pathways and antioxidant protection. Arterioscler Thromb Vasc Biol. 2000;20:1716–1723. doi: 10.1161/01.atv.20.7.1716. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B. How to characterize a biological antioxidant. Free Radic Res Commun. 1990;9:1–32. doi: 10.3109/10715769009148569. [DOI] [PubMed] [Google Scholar]
- 18.Punithavathi D, Venkatesan N, Babu M. Protective effects of curcumin against amiodarone-induced pulmonary fibrosis in rats. Br J Pharmacol. 2003;139:1342–1350. doi: 10.1038/sj.bjp.0705362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Chainani-Wu N. Safety and anti-inflammatory activity of curcumin: a component of tumeric (Curcuma longa) J Altern Complement Med. 2003;9:161–168. doi: 10.1089/107555303321223035. [DOI] [PubMed] [Google Scholar]
- 21.Ravindranath V, Chandrasekhara N. Metabolism of curcumin--studies with [3H]curcumin. Toxicology. 1982;22:337–344. doi: 10.1016/0300-483x(81)90027-5. [DOI] [PubMed] [Google Scholar]
- 22.Ireson C, Orr S, Jones DJ, Verschoyle R, Lim CK, Luo JL, Howells L, Plummer S, Jukes R, Williams M, et al. Characterization of metabolites of the chemopreventive agent curcumin in human and rat hepatocytes and in the rat in vivo, and evaluation of their ability to inhibit phorbol ester-induced prostaglandin E2 production. Cancer Res. 2001;61:1058–1064. [PubMed] [Google Scholar]
- 23.Aggarwal B, Bhatt ID, Ichikawa H, Anh KS, Sethi G, Sandur SK, Natarajan C, Seeram N, Shishodia S. Curcumin-biological and medicinal properties. In: Ravindran PN, Nirmal-Babu K, Sivaraman K, editors. Tumeric: The Genus Curcuma. Boca Raton, USA: CRC Press; 2007. pp. 297–368. [Google Scholar]
- 24.Arcaro CA, Gutierres VO, Assis RP, Moreira TF, Costa PI, Baviera AM, Brunetti IL. Piperine, a natural bioenhancer, nullifies the antidiabetic and antioxidant activities of curcumin in streptozotocin-diabetic rats. PLoS One. 2014;9:e113993. doi: 10.1371/journal.pone.0113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sehgal A, Kumar M, Jain M, Dhawan DK. Piperine as an adjuvant increases the efficacy of curcumin in mitigating benzo(a)pyrene toxicity. Hum Exp Toxicol. 2012;31:473–482. doi: 10.1177/0960327111421943. [DOI] [PubMed] [Google Scholar]
- 26.Matés JM, Segura JA, Alonso FJ, Márquez J. Natural antioxidants: therapeutic prospects for cancer and neurological diseases. Mini Rev Med Chem. 2009;9:1202–1214. doi: 10.2174/138955709789055180. [DOI] [PubMed] [Google Scholar]
- 27.Wang ME, Chen YC, Chen IS, Hsieh SC, Chen SS, Chiu CH. Curcumin protects against thioacetamide-induced hepatic fibrosis by attenuating the inflammatory response and inducing apoptosis of damaged hepatocytes. J Nutr Biochem. 2012;23:1352–1366. doi: 10.1016/j.jnutbio.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Reyes-Gordillo K, Segovia J, Shibayama M, Vergara P, Moreno MG, Muriel P. Curcumin protects against acute liver damage in the rat by inhibiting NF-kappaB, proinflammatory cytokines production and oxidative stress. Biochim Biophys Acta. 2007;1770:989–996. doi: 10.1016/j.bbagen.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Jiao Y, Wilkinson J, Christine Pietsch E, Buss JL, Wang W, Planalp R, Torti FM, Torti SV. Iron chelation in the biological activity of curcumin. Free Radic Biol Med. 2006;40:1152–1160. doi: 10.1016/j.freeradbiomed.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 30.Bernabé-Pineda M, Ramírez-Silva MT, Romero-Romo MA, González-Vergara E, Rojas-Hernández A. Spectrophotometric and electrochemical determination of the formation constants of the complexes Curcumin-Fe(III)-water and Curcumin-Fe(II)-water. Spectrochim Acta A Mol Biomol Spectrosc. 2004;60:1105–1113. doi: 10.1016/S1386-1425(03)00344-5. [DOI] [PubMed] [Google Scholar]
- 31.Rivera-Espinoza Y, Muriel P. Pharmacological actions of curcumin in liver diseases or damage. Liver Int. 2009;29:1457–1466. doi: 10.1111/j.1478-3231.2009.02086.x. [DOI] [PubMed] [Google Scholar]
- 32.Baum L, Ng A. Curcumin interaction with copper and iron suggests one possible mechanism of action in Alzheimer’s disease animal models. J Alzheimers Dis. 2004;6:367–377; discussion 443-449. doi: 10.3233/jad-2004-6403. [DOI] [PubMed] [Google Scholar]
- 33.Li B, Wang L, Lu Q, Da W. Liver injury attenuation by curcumin in a rat NASH model: an Nrf2 activation-mediated effect? Ir J Med Sci. 2014:Epub ahead of print. doi: 10.1007/s11845-014-1226-9. [DOI] [PubMed] [Google Scholar]
- 34.Charoensuk L, Pinlaor P, Prakobwong S, Hiraku Y, Laothong U, Ruangjirachuporn W, Yongvanit P, Pinlaor S. Curcumin induces a nuclear factor-erythroid 2-related factor 2-driven response against oxidative and nitrative stress after praziquantel treatment in liver fluke-infected hamsters. Int J Parasitol. 2011;41:615–626. doi: 10.1016/j.ijpara.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 35.Reddy ACP, Lokesh BR. Alterations in lipid peroxides in rat liver by dietary n-3 fatty acids: modulation of antioxidant enzymes by curcumin, eugenol, and vitamin E. J Nutr Biochem. 1994;5:181–188. [Google Scholar]
- 36.Koiram PR, Veerapur VP, Kunwar A, Mishra B, Barik A, Priyadarsini IK, Mazhuvancherry UK. Effect of curcumin and curcumin copper complex (1: 1) on radiation-induced changes of anti-oxidant enzymes levels in the livers of Swiss albino mice. J Radiat Res. 2007;48:241–245. doi: 10.1269/jrr.06103. [DOI] [PubMed] [Google Scholar]
- 37.Hassan ZK, Al-Olayan EM. Curcumin reorganizes miRNA expression in a mouse model of liver fibrosis. Asian Pac J Cancer Prev. 2012;13:5405–5408. doi: 10.7314/apjcp.2012.13.11.5405. [DOI] [PubMed] [Google Scholar]
- 38.Lee HI, McGregor RA, Choi MS, Seo KI, Jung UJ, Yeo J, Kim MJ, Lee MK. Low doses of curcumin protect alcohol-induced liver damage by modulation of the alcohol metabolic pathway, CYP2E1 and AMPK. Life Sci. 2013;93:693–699. doi: 10.1016/j.lfs.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Guangwei X, Rongzhu L, Wenrong X, Suhua W, Xiaowu Z, Shizhong W, Ye Z, Aschner M, Kulkarni SK, Bishnoi M. Curcumin pretreatment protects against acute acrylonitrile-induced oxidative damage in rats. Toxicology. 2010;267:140–146. doi: 10.1016/j.tox.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Sugiyama T, Nagata J, Yamagishi A, Endoh K, Saito M, Yamada K, Yamada S, Umegaki K. Selective protection of curcumin against carbon tetrachloride-induced inactivation of hepatic cytochrome P450 isozymes in rats. Life Sci. 2006;78:2188–2193. doi: 10.1016/j.lfs.2005.09.025. [DOI] [PubMed] [Google Scholar]
- 41.Oetari S, Sudibyo M, Commandeur JN, Samhoedi R, Vermeulen NP. Effects of curcumin on cytochrome P450 and glutathione S-transferase activities in rat liver. Biochem Pharmacol. 1996;51:39–45. doi: 10.1016/0006-2952(95)02113-2. [DOI] [PubMed] [Google Scholar]
- 42.Matés JM, Segura JA, Alonso FJ, Márquez J. Anticancer antioxidant regulatory functions of phytochemicals. Curr Med Chem. 2011;18:2315–2338. doi: 10.2174/092986711795656036. [DOI] [PubMed] [Google Scholar]
- 43.Chávez E, Reyes-Gordillo K, Segovia J, Shibayama M, Tsutsumi V, Vergara P, Moreno MG, Muriel P. Resveratrol prevents fibrosis, NF-kappaB activation and TGF-beta increases induced by chronic CCl4 treatment in rats. J Appl Toxicol. 2008;28:35–43. doi: 10.1002/jat.1249. [DOI] [PubMed] [Google Scholar]
- 44.Walle T. Bioavailability of resveratrol. Ann N Y Acad Sci. 2011;1215:9–15. doi: 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- 45.Yu C, Shin YG, Chow A, Li Y, Kosmeder JW, Lee YS, Hirschelman WH, Pezzuto JM, Mehta RG, van Breemen RB. Human, rat, and mouse metabolism of resveratrol. Pharm Res. 2002;19:1907–1914. doi: 10.1023/a:1021414129280. [DOI] [PubMed] [Google Scholar]
- 46.Wenzel E, Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol Nutr Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 47.Marier JF, Vachon P, Gritsas A, Zhang J, Moreau JP, Ducharme MP. Metabolism and disposition of resveratrol in rats: extent of absorption, glucuronidation, and enterohepatic recirculation evidenced by a linked-rat model. J Pharmacol Exp Ther. 2002;302:369–373. doi: 10.1124/jpet.102.033340. [DOI] [PubMed] [Google Scholar]
- 48.Cottart CH, Nivet-Antoine V, Laguillier-Morizot C, Beaudeux JL. Resveratrol bioavailability and toxicity in humans. Mol Nutr Food Res. 2010;54:7–16. doi: 10.1002/mnfr.200900437. [DOI] [PubMed] [Google Scholar]
- 49.Crowell JA, Korytko PJ, Morrissey RL, Booth TD, Levine BS. Resveratrol-associated renal toxicity. Toxicol Sci. 2004;82:614–619. doi: 10.1093/toxsci/kfh263. [DOI] [PubMed] [Google Scholar]
- 50.Williams LD, Burdock GA, Edwards JA, Beck M, Bausch J. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47:2170–2182. doi: 10.1016/j.fct.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 51.Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, Gharbi N, Kamoun A, El-Fazaa S. Protective effect of resveratrol on ethanol-induced lipid peroxidation in rats. Alcohol Alcohol. 2006;41:236–239. doi: 10.1093/alcalc/agh256. [DOI] [PubMed] [Google Scholar]
- 52.Sener G, Toklu HZ, Sehirli AO, Velioğlu-Oğünç A, Cetinel S, Gedik N. Protective effects of resveratrol against acetaminophen-induced toxicity in mice. Hepatol Res. 2006;35:62–68. doi: 10.1016/j.hepres.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 53.Ara C, Kirimlioglu H, Karabulut AB, Coban S, Ay S, Harputluoglu M, Kirimlioglu V, Yilmaz S. Protective effect of resveratrol against oxidative stress in cholestasis. J Surg Res. 2005;127:112–117. doi: 10.1016/j.jss.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Rivera H, Shibayama M, Tsutsumi V, Perez-Alvarez V, Muriel P. Resveratrol and trimethylated resveratrol protect from acute liver damage induced by CCl4 in the rat. J Appl Toxicol. 2008;28:147–155. doi: 10.1002/jat.1260. [DOI] [PubMed] [Google Scholar]
- 55.Walle T, Hsieh F, DeLegge MH, Oatis JE, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 56.Cheng AS, Cheng YH, Chiou CH, Chang TL. Resveratrol upregulates Nrf2 expression to attenuate methylglyoxal-induced insulin resistance in Hep G2 cells. J Agric Food Chem. 2012;60:9180–9187. doi: 10.1021/jf302831d. [DOI] [PubMed] [Google Scholar]
- 57.Bagul PK, Middela H, Matapally S, Padiya R, Bastia T, Madhusudana K, Reddy BR, Chakravarty S, Banerjee SK. Attenuation of insulin resistance, metabolic syndrome and hepatic oxidative stress by resveratrol in fructose-fed rats. Pharmacol Res. 2012;66:260–268. doi: 10.1016/j.phrs.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 58.Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 59.Price NL, Gomes AP, Ling AJ, Duarte FV, Martin-Montalvo A, North BJ, Agarwal B, Ye L, Ramadori G, Teodoro JS, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu W, Chen S, Li Z, Zhao X, Li W, Sun Y, Zhang Z, Ling W, Feng X. Effects and mechanisms of resveratrol on the amelioration of oxidative stress and hepatic steatosis in KKAy mice. Nutr Metab (Lond) 2014;11:35. doi: 10.1186/1743-7075-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piver B, Berthou F, Dreano Y, Lucas D. Inhibition of CYP3A, CYP1A and CYP2E1 activities by resveratrol and other non volatile red wine components. Toxicol Lett. 2001;125:83–91. doi: 10.1016/s0378-4274(01)00418-0. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Jiang Y, Fan X, Tan H, Zeng H, Wang Y, Chen P, Huang M, Bi H. Hepato-protective effect of resveratrol against acetaminophen-induced liver injury is associated with inhibition of CYP-mediated bioactivation and regulation of SIRT1-p53 signaling pathways. Toxicol Lett. 2015;236:82–89. doi: 10.1016/j.toxlet.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 63.Wu X, Li C, Xing G, Qi X, Ren J. Resveratrol Downregulates Cyp2e1 and Attenuates Chemically Induced Hepatocarcinogenesis in SD Rats. J Toxicol Pathol. 2013;26:385–392. doi: 10.1293/tox.2013-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Faghihzadeh F, Adibi P, Rafiei R, Hekmatdoost A. Resveratrol supplementation improves inflammatory biomarkers in patients with nonalcoholic fatty liver disease. Nutr Res. 2014;34:837–843. doi: 10.1016/j.nutres.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Chachay VS, Macdonald GA, Martin JH, Whitehead JP, O’Moore-Sullivan TM, Lee P, Franklin M, Klein K, Taylor PJ, Ferguson M, et al. Resveratrol does not benefit patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2014;12:2092–103.e1-6. doi: 10.1016/j.cgh.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 66.Chan CC, Cheng LY, Lin CL, Huang YH, Lin HC, Lee FY. The protective role of natural phytoalexin resveratrol on inflammation, fibrosis and regeneration in cholestatic liver injury. Mol Nutr Food Res. 2011;55:1841–1849. doi: 10.1002/mnfr.201100374. [DOI] [PubMed] [Google Scholar]
- 67.Shin JW, Wang JH, Kang JK, Son CG. Experimental evidence for the protective effects of coffee against liver fibrosis in SD rats. J Sci Food Agric. 2010;90:450–455. doi: 10.1002/jsfa.3838. [DOI] [PubMed] [Google Scholar]
- 68.Spiller MA. The chemical components of coffee. In: Spiller GA, editor. Caffeine. Boca Raton, USA: CRC Press; 1998. pp. 97–161. [Google Scholar]
- 69.Daglia M, Papetti A, Gregotti C, Bertè F, Gazzani G. In vitro antioxidant and ex vivo protective activities of green and roasted coffee. J Agric Food Chem. 2000;48:1449–1454. doi: 10.1021/jf990510g. [DOI] [PubMed] [Google Scholar]
- 70.McCusker RR, Goldberger BA, Cone EJ. Caffeine content of specialty coffees. J Anal Toxicol. 2003;27:520–522. doi: 10.1093/jat/27.7.520. [DOI] [PubMed] [Google Scholar]
- 71.Muriel P, Arauz J. Coffee and liver diseases. Fitoterapia. 2010;81:297–305. doi: 10.1016/j.fitote.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 72.Arauz J, Moreno MG, Cortés-Reynosa P, Salazar EP, Muriel P. Coffee attenuates fibrosis by decreasing the expression of TGF-β and CTGF in a murine model of liver damage. J Appl Toxicol. 2013;33:970–979. doi: 10.1002/jat.2788. [DOI] [PubMed] [Google Scholar]
- 73.Ibrahim NK, Iftikhar R. Energy drinks: Getting wings but at what health cost? Pak J Med Sci. 2014;30:1415–1419. doi: 10.12669/pjms.306.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Worthley MI, Prabhu A, De Sciscio P, Schultz C, Sanders P, Willoughby SR. Detrimental effects of energy drink consumption on platelet and endothelial function. Am J Med. 2010;123:184–187. doi: 10.1016/j.amjmed.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 75.Smith A. Effects of caffeine on human behavior. Food Chem Toxicol. 2002;40:1243–1255. doi: 10.1016/s0278-6915(02)00096-0. [DOI] [PubMed] [Google Scholar]
- 76.Papadelis C, Kourtidou-Papadeli C, Vlachogiannis E, Skepastianos P, Bamidis P, Maglaveras N, Pappas K. Effects of mental workload and caffeine on catecholamines and blood pressure compared to performance variations. Brain Cogn. 2003;51:143–154. doi: 10.1016/s0278-2626(02)00530-4. [DOI] [PubMed] [Google Scholar]
- 77.Rogers PJ. Caffeine, mood and mental performance in everyday life. Nutrition Bulletin. 2007;32:84–89. [Google Scholar]
- 78.Gajewska M, Paini A, Sala Benito JV, Burton J, Worth A, Urani C, Briesen H, Schramm KW. In vitro-to-in vivo correlation of the skin penetration, liver clearance and hepatotoxicity of caffeine. Food Chem Toxicol. 2015;75:39–49. doi: 10.1016/j.fct.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 79.McCall AL, Millington WR, Wurtman RJ. Blood-brain barrier transport of caffeine: dose-related restriction of adenine transport. Life Sci. 1982;31:2709–2715. doi: 10.1016/0024-3205(82)90715-9. [DOI] [PubMed] [Google Scholar]
- 80.Stalmach A. Bioavailability of coffee chlorogenic acid. In: Chu YF, editor. Coffee: Emerging health effects and disease prevention. West Sussex, UK: IFT Press/Wiley-Blackwell; 2012. pp. 60–76. [Google Scholar]
- 81.Klatsky AL, Armstrong MA. Alcohol, smoking, coffee, and cirrhosis. Am J Epidemiol. 1992;136:1248–1257. doi: 10.1093/oxfordjournals.aje.a116433. [DOI] [PubMed] [Google Scholar]
- 82.Corrao G, Lepore AR, Torchio P, Valenti M, Galatola G, D’Amicis A, Aricó S, di Orio F. The effect of drinking coffee and smoking cigarettes on the risk of cirrhosis associated with alcohol consumption. A case-control study. Provincial Group for the Study of Chronic Liver Disease. Eur J Epidemiol. 1994;10:657–664. doi: 10.1007/BF01719277. [DOI] [PubMed] [Google Scholar]
- 83.Morisco F, Lembo V, Mazzone G, Camera S, Caporaso N. Coffee and liver health. J Clin Gastroenterol. 2014;48 Suppl 1:S87–S90. doi: 10.1097/MCG.0000000000000240. [DOI] [PubMed] [Google Scholar]
- 84.La Vecchia C. Coffee, liver enzymes, cirrhosis and liver cancer. J Hepatol. 2005;42:444–446. doi: 10.1016/j.jhep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 85.Ruhl CE, Everhart JE. Coffee and caffeine consumption reduce the risk of elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2005;128:24–32. doi: 10.1053/j.gastro.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 86.Moreno MG, Chávez E, Aldaba-Muruato LR, Segovia J, Vergara P, Tsutsumi V, Shibayama M, Rivera-Espinoza Y, Muriel P. Coffee prevents CCl(4)-induced liver cirrhosis in the rat. Hepatol Int. 2011;5:857–863. doi: 10.1007/s12072-010-9247-6. [DOI] [PubMed] [Google Scholar]
- 87.Cavin C, Marin-Kuan M, Langouët S, Bezençon C, Guignard G, Verguet C, Piguet D, Holzhäuser D, Cornaz R, Schilter B. Induction of Nrf2-mediated cellular defenses and alteration of phase I activities as mechanisms of chemoprotective effects of coffee in the liver. Food Chem Toxicol. 2008;46:1239–1248. doi: 10.1016/j.fct.2007.09.099. [DOI] [PubMed] [Google Scholar]
- 88.Higgins LG, Cavin C, Itoh K, Yamamoto M, Hayes JD. Induction of cancer chemopreventive enzymes by coffee is mediated by transcription factor Nrf2. Evidence that the coffee-specific diterpenes cafestol and kahweol confer protection against acrolein. Toxicol Appl Pharmacol. 2008;226:328–337. doi: 10.1016/j.taap.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 89.Hertog MG, Hollman PC, Katan MB, Kromhout D. Intake of potentially anticarcinogenic flavonoids and their determinants in adults in The Netherlands. Nutr Cancer. 1993;20:21–29. doi: 10.1080/01635589309514267. [DOI] [PubMed] [Google Scholar]
- 90.Harwood M, Danielewska-Nikiel B, Borzelleca JF, Flamm GW, Williams GM, Lines TC. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 91.Hernández-Ortega LD, Alcántar-Díaz BE, Ruiz-Corro LA, Sandoval-Rodriguez A, Bueno-Topete M, Armendariz-Borunda J, Salazar-Montes AM. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J Gastroenterol Hepatol. 2012;27:1865–1872. doi: 10.1111/j.1440-1746.2012.07262.x. [DOI] [PubMed] [Google Scholar]
- 92.Bors W, Heller W, Michel C, Saran M. Radical chemistry of flavonoid antioxidants. In: Emerit I, Packer L, Auclair C, editors. Antioxidants in Therapy and Preventive Medicine (Advances in Experimental Medicine and Biology) New York, USA: Plenum Press; 1990. pp. 165–170. [DOI] [PubMed] [Google Scholar]
- 93.Mira L, Fernandez MT, Santos M, Rocha R, Florêncio MH, Jennings KR. Interactions of flavonoids with iron and copper ions: a mechanism for their antioxidant activity. Free Radic Res. 2002;36:1199–1208. doi: 10.1080/1071576021000016463. [DOI] [PubMed] [Google Scholar]
- 94.Murota K, Terao J. Quercetin appears in the lymph of unanesthetized rats as its phase II metabolites after administered into the stomach. FEBS Lett. 2005;579:5343–5346. doi: 10.1016/j.febslet.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 95.Murota K, Shimizu S, Chujo H, Moon JH, Terao J. Efficiency of absorption and metabolic conversion of quercetin and its glucosides in human intestinal cell line Caco-2. Arch Biochem Biophys. 2000;384:391–397. doi: 10.1006/abbi.2000.2123. [DOI] [PubMed] [Google Scholar]
- 96.Crespy V, Morand C, Manach C, Besson C, Demigne C, Remesy C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am J Physiol. 1999;277:G120–G126. doi: 10.1152/ajpgi.1999.277.1.G120. [DOI] [PubMed] [Google Scholar]
- 97.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radic Biol Med. 2002;33:220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 98.Pavanato A, Tuñón MJ, Sánchez-Campos S, Marroni CA, Llesuy S, González-Gallego J, Marroni N. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824–829. doi: 10.1023/a:1022869716643. [DOI] [PubMed] [Google Scholar]
- 99.de David C, Rodrigues G, Bona S, Meurer L, González-Gallego J, Tuñón MJ, Marroni NP. Role of quercetin in preventing thioacetamide-induced liver injury in rats. Toxicol Pathol. 2011;39:949–957. doi: 10.1177/0192623311418680. [DOI] [PubMed] [Google Scholar]
- 100.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem Biol Interact. 2012;195:154–164. doi: 10.1016/j.cbi.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 101.Tanigawa S, Fujii M, Hou DX. Action of Nrf2 and Keap1 in ARE-mediated NQO1 expression by quercetin. Free Radic Biol Med. 2007;42:1690–1703. doi: 10.1016/j.freeradbiomed.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 102.Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23. doi: 10.1016/j.freeradbiomed.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 103.Tang Y, Tian H, Shi Y, Gao C, Xing M, Yang W, Bao W, Wang D, Liu L, Yao P. Quercetin suppressed CYP2E1-dependent ethanol hepatotoxicity via depleting heme pool and releasing CO. Phytomedicine. 2013;20:699–704. doi: 10.1016/j.phymed.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 104.Surapaneni KM, Priya VV, Mallika J. Pioglitazone, quercetin and hydroxy citric acid effect on cytochrome P450 2E1 (CYP2E1) enzyme levels in experimentally induced non alcoholic steatohepatitis (NASH) Eur Rev Med Pharmacol Sci. 2014;18:2736–2741. [PubMed] [Google Scholar]
- 105.Zhou JQ, Tang ZQ. Effect of quercetin on CYP1A2, CYP2E1, CYP3A2 activities and its inhibitory mechanism studies in rat liver microsomes. J Chin Pharm Sci. 2005;14:231–236. [Google Scholar]
- 106.Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem Toxicol. 2013;52:53–60. doi: 10.1016/j.fct.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 107.Bahmani M, Shirzad H, Rafieian S, Rafieian-Kopaei M. Silybum marianum: Beyond Hepatoprotection. J Evid Based Complementary Altern Med. 2015:Epub ahead of print. doi: 10.1177/2156587215571116. [DOI] [PubMed] [Google Scholar]
- 108.El-Kamary SS, Shardell MD, Abdel-Hamid M, Ismail S, El-Ateek M, Metwally M, Mikhail N, Hashem M, Mousa A, Aboul-Fotouh A, et al. A randomized controlled trial to assess the safety and efficacy of silymarin on symptoms, signs and biomarkers of acute hepatitis. Phytomedicine. 2009;16:391–400. doi: 10.1016/j.phymed.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dixit N, Baboota S, Kohli K, Ahmad S, Ali J. Silymarin: A review of pharmacological aspects and bioavailability enhancement approaches. Indian J Pharmacol. 2007;39:172–179. [Google Scholar]
- 110.Hutchinson C, Bomford A, Geissler CA. The iron-chelating potential of silybin in patients with hereditary haemochromatosis. Eur J Clin Nutr. 2010;64:1239–1241. doi: 10.1038/ejcn.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Pietrangelo A, Borella F, Casalgrandi G, Montosi G, Ceccarelli D, Gallesi D, Giovannini F, Gasparetto A, Masini A. Antioxidant activity of silybin in vivo during long-term iron overload in rats. Gastroenterology. 1995;109:1941–1949. doi: 10.1016/0016-5085(95)90762-9. [DOI] [PubMed] [Google Scholar]
- 112.Hagag AA, Elfatah MA. Therapeutic value of silymarin as iron chelator in children with beta thalassemia with iron overload. J Leuk. 2014;42:1–6. [Google Scholar]
- 113.Najafzadeh H, Jalali MR, Morovvati H, Taravati F. Comparison of the prophylactic effect of silymarin and deferoxamine on iron overload-induced hepatotoxicity in rat. J Med Toxicol. 2010;6:22–26. doi: 10.1007/s13181-010-0030-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mata-Santos HA, Dutra FF, Rocha CC, Lino FG, Xavier FR, Chinalia LA, Hossy BH, Castelo-Branco MT, Teodoro AJ, Paiva CN, et al. Silymarin reduces profibrogenic cytokines and reverses hepatic fibrosis in chronic murine schistosomiasis. Antimicrob Agents Chemother. 2014;58:2076–2083. doi: 10.1128/AAC.01936-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Muriel P, Garciapiña T, Perez-Alvarez V, Mourelle M. Silymarin protects against paracetamol-induced lipid peroxidation and liver damage. J Appl Toxicol. 1992;12:439–442. doi: 10.1002/jat.2550120613. [DOI] [PubMed] [Google Scholar]
- 116.Mourelle M, Muriel P, Favari L, Franco T. Prevention of CCL4-induced liver cirrhosis by silymarin. Fundam Clin Pharmacol. 1989;3:183–191. doi: 10.1111/j.1472-8206.1989.tb00449.x. [DOI] [PubMed] [Google Scholar]
- 117.Muriel P, Mourelle M. Prevention by silymarin of membrane alterations in acute CCl4 liver damage. J Appl Toxicol. 1990;10:275–279. doi: 10.1002/jat.2550100408. [DOI] [PubMed] [Google Scholar]
- 118.Muriel P, Mourelle M. The role of membrane composition in ATPase activities of cirrhotic rat liver: effect of silymarin. J Appl Toxicol. 1990;10:281–284. doi: 10.1002/jat.2550100409. [DOI] [PubMed] [Google Scholar]
- 119.Muriel P, Moreno MG, Hernández Mdel C, Chávez E, Alcantar LK. Resolution of liver fibrosis in chronic CCl4 administration in the rat after discontinuation of treatment: effect of silymarin, silibinin, colchicine and trimethylcolchicinic acid. Basic Clin Pharmacol Toxicol. 2005;96:375–380. doi: 10.1111/j.1742-7843.2005.pto_06.x. [DOI] [PubMed] [Google Scholar]
- 120.Kim M, Yang SG, Kim JM, Lee JW, Kim YS, Lee JI. Silymarin suppresses hepatic stellate cell activation in a dietary rat model of non-alcoholic steatohepatitis: analysis of isolated hepatic stellate cells. Int J Mol Med. 2012;30:473–479. doi: 10.3892/ijmm.2012.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tzeng JI, Chen MF, Chung HH, Cheng JT. Silymarin decreases connective tissue growth factor to improve liver fibrosis in rats treated with carbon tetrachloride. Phytother Res. 2013;27:1023–1028. doi: 10.1002/ptr.4829. [DOI] [PubMed] [Google Scholar]
- 122.Kiruthiga PV, Pandian SK, Devi KP. Silymarin protects PBMC against B(a)P induced toxicity by replenishing redox status and modulating glutathione metabolizing enzymes--an in vitro study. Toxicol Appl Pharmacol. 2010;247:116–128. doi: 10.1016/j.taap.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 123.Vailati A, Aristia L, Sozze E, Milani F, Inglese V, Calenda PO, Gossolo PA, Ascari E. Randomized open study of the dose-effect relationship of a short course of IdB 1016 (Silybin Phytosome) in patients with viral or alcoholic hepatitis. Fitoterapia. 1993;94:219–228. [Google Scholar]
- 124.Magliulo E, Gagliardi B, Fiori GP. [Results of a double blind study on the effect of silymarin in the treatment of acute viral hepatitis, carried out at two medical centres (author’s transl)] Med Klin. 1978;73:1060–1065. [PubMed] [Google Scholar]
- 125.Ferenci P, Dragosics B, Dittrich H, Frank H, Benda L, Lochs H, Meryn S, Base W, Schneider B. Randomized controlled trial of silymarin treatment in patients with cirrhosis of the liver. J Hepatol. 1989;9:105–113. doi: 10.1016/0168-8278(89)90083-4. [DOI] [PubMed] [Google Scholar]
- 126.Parés A, Planas R, Torres M, Caballería J, Viver JM, Acero D, Panés J, Rigau J, Santos J, Rodés J. Effects of silymarin in alcoholic patients with cirrhosis of the liver: results of a controlled, double-blind, randomized and multicenter trial. J Hepatol. 1998;28:615–621. doi: 10.1016/s0168-8278(98)80285-7. [DOI] [PubMed] [Google Scholar]
- 127.Patel K, Singh GK, Patel DK. A review on pharmacological and analytical aspects of naringenin. Chin J Integr Med. 2014:Epub ahead of print. doi: 10.1007/s11655-014-1960-x. [DOI] [PubMed] [Google Scholar]
- 128.Yang CP, Liu MH, Zou W, Guan XL, Lai L, Su WW. Toxicokinetics of naringin and its metabolite naringenin after 180-day repeated oral administration in beagle dogs assayed by a rapid resolution liquid chromatography/tandem mass spectrometric method. J Asian Nat Prod Res. 2012;14:68–75. doi: 10.1080/10286020.2011.632369. [DOI] [PubMed] [Google Scholar]
- 129.Surampalli G, Nanjwade BK, Patil PA. Safety evaluation of naringenin upon experimental exposure on rat gastrointestinal epithelium for novel optimal drug delivery. Drug Deliv. 2014:Epub ahead of print. doi: 10.3109/10717544.2014.923957. [DOI] [PubMed] [Google Scholar]
- 130.Erlund I. Review of the flavonoids quercetin, hesperetin, and naringenin. Dietary sources, bioactivities, bioavailability, and epidemiology. Nutr Res. 2004;24:851–874. [Google Scholar]
- 131.Chtourou Y, Fetoui H, Jemai R, Ben Slima A, Makni M, Gdoura R. Naringenin reduces cholesterol-induced hepatic inflammation in rats by modulating matrix metalloproteinases-2, 9 via inhibition of nuclear factor κB pathway. Eur J Pharmacol. 2015;746:96–105. doi: 10.1016/j.ejphar.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 132.Yen FL, Wu TH, Lin LT, Cham TM, Lin CC. Naringenin-loaded nanoparticles improve the physicochemical properties and the hepatoprotective effects of naringenin in orally-administered rats with CCl(4)-induced acute liver failure. Pharm Res. 2009;26:893–902. doi: 10.1007/s11095-008-9791-0. [DOI] [PubMed] [Google Scholar]
- 133.Goldwasser J, Cohen PY, Lin W, Kitsberg D, Balaguer P, Polyak SJ, Chung RT, Yarmush ML, Nahmias Y. Naringenin inhibits the assembly and long-term production of infectious hepatitis C virus particles through a PPAR-mediated mechanism. J Hepatol. 2011;55:963–971. doi: 10.1016/j.jhep.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Cho KW, Kim YO, Andrade JE, Burgess JR, Kim YC. Dietary naringenin increases hepatic peroxisome proliferators-activated receptor α protein expression and decreases plasma triglyceride and adiposity in rats. Eur J Nutr. 2011;50:81–88. doi: 10.1007/s00394-010-0117-8. [DOI] [PubMed] [Google Scholar]
- 135.Han X, Pan J, Ren D, Cheng Y, Fan P, Lou H. Naringenin-7-O-glucoside protects against doxorubicin-induced toxicity in H9c2 cardiomyocytes by induction of endogenous antioxidant enzymes. Food Chem Toxicol. 2008;46:3140–3146. doi: 10.1016/j.fct.2008.06.086. [DOI] [PubMed] [Google Scholar]
- 136.Esmaeili MA, Alilou M. Naringenin attenuates CCl4 -induced hepatic inflammation by the activation of an Nrf2-mediated pathway in rats. Clin Exp Pharmacol Physiol. 2014;41:416–422. doi: 10.1111/1440-1681.12230. [DOI] [PubMed] [Google Scholar]
- 137.Motawi TK, Teleb ZA, El-Boghdady NA, Ibrahim SA. Effect of simvastatin and naringenin coadministration on rat liver DNA fragmentation and cytochrome P450 activity: an in vivo and in vitro study. J Physiol Biochem. 2014;70:225–237. doi: 10.1007/s13105-013-0296-x. [DOI] [PubMed] [Google Scholar]
- 138.Hermenean A, Ardelean A, Stan M, Hadaruga N, Mihali CV, Costache M, Dinischiotu A. Antioxidant and hepatoprotective effects of naringenin and its β-cyclodextrin formulation in mice intoxicated with carbon tetrachloride: a comparative study. J Med Food. 2014;17:670–677. doi: 10.1089/jmf.2013.0007. [DOI] [PubMed] [Google Scholar]
- 139.Castro J, Pregibon T, Chumanov K, Marcus RK. Determination of catechins and caffeine in proposed green tea standard reference materials by liquid chromatography-particle beam/electron ionization mass spectrometry (LC-PB/EIMS) Talanta. 2010;82:1687–1695. doi: 10.1016/j.talanta.2010.07.054. [DOI] [PubMed] [Google Scholar]
- 140.Mazzanti G, Sotto AD, Vitalone A. Hepatoxicity of green tea: an update. Arch Toxicol. 2015:Epub ahead of print. doi: 10.1007/s00204-015-1521-x. [DOI] [PubMed] [Google Scholar]
- 141.Pérez-Vargas JE, Zarco N, Vergara P, Shibayama M, Segovia J, Tsutsumi V, Muriel P. l-Theanine prevents carbon tetrachloride-induced liver fibrosis via inhibition of nuclear factor κB and down-regulation of transforming growth factor β and connective tissue growth factor. Hum Exp Toxicol. 2015:Epub ahead of print. doi: 10.1177/0960327115578864. [DOI] [PubMed] [Google Scholar]
- 142.Yu DK, Zhang CX, Zhao SS, Zhang SH, Zhang H, Cai SY, Shao RG, He HW. The anti-fibrotic effects of epigallocatechin-3-gallate in bile duct-ligated cholestatic rats and human hepatic stellate LX-2 cells are mediated by the PI3K/Akt/Smad pathway. Acta Pharmacol Sin. 2015;36:473–482. doi: 10.1038/aps.2014.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bin Dajem SM, Shati AA, Adly MA, Ahmed OM, Ibrahim EH, Mostafa OM. Green tea (Camellia sinesis) ameliorates female Schistosoma mansoni-induced changes in the liver of Balb/C mice. Saudi J Biol Sci. 2011;18:361–368. doi: 10.1016/j.sjbs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, Enjoji M, Kobayashi N, Nakamuta M. Epigallocatechin-3-gallate, a green-tea polyphenol, suppresses Rho signaling in TWNT-4 human hepatic stellate cells. J Lab Clin Med. 2005;145:316–322. doi: 10.1016/j.lab.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 145.Sahin K, Tuzcu M, Gencoglu H, Dogukan A, Timurkan M, Sahin N, Aslan A, Kucuk O. Epigallocatechin-3-gallate activates Nrf2/HO-1 signaling pathway in cisplatin-induced nephrotoxicity in rats. Life Sci. 2010;87:240–245. doi: 10.1016/j.lfs.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 146.Hosseini A, Ghorbani A. Cancer therapy with phytochemicals: evidence from clinical studies. Avicenna J Phytomed. 2015;5:84–97. [PMC free article] [PubMed] [Google Scholar]
- 147.Darvesh AS, Bishayee A. Chemopreventive and therapeutic potential of tea polyphenols in hepatocellular cancer. Nutr Cancer. 2013;65:329–344. doi: 10.1080/01635581.2013.767367. [DOI] [PubMed] [Google Scholar]
- 148.Halegoua-De Marzio D, Kraft WK, Daskalakis C, Ying X, Hawke RL, Navarro VJ. Limited sampling estimates of epigallocatechin gallate exposures in cirrhotic and noncirrhotic patients with hepatitis C after single oral doses of green tea extract. Clin Ther. 2012;34:2279–2285.e1. doi: 10.1016/j.clinthera.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 149.Hackett ES, Twedt DC, Gustafson DL. Milk thistle and its derivative compounds: a review of opportunities for treatment of liver disease. J Vet Intern Med. 2013;27:10–16. doi: 10.1111/jvim.12002. [DOI] [PubMed] [Google Scholar]
- 150.Lambert JD, Elias RJ. The antioxidant and pro-oxidant activities of green tea polyphenols: a role in cancer prevention. Arch Biochem Biophys. 2010;501:65–72. doi: 10.1016/j.abb.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ak T, Gülçin I. Antioxidant and radical scavenging properties of curcumin. Chem Biol Interact. 2008;174:27–37. doi: 10.1016/j.cbi.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 152.Gülçin I. Antioxidant properties of resveratrol: A structure-activity insight. Innov Food Sci Emerg Technol. 2010;11:210–218. [Google Scholar]
- 153.Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med. 1997;22:749–760. doi: 10.1016/s0891-5849(96)00351-6. [DOI] [PubMed] [Google Scholar]
- 154.Chu YF, Chen Y, Brown PH, Lyle BJ, Black RM, Cheng IH, Ou B, Prior RL. Bioactivities of crude caffeine: Antioxidant activity, ciclooxygenase-2 inhibition, and enhanced glucose uptake. Food Chem. 2012;131:564–568. [Google Scholar]


