Abstract
Postmenopausal disorders are frequently observed in various organs, but their relationship with estrogen deficiency and mechanisms remain unclear. As tissue-specific stem cells have been found to express estrogen receptors, we examined the hypothesis that estrogen deficiency impairs stem cells, which consequently contributes to postmenopausal disorders. Six-week-old C57BL/6 female mice were ovariectomized, following which they received 17β-estradiol replacement or vehicle (control). Sham-operated mice were used as healthy controls. All mice were killed for evaluation 2 months after treatments. Compared with the healthy control, ovariectomy significantly decreased uterine weight, which was partially recovered by 17β-estradiol replacement. Ovariectomy significantly increased the numbers of c-kit-positive hematopoietic stem/progenitor cells in bone marrow, but impaired their capacity to grow mixed cell-type colonies in vitro. Estrogen replacement further increased the numbers of c-kit-positive hematopoietic stem/progenitor cells in bone marrow, without significantly affecting colony growth in vitro. The number of CD105-positive mesenchymal stem cells in bone marrow also significantly decreased after ovariectomy, but completely recovered following estrogen replacement. Otherwise, neither ovariectomy nor estrogen replacement changed the number of Pax7-positive satellite cells, which are a skeletal muscle-type stem cell. Estrogen deficiency heterogeneously affected tissue-specific stem cells, suggesting a likely and direct relationship with postmenopausal disorders.
The physiological changes caused by urogenital atrophy can induce a number of pathological disorders, such as osteoporosis, thromboembolism, arteriosclerosis, and muscle atrophy, all of which are frequently observed following menopause1. These pathological disorders are considered to be associated, at least partially, with ovarian dysfunction, especially estrogen deficiency2. Therefore, hormone replacement therapy (HRT) is popularly accepted to prevent/alleviate these postmenopausal disorders3. HRT has been found to be beneficial, but is also accompanied by side effects and may induce some complications of its own4. As many other common pathogenic factors are also well known to contribute to these disorders, we must confirm their relationship with estrogen deficiency and gain further understanding of the relevant mechanisms at the molecular and cellular levels.
The rapid advancement on stem cell research in past decades has clearly identified stem cells in almost all tissues/organs of our body, and these tissue specific stem/progenitor cells are functionally known to be critical in the repair/regeneration of tissue/organs due to physiological turnover or pathological damage5,6. Aging and many other pathogenic factors, such as diabetes mellitus, can impair these tissue-specific stem cells7. Interestingly, recent studies have found that the resident stem cells in different tissues/organs, including hematopoietic stem cells, endothelial progenitors, mesenchymal stem cells, and satellite cells (skeletal muscle-type stem cells) express estrogen receptors8,9,10,11. It has also been reported that estrogen plays critical roles in regulating the survival and differentiation of either embryonic stem cells or tissue-specific stem/progenitor cells12. Considering the potential roles of estrogen in maintaining stem cells, it is quite possible that estrogen deficiency causes a decrease in the quantity and quality of stem/progenitor cells, which consequently contributes to postmenopausal disorders in various tissues/organs.
Using an ovariectomy model in young healthy female mice, we herein examined how estrogen deficiency could affect hematopoietic and mesenchymal stem cells in bone marrow, as well as satellite cells in muscles.
Results
Estrogen deficiency induced uterine atrophy and increased body weight
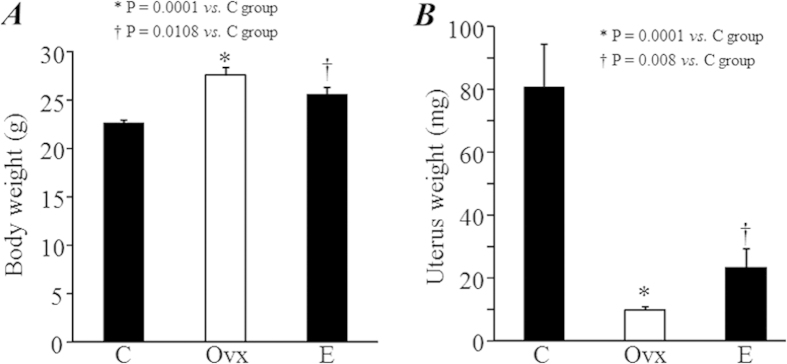
The body weight of mice was significantly higher in the Ovx and E groups compared with C group (P = 0.0001 and 0.0108, Fig. 1A). The uterine weight was significantly lower in the Ovx group compared with E and C groups (P = 0.0001, Fig. 1A), although the uterine weight was also significantly lower in the E group compared with the C group (P = 0.008, Fig. 1B). These results indicate that partial atrophy of the uterus occurred following estrogen replacement during the 2 months after ovariectomy.
Figure 1. Body weight and uterine weight.
Body weight (A) and uterine weight (B) were measured 2 months after treatments.
Estrogen deficiency increased the number, but impaired the function, of c-kit+ hematopoietic stem/progenitor cells
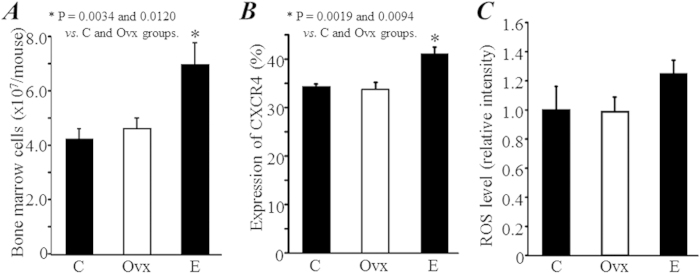
Although all procedures were performed with well-trained skill and a defined protocol, we collected significantly more BM-MNCs in the E group compared with the C and Ovx groups (P = 0.0019 and 0.0094, Fig. 2A). The expression of CXCR4 in BM-MNCs did not differ between the C and Ovx groups, but was significantly enhanced in the E group (P = 0.002 and 0.009 vs. C and Ovx groups, Fig. 2B). The intracellular ROS level in BM-MNCs was not significantly different among groups, although it was slightly increased in the E group (Fig. 2C).
Figure 2. The expression of CXCR4 and intracellular ROS levesl in bone marrow mononuclear cells (BM-MNCs).
(A) The bone marrow was collected from the femur and tibia 2 months after treatments, and the number of total collected mononuclear cells from each mouse was directly counted. (B) The expression of CXCR4 was detected in freshly collected BM-MNCs by flow cytometry. (C) The intracellular ROS level was measured as the mean fluorescence intensity in the freshly collected BM-MNCs after 30 min loading with 10-μM CM-H2DCFDA.
Moreover, compared with healthy mice in the C group, the expression of c-kit, a marker popularly used for identifying hematopoietic stem/progenitors cells, was detected to be significantly higher in the Ovx group (3.27 ± 0.14% vs. 2.65 ± 0.09%, P = 0.0026; Fig. 3). Strangely, the expression of c-kit was even further increased by estrogen replacement (4.87 ± 0.16%, P = 0.0001 vs. C and Ovx groups, Fig. 3).
Figure 3. The number of hematopoietic stem/progenitor cells.
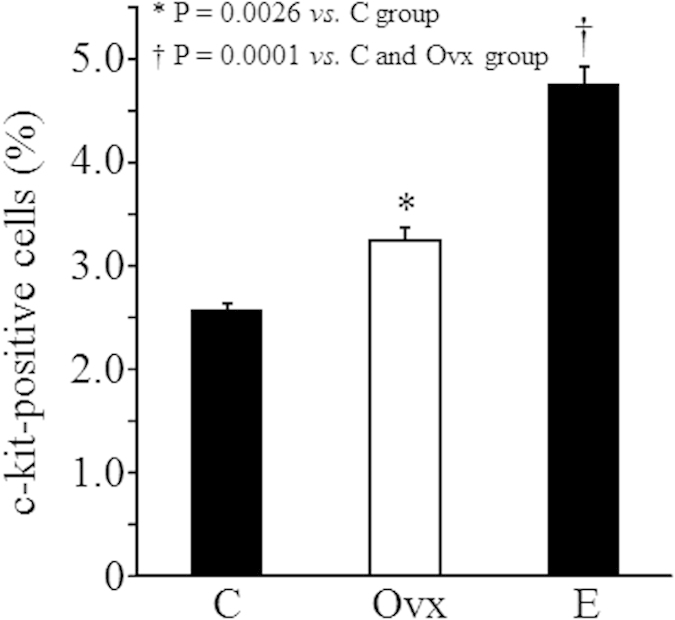
The expression of c-kit, a marker of hematopoietic stem/progenitor cells in bone marrow mononuclear cells, was measured by flow cytometry.
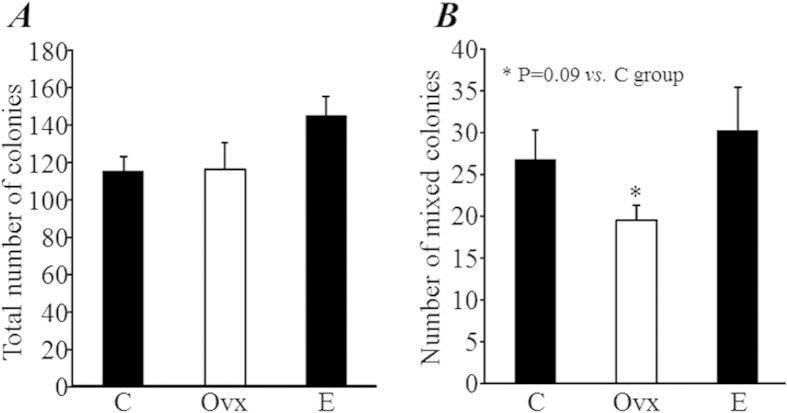
The results of the colony-forming assay, a method well used for evaluating the function of hematopoietic stem/progenitor cells in vitro, showed that the total number of colonies formed from 2 × 104 BM-MNCs was not significantly different among groups (Fig. 4A). Interestingly, the number of colonies of mixed cell type, a subtype of colonies that formed from an even earlier stage of stem/progenitor cells, tended to decrease in the Ovx group compared with the C group (19.5 ± 4.6 vs. 26.7 ± 3.5, P = 0.09; Fig. 4B), but the decrease was completely recovered by estrogen replacement treatment (30.2 ± 5.1, P = 0.57 vs. C group; Fig. 4B).
Figure 4. Colony-forming assay.
Bone marrow mononuclear cells were isolated from mice 2 months after treatments. Colony formation was observed under microscopy at 7 days after incubation. The number of all types of colonies (30 cells, (A)) and mixed cell type colonies (at least two different types of cell in the colony, (B)) were counted.
Estrogen deficiency increased the number of CD105+ mesenchymal stem cells in the bone marrow
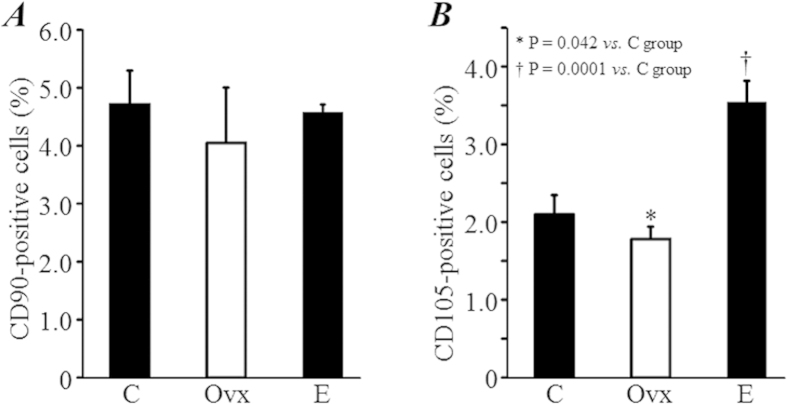
We also measured the expressions of CD90 and CD105, two popular markers used for the identifying mesenchymal stem cells. The expression of CD90 in BM-MNCs did not significantly differ among groups (Fig. 5A). However, the expression of CD105 in BM-MNCs was significantly lower in the Ovx group compared with the C group (1.78 ± 0.25% vs. 2.10 ± 0.16%, P = 0.042; Fig. 5B), and was at the highest level in the E group (3.53 ± 0.29%, P = 0.0001 vs. C group; Fig. 5B).
Figure 5. The number of mesenchymal stem cells.
The expression of CD90 (A) and CD105 (B), two markers for mesenchymal stem cells in bone marrow mononuclear cells, were measured by flow cytometry.
Estrogen deficiency did not change the number of satellite cells in skeletal muscle
By counting the number of Pax7+ satellite cells within the tibialis, we found that there was no significant difference among groups (Fig. 6).
Figure 6. The number of satellite cells.
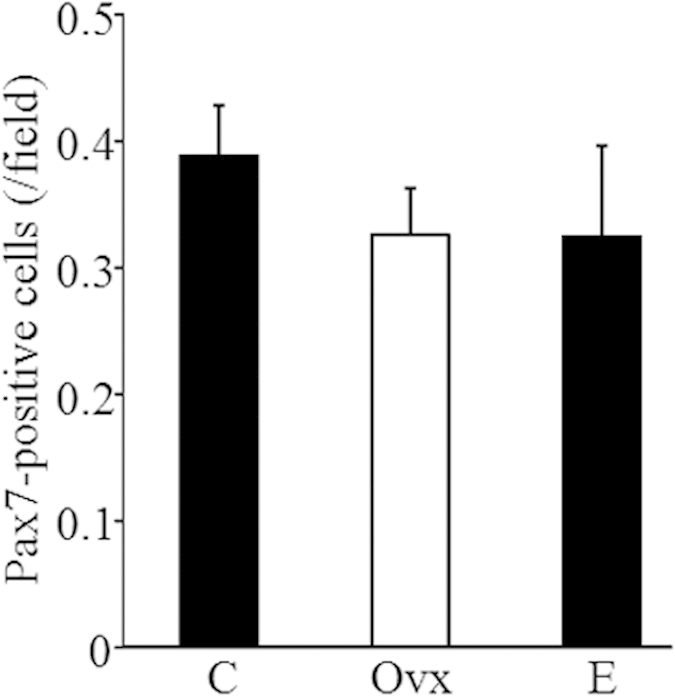
Satellite cells were detected in tibialis anterior muscles by immunostaining with an anti-Pax7 antibody, and the Pax7-positive cells in randomly selected fields was counted under fluorescence microscopy.
Discussion
The present study was designed to examine the hypothesis that estrogen deficiency induces a decrease in the quantity and quality of tissue-specific stem cells, thereby contributing to postmenopausal secondary disorders in different tissues/organs. Using an ovariectomy model in young healthy female mice, we found that estrogen deficiency increased the number, but likely impaired the function, of hematopoietic stem/progenitor cells. Estrogen deficiency also significantly decreased the number of CD105+ mesenchymal stem cells in bone marrow, but did not change the number of Pax7+ satellite cells in skeletal muscles. Our data shows the heterogeneous effects of estrogen deficiency in different types of tissue-specific stem cells, suggesting a likely and direct relationship between the estrogen deficiency-induced impairment of stem cells and postmenopausal disorders.
Although estrogens are generally known as female reproductive hormones, the functions of estrogens in non-reproductive tissues, such as brain, bone, and cardiovascular systems, are well-defined from previous studies13,14,15. The biological activities of estrogens are mediated by two estrogen receptor (ER) isoforms, namely ERα and ERβ15. As stem cells in various tissues express ERs, we examined the role of estrogen in stem cells in vivo. We used the well-established ovariectomy-induced estrogen deficiency model in young healthy female mice, which were subsequently treated with estrogen replacement or placebo. Estrogen deficiency was confirmed by the presence of uterine hypotrophy following ovariectomy. Uterine size was significantly increased by subcutaneous insertion with a slow-release pellet of 17β-estradiol, an easy method popularly used for estrogen replacement. However, compared with healthy mice in the control group, the uterine weight was much lower in mice receiving estrogen replacement after ovariectomy. Although we did not monitor the levels of estrogen in mice, the small size of the uterus might be due to not enough estrogen being released from the slow-release pellet to maintain it at physiological levels, especially during the later periods after subcutaneous insertion.
A recent study found that the treatment with 17β-estradiol enhances the cell cycle and increases the numbers of LSK (lin-/Sca-1+/c-kit+) hematopoietic stem cells in wild-type healthy mice, but not in ERα-knockout mice8. Unexpectedly, our data revealed that bone marrow mononuclear cells from ovariectomized mice contained more c-kit+ hematopoietic stem/progenitor cells, but showed an impaired capacity of growth for colonies of mixed cell type in vitro. Estrogen replacement treatment increased c-kit+ hematopoietic stem/progenitor cells to an even higher level, and also improved the colony forming capacity. Similar to our data, a very recently published study has also observed an increased number of hematopoietic stem cells with impaired function in aged mice16. It is quite possible that estrogen deficiency may also increase the number, but decrease the quality of hematopoietic stem/progenitor cells. However, further study with longer follow-up is required to answer whether estrogen deficiency decreases the number of hematopoietic stem cells. It is also highly required to understand the molecular mechanism on estrogen deficiency-induced increase of c-kit-positive stem/progenitor cells.
The expression of CD105, one of the most popularly used markers for the identifying mesenchymal stem cells, was significantly decreased in BM-MNCs from ovariectomized mice, but increased with estrogen replacement. This suggests that estrogen deficiency might inhibit mesenchymal stem cell function, as it did not change the expression of CD90, another marker of mesenchymal stem cells. It is possible that some mononuclear cells that are not the phenotype of mesenchymal stem cells, such as matured T cells, were positive for CD90, also known as Thy-1, which was originally identified on murine thymocytes and is expressed in T cells17. If this is possible, then estrogen deficiency may reduce the number of mesenchymal stem cells in bone marrow.
Satellite cells are the myogenic progenitor cells of postnatal skeletal muscles and reside at the periphery of myofibers in a quiescent state18. Controversial results have previously been reported on the role of estrogen in regulating satellite cells. It has been found that estrogen increases the number of satellite cells after exercise19, and selective activation of ERβ stimulates skeletal muscle growth and regeneration11. In contrast, estrogen has also been demonstrated to repress myogenic differentiation of satellite cells20. Although these results lack functional analysis, our data revealed that estrogen deficiency did not change the number of satellite cells within 2 months of follow-up. Long-term follow-up is needed to confirm this finding.
The reason for the heterogeneity of estrogen deficiency effects in different types of stem cells remains unclear. Many studies have reported anti-oxidative and anti-inflammatory effects of estrogens, which may be attributed to their phenolic hydroxyl group21. However, the phenolic hydroxyl group present at the C3 position of the A ring of estrogen can be oxidized, either by accepting an electron or by losing a proton22. In addition, estrogens also produce ROS by increasing mitochondrial activity, thus meaning that they also act as pro-oxidants. The controversial role of estrogens as pro-oxidants or anti-oxidants is largely dependent on cell type and different expression levels of ERs in a particular cell. Our data indicate that estrogen deficiency does not significantly change the intracellular ROS level and the expression of CXCR4 in BM-MNCs, suggesting a mineral role of estrogens as an anti-oxidant and anti-inflammatory.
It is critical to compare the expression levels of ERs in different cells after treatments. Unfortunately, we failed to quantify the expression of ERS by flow cytometry due to the quality problem of antibodies. Therefore, we tried to measure the mRNA expression of ERs in c-kit+ hematopoietic stem cells and satellite cells (Supplementary Figure). Interestingly, the expression of ERβ was higher in c-kit+ hematopoietic stem/progenitor cells than the matured c-kit− mononuclear cells from bone marrow. However, the expressions of ERα and ERβ were even lower in the satellite cells compared with differentiated/matured muscle fibers. Although we did not measure the changes of ERs in these cells after different treatments, the effect of estrogen deficiency in stem cells may depend, at least partially, on the expression levels of ERs, which provides reasonable explanation on the heterogeneity of estrogen deficiency-induced changes in different types of stem cells.
In summary, the results of this study revealed that estrogen deficiency heterogeneously affects tissue-specific stem cells. Further study, including a longer follow-up, is required to ascertain the estrogen deficiency-induced impair of tissue-specific stem cells. This may finally uncover the relevant molecular and cellular mechanisms, and provide novel strategy for the prevention and treatment of postmenopausal disorders.
Materials and Methods
Animals
Six-week-old female C57BL/6 mice were used in this study. All animals were purchased from Charles River Laboratories, Kanagawa Japan. All experiments were approved by the Institutional Animal Care and Use Committee of Nagasaki University (No. 120721004), and experiments were performed in accordance with the institutional and national guidelines.
Ovariectomy and estrogen replacement treatment
Mice were ovariectomized under anesthesia, and then randomly received subcutaneous insertion of a placebo pellet (Ovx group, n = 8), or a slow-release 17β-estradiol pellet (0.01 mg/60 days, Innovative Research of America, FL USA) for estrogen replacement (E group, n = 7), as previously described23. Sham-operated mice were used as the healthy control (C group, n = 8). All mice were killed 2 months after treatments. The body weight and uterine weight were measured, and other tissue samplings and assessments were performed as described in the following sections.
Collection of mononuclear cells from bone marrow
Bone marrow cells were collected from the femur and tibia. Bone marrow mononuclear cells (BM-MNCs) were isolated by density gradient centrifugation24,25, and then counted using a Nucleo Counter cell-counting device (Chemotetec A/S, Denmark).
Flow cytometry
To measure the number of c-kit-positive (c-kit+) stem/progenitor cells in the freshly isolated BM-MNCs, we labeled cells with a PE-conjugated anti-mouse c-kit antibody (eBioscience, CA USA) for 30 min. Respective isotype controls were used as a negative control. After washing, quantitative flow cytometry analysis was performed using a FACSCalibur instrument (Becton Dickinson, USA)24,25. The acquired data was analyzed using Cell Quest software (Becton Dickinson).
BM-MNCs were also stained with goat and rat monoclonal antibodies against CD90 (Abcam, UK), CD105 (Abcam), CXCR4 (R&D Systems, USA), followed by their respective FITC-conjugated secondary antibodies. The expressions of CD90, CD105, and CXCR4 in BM-MNCs were analyzed by flow cytometry as described above.
Detection of intracellular levels of reactive oxygen species (ROS)
To measure the intracellular levels of ROS, freshly isolated BM-MNCs were incubated with 10 μM CM-H2DCFDA (Molecular Probes Inc., OR USA), at 37 °C for 30 min26. After washing, the fluorescence intensity in cells was measured by flow cytometry as described above.
Colony-forming assay
The colony-forming capacity of the isolated BM-MNCs was estimated using mouse methylcellulose complete medium27, according to the manufacturer’s instructions (R&D System, MN USA). Briefly, 2 × 104 cells were mixed well with 1 ml of medium, plated in 3-cm culture dishes, and incubated at 37 °C in a 5% CO2 incubator. The formation of colonies was observed under a microscope, and the total number of colonies in each dish was counted after 7 days of incubation. We also counted the number of colonies of mixed cell type, in which at least two different types of cell were growing from a single stem/progenitor cell. The mean number of colonies in duplicate assays was used for statistical analysis.
Immunohistological staining
To detect the presence of satellite cells, tibialis anterior muscles were dissected, frozen in liquid nitrogen, and stored in a −80 °C refrigerator. Muscle tissues were sectioned into 5-μm-thick sections and fixed with 4% paraformaldehyde for 10 min. After blocking with DAKO Protein Block (DAKO, Denmark), sections were incubated with anti-laminin α2 antibody (Alexis, CA, USA) to stain the basal membrane of muscle fibers. Satellite cells that localized to the inside of the skeletal basal membrane of muscle fibers were stained using an anti-Pax7 antibody (Santa Cruz, CA, USA). All immunostained samples were visualized using their respective secondary antibodies conjugated with Alexa Fluor 488 or 568 (Life Technologies, Tokyo, Japan). The nuclei were labeled with DAPI. We counted the number of Pax7-positive satellite cells directly under a fluorescence microscope with 200-fold magnification. We randomly selected at least 10 fields from three sections of each animal, and the average cell count per field was used for statistical analysis.
Statistical analyses
All results are presented as the means ± standard error mean (SEM). The statistical significance was determined by one-way analysis of variance followed by the Bonferroni post hoc test (Dr. SPSS II, Chicago, IL). Differences were considered significant when P < 0.05.
Additional Information
How to cite this article: Kitajima, Y. et al. Estrogen deficiency heterogeneously affects tissue specific stem cells in mice. Sci. Rep. 5, 12861; doi: 10.1038/srep12861 (2015).
Supplementary Material
Acknowledgments
This study was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science, and Technology, Japan. Funding from the Uehara Memorial Foundation and Mochida Memorial Foundation was also received. No additional external funding was received for this study. The founders did not participate in this study.
Footnotes
The authors declare no competing financial interests.
Author Contributions T.L. and H.M. conceived and designed the experiments. Y.K., Y.O., Y.U., S.G., H.D., M.K., K.M. and T.L. performed the experiments and analyzed the data. Y.K. and T.L. wrote the main manuscript text. All authors reviewed the manuscript.
References
- Lobo R. A. Yen and Jaffe’s reproductive endocrinology: physiology, pathophysiology, and clinical management. 5th edn Ch. 14, 421–460 (Elsevier Sanders, 2004).
- Burger H. The menopausal transition-endocrinology. J Sex Med 5, 2266–2273 (2008). [DOI] [PubMed] [Google Scholar]
- American College of Physicians: Guidline for counseling postmenopausal women about preventive hormone therapy. Ann Intern Med. 12, 1038–1041 (1992). [DOI] [PubMed] [Google Scholar]
- Rossouw J. E. et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 288, 321–333 (2002). [DOI] [PubMed] [Google Scholar]
- Prockop D. J. & Olson S. D. Clinical trials with adult stem/progenitor cells for tissue repair: let’s not overlook some essential precautions. Blood. 109, 3147–3151 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. et al. Concise review: bone marrow-derived stem/progenitor cells in cutaneous repair and regeneration. Stem Cells. 28, 905–915 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T. S. et al. Diabetic impairment of c-kit+ bone marrow stem cells involves the disorders of inflammatory factors, cell adhesion and extracellular matrix molecules. PLoS One. 6, e25543 (2011). 10.1371/journal.pone.0025543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illing A. et al. Estradiol increases hematopoietic stem and progenitor cells independent of its actions on bone. Haematologica. 8, 1131–1135 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolego C. et al. Selective estrogen receptor-alpha agonist provides widespread heart and vascular protection with enhanced endothelial progenitor cell mobilization in the absence of uterotrophic action. FASEB J. 7, 2262–2272 (2010). [DOI] [PubMed] [Google Scholar]
- Zhou S. et al. Estrogen modulates estrogen receptor alpha and beta expressiion, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J Cell Biochem. 36 Suppl, 144–155 (2001). [DOI] [PubMed] [Google Scholar]
- Velders M. et al. Selective estrogen receptor-β activation stimulates skeletal muscle growth and regeneration. FASEB J. 26, 1909–1920 (2012). [DOI] [PubMed] [Google Scholar]
- Hong S. H. et al. Expression of estrogen receptor-alpha and -beta, glucocorticoid receptor, and progesterone receptor genes in human embryonic stem cells and embryoid bodies. Mol Cells. 18, 320–325 (2004). [PubMed] [Google Scholar]
- Comasco E. et al. Functional and molecular neuroimaging of menopause and hormone replacement therapy. Front Neurosci. 8, 388 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedi S. et al. The effects of hormone replacement therapy on cortical bone in postmenopausal women. A histomorphometric study. Bone. 33, 330–334 (2003). [DOI] [PubMed] [Google Scholar]
- Teede H. J. Sex hormones and cardiovascular system: effects on arterial function in women. Clin Exp Pharmacol Physiol. 34, 672–676 (2007). [DOI] [PubMed] [Google Scholar]
- Flach J. et al. Replication stress is a potent driver of functional decline in aging haematopoietic stem cells. Nature. 512, 198–202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeryfar S. M. et al. Thy-1: more than a mouse pan-T cell marker. J Immunol. 173, 3581–3588 (2004). [DOI] [PubMed] [Google Scholar]
- Dhawan J. et al. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 12, 666–673 (2005). [DOI] [PubMed] [Google Scholar]
- Mangan G. et al. Oestrogen-dependent satellite cell activation and proliferation following a running exercise occurs via the PI3K signalling pathway and not IGF-1. Acta Physiol (Oxf). 212, 75–85 (2014). [DOI] [PubMed] [Google Scholar]
- Ogawa M. et al. 17β-estradiol represses myogenic differentiation by increasing ubiquitin-specific peptidase 19 through estrogen receptor α. J Biol Chem. 286, 41455–41465 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speir E. et al. Antioxidant effect of estrogen on cytomegalovirus-induced gene expression in coronary artery smooth muscle cells. Circulation. 102, 2990–2996 (2000). [DOI] [PubMed] [Google Scholar]
- Kumar S. et al. Role of estrogen receptors in pro-oxidative and anti-oxidative actions of estrogens: aperspective. Biochim Biophys Acta. 1800, 1127–1135 (2010). [DOI] [PubMed] [Google Scholar]
- Ingberg E. et al. Methods for long-term 17β-estradiol administration to mice. Gen Comp Endocrinol. 175, 188–193 (2012). [DOI] [PubMed] [Google Scholar]
- Li T. S. et al. Regeneration of infarcted myocardium by intramyocardial implantation of ex vivo transforming growth factor-beta-preprogrammed bone marrow stem cells. Circulation. 111, 2438–2445 (2005). [DOI] [PubMed] [Google Scholar]
- Kamota T. et al. Ischemic preconditioning enhances the mobilization and recruitment of bone marrow stem cells to protect against ischemia/reperfusion injury in the late phase. J Am Coll Cardiol. 53, 1814–1822 (2009). [DOI] [PubMed] [Google Scholar]
- Yoshida T. et al. Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res. 46, 147–153 (2012). [DOI] [PubMed] [Google Scholar]
- Guo C. Y. et al. Effects of antioxidants on the quality and genomic stability of induced pluripotent stem cells. Sci Rep. 5, 8055 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.