Abstract
The interaction between floral oil secreting plants and oil-collecting bees is one of the most specialized of all pollination mutualisms. Yet, the specific stimuli used by the bees to locate their host flowers have remained elusive. This study identifies diacetin, a volatile acetylated glycerol, as a floral signal compound shared by unrelated oil plants from around the globe. Electrophysiological measurements of antennae and behavioural assays identified diacetin as the key volatile used by oil-collecting bees to locate their host flowers. Furthermore, electrophysiological measurements indicate that only oil-collecting bees are capable of detecting diacetin. The structural and obvious biosynthetic similarity between diacetin and associated floral oils make it a reliable cue for oil-collecting bees. It is easily perceived by oil bees, but can’t be detected by other potential pollinators. Therefore, diacetin represents the first demonstrated private communication channel in a pollination system.
Most angiosperms are pollinated by a diverse subset of all potential flower-visiting animals1. However, some plants exhibit extreme specialization for pollination in that only one or a few animal species belonging to a single functional group (e.g. bees, butterflies, or birds) act as their pollinators2,3. Benefits to plants that are highly specialized for pollination include reduction of pollen loss and clogging of stigmas with foreign pollen, and a decrease in interspecific gene flow, especially if the pollinators show fidelity and are equally specialized in the choice of their host plants2,4. Advantages of being a specialist pollinator are a higher foraging efficiency, potentially reduced interspecific competition from other pollinators, and the possibility of evolving reciprocal adaptations for exploitation of particular host plants4,5. Examples of specialized pollination systems include the interactions between figs and fig wasp pollinators, between long-spurred flowers and their long-tongued fly or moth pollinators, and between oil secreting plants and their oil-collecting bee pollinators2,4. In many specialized pollination systems, floral scent is the most important floral signal for pollinator attraction and this allows recognition of the host by the pollinator6, however, other modalities, such as visual cues, are also typically involved in pollinator attraction7. Scent-mediated specificity in pollinator attraction has been suggested to occur through either one of two mechanisms: 1) the production of unique compounds or 2) the production of specific blends of common compounds. The first type can be viewed as a sensory ‘private channel’ between the plant and its intended pollinator if the critical scent components are easily detected by the intended receivers (i.e., pollinators) while remaining undetected by unintended receivers6. These two alternative mechanisms (unique compounds vs. blends of common compounds) have been variously implicated in case studies of specialized pollination systems. For example, sexually deceptive orchids mimic female sex pheromones of various Hymenoptera by emitting either uncommon compounds (e.g., chiloglottone8, pyrazines9), or blends of commonly occurring hydrocarbons such as alkenes or alkanes10 to attract pollinating males that are searching for females. Although unusual or unique compounds are good candidates for private communication channels, the assumption that these are readily detected by pollinators and undetected by other potential flower visitors has not been tested previously in any pollination system where private channels are assumed to operate6. In (sexually) deceptive systems mediated by uncommon compounds, the plants exploit existing olfactory capabilities and preferences of specific pollinators. In non-deceptive, reward-based pollination systems, the olfactory capability for detecting uncommon or unique compound(s) may be the result of an adaptation in the olfactory circuitry (receptors, binding proteins, neurons) that evolved to recognize the specific food plant(s). Although such adaptations to specific scent compounds of non-deceptive host plants have not been demonstrated in any pollinator, it is known that different insects detect and respond differently to specific compounds from their habitat. This variability in the periphery of the olfactory circuitry of insects demonstrates the evolutionary potential for divergence in response to scent components, even among insects that are closely related11,12,13.
The highly specialized pollination mutualism between floral oil secreting plants (henceforth oil plants) and oil-collecting bees (henceforth oil bees) has evolved in more than ten plant families and two families of bees14,15,16,17. Plant species which produce and secrete floral fatty oils, (mostly) in lieu of nectar, occur throughout the globe in Neotropical, Palaeotropical, Afrotemperate, and Holarctic floristic regions18. In each area, this oil is collected by females of only a few specialized oil bee species and these are either members of the Apidae (Palaeotropical and Neotropical regions) or Melittidae (Holarctic and Afrotemperate regions). The oil is used by these bees as larval food provisions (e. g.19) and as a constituent of the cell lining within the nest19,20. The function of this cell lining is to protect the larval provision and the immature stages from water and pathogens, such as fungi15. The use of floral oils in nest cell lining is exceptional in bees, and only found in oil-collecting bees, and not in other bees, which usually use secretions of the large Dufour’s gland for the cell lining21. In Macropis and other oil bees, the Dufour’s gland is small and strongly reduced20,22. In bees, oil collection has evolved at least seven times, and, in plants, oil as a floral reward has developed independently at least 28 times18. Despite the widespread nature and repeated evolution of this pollination system, it is generally rather rare compared to nectar- or pollen-based reward systems18, involving approximately 1700 species of plants and 370 species of bees.
Most interactions between oil plants and oil bees are obligate mutualisms. The oil bees are the sole pollinators of the plants and the oil plants are essential hosts for the bees23,24. Although some oil plants are also visited to a small extent by non-oil bees, such bees play only a minor role as pollinators24. As oil plants and oil bees are dependent on each other, an effective communication system can be expected to have (co)evolved in this pollination system. As in other specialized pollination systems, floral scent is important for the interaction between oil plants and their bee pollinators. Behavioural experiments with naive females of the European Macropis fulvipes with no prior Lysimachia experience revealed that olfactory cues of their host plant, Lysimachia punctata, are more important for host location than visual cues. This use of olfactory cues for locating hosts seems to have a genetic basis, as scent-based attraction of M. fulvipes bees to Lysimachia flowers was not dependent on their previous foraging experiences7,25.
Compounds responsible for attracting M. fulvipes are present in solvent extracts of complete flowers and in extracts of the floral oils25 (Schäffler unpublished). Both complete flowers and floral oils release a wide variety of compounds25, however, the specific compound(s) eliciting the behavioural response in oil bees have remained unknown. One uncommon compound, 1-hydroxy-1-phenyl-2-propanone, present in both flower and oil samples has been suggested to play a role in Macropis bees attraction6,25, however, it has not been found to attract bees in behavioural tests26.
The finding that solvent extracts of oil are capable of attracting Macropis bees led us to speculate that the floral oils or compounds involved in the biosynthesis of these oils are involved in pollinator attraction. Such compounds would be an ideal signal for Macropis to locate oils, because it would directly indicate the presence of oils (i.e., be an honest signal sensu Raguso27). In animal communication terminology, the biosynthetic link between such a compound and the reward would make the compound an “index signal”28,29. Interestingly, oil plants around the world all produce quite similar oils. These consist typically of mono-, di-, or triacetylated glycerols or free fatty acids (typical chain lengths: C16, C18) with a hydroxyl or acetyloxy group on the beta carbon14,15,16,30,31. Thus, oil flowers around the world may advertise their oil rewards with a similar signal. Generally, in most pollination systems, pollinators locate rewards using volatile signals derived from biosynthetic pathways (e.g. terpenoids, aromatics, aliphatics) that are not directly linked to production of rewards (e.g. sugars and/or protein)32. Such “conventional signals”28, however, can also honestly indicate a reward33. Pollination systems in which the signal itself is the reward or that are based on compounds that are biosynthetically very similar to the rewards are very rare, but can be found in systems involving male perfume collecting euglossine bees34 and male tephritid flies35. Euglossine males use these compounds during courtship behaviour36, whereas male flies use them directly37 or after conversion into sex pheromones38, for mate attraction.
Based on (i) the similarity in chemical structure of floral oils among oil plants around the world, (ii) the observation that floral scent extracted from oil attracts oil bees, and (iii) the fact that oil flowers are pollinated almost exclusively by oil bees, we address the hypothesis that the oil flower/oil bee pollination system is mediated by a volatile private communication channel which is derived from the pollinator reward (viz. oil).
Results
Detection of EAD-active compounds in oil flowers
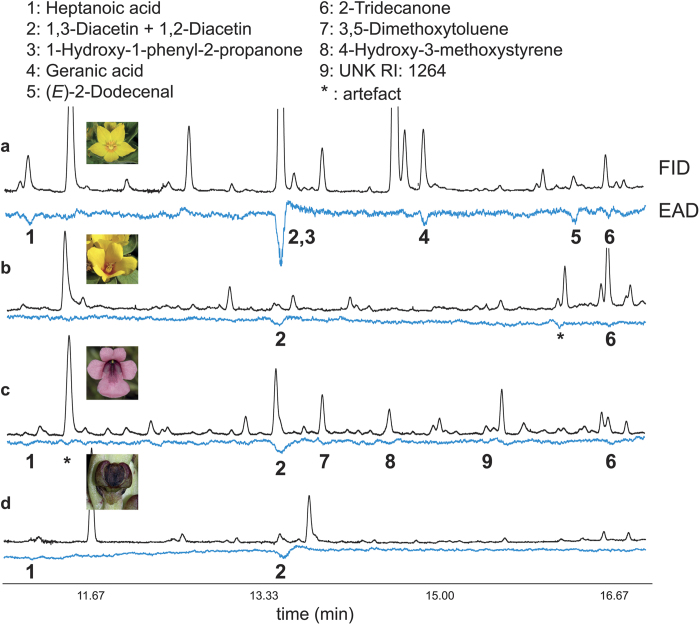
In the GC-EAD analyses with antennae of M. fulvipes and scent samples collected from four different oil plants, we found only one EAD-active compound, diacetin, that occurred in all of these plant species (Fig. 1). Two EAD-active compounds (heptanoic acid, 2-tridecanone) occurred in three of the plant species, whereas six compounds (1-hydroxy-1-phenyl-2-propanone, triacetin, (E)-2-dodecenal, 3,5-dimethoxytoluene, 4-hydroxy-3-methoxystyrene, UNK RI: 1264) occurred in one of the four plant species.
Figure 1. Electroantennographic responses of Macropis fulvipes oil bees to scents of different oil flowers.
GC-FID (black line) and GC-EAD responses of M. fulvipes antennae (inverted blue line) to floral extracts of L. punctata (a), L. congestiflora (b), Diascia integerrima (c), and Corycium dracomontanum (d); (UNK RI 1264: unknown compound, kovats retention index 1264; m/z: 122, 78, 106, 51, 50). Plant photographs by Irmgard Schäffler (a,b) and Kim E. Steiner (c,d).
Occurrence of EAD-active compounds
The most widespread EAD-active compound was diacetin, which occurred in 41 of the 50 (82%) studied oil species, but in only one of the eight (12.5%) related non-oil species examined. It was present in all of the Holarctic (seven) and South African (18) oil species, as well as in 16 (73%) of the Neotropical oil species (Table 1, for complete list see Table S1). Nearly as widespread as diacetin was 2-tridecanone, which was found in 34 (68%) of the oil species and in one non-oil species. Heptanoic acid was detected in 20 (40%) and 4-hydroxy-3-methoxystyrene in 18 (36%) of the oil species, whereas the remaining EAD-active compounds occurred in less than 10 (20%) of the oil secreting species.
Table 1. Occurrence of EAD-active substances (see Fig. 1) in oil and non-oil plants of different families/genera and floristic regions.
| Holarctic | Afrotemp. | Neotropics | Palaeotropics | |
|---|---|---|---|---|
| Number of taxa studied in floristic regions | ||||
| families | 1 | 3 | 6 | 1 |
| genera | 1 | 7 | 13 | 1 |
| oil/non-oil species | 7/7 | 18/0 | 22/0 | 3/1 |
| Percentage of oil/non-oil species with a specific EAD-active compound | ||||
| diacetin | 100/14 | 100/– | 73/– | 0/0 |
| 2-tridecanone | 100/0 | 72/– | 55/– | 100/100 |
| heptanoic acid | 71/0 | 55/– | 23/– | 0/0 |
| 4-hydroxy-3-methoxystyrene | 29/0 | 50/– | 18/– | 100/0 |
| unkown (RI 1264) | 0/0 | 17/– | 0/– | 66/0 |
| geranic acid | 29/0 | 0/– | 2/– | 0/0 |
| (E)-2-dodecenal | 43/0 | 0/– | 0/– | 0/0 |
| 1-hydroxy-1-phenyl-2-propanone | 29/0 | 0/– | 0/– | 0/0 |
| 3,5-dimethoxytoluene | 0/0 | 11/– | 0/– | 0/0 |
Afrotemp.: Afrotemperate region. (For detailed information see Table S1).
EAG - antennal responses to diacetin among oil and non-oil bees
Overall analysis revealed significant effects of bee species (F2,17 = 15.97, P < 0.001), dilution (F3,51 = 60.21, P < 0.001), and the bee species × dilution interaction (F6,51 = 17.94, P < 0.001) on antennal responses to diacetin. Antennal responses increased with increasing concentration of diacetin for M. fulvipes and Rediviva neliana oil bees, but not for honey bees (Fig. 2). As was the case for honey bees, there was no dilution effect of diacetin for the non-oil bee Melitta haemorrhoidalis (t = 1.00, df = 4, P = 0.37).
Figure 2. Physiological dose-response curves of different bees for diacetin.
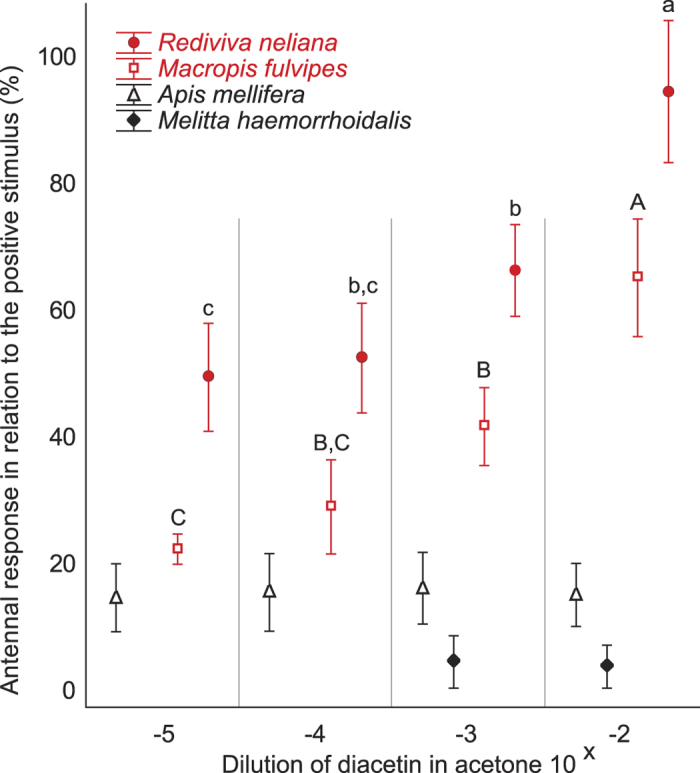
Electroantennographic responses (EAG) of oil bees (Macropis fulvipes/Rediviva neliana) and non-oil bees (Melitta haemorrhoidalis/Apis mellifera) to different dilutions of diacetin. SEM = standard error of the mean. Means (within the same species) that share same letters or do not have letters are not significantly different.
Responses to the highest concentration of diacetin were stronger than to acetone in antennae of M. fulvipes (t = 6.32, df = 4, P < 0.01) and R. neliana (t = 9.44, df = 5, P < 0.001), but not in those of M. haemorrhoidalis (t-test: t = 1.00, df = 4, P = 0.37) and A. mellifera (t-test: t = 1.13, df = 8, P = 0.29).
Behavioural experiments
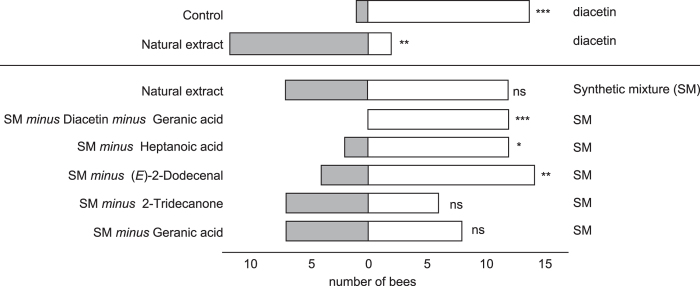
In two-choice experiments conducted in the flight cage, diacetin alone attracted significantly more bees than did a negative control, but significantly less bees than did a natural floral extract (Fig. 3). However, the creation of a synthetic mixture with diacetin and four additional EAD-active compounds increased the attractiveness to the same level as the natural floral extract (see also supplementary data, Movie 1).
Figure 3. Behavioural experiments testing the attractiveness of floral compounds of Lysimachia oil flowers to Macropis oil bees.
Approaches of naive Macropis fulvipes females to diacetin alone (against acetone as a control), natural floral extracts of Lysimachia punctata, and complete (diacetin and four other compounds) and partially depleted synthetic mixtures of EAD-active compounds identified in L. punctata floral extracts. Exact binomial test: ns: P > 0.05; **P < 0.01; ***P < 0.001).
Bees responded differently to samples (natural extract, reduced synthetic mixtures) that were tested against the complete synthetic mixture (Fisher’s exact test: P = 0.01). Removal of geranic acid or 2-tridecanone from the mixture had no effect on attractiveness to M. fulvipes females, but removal of heptanoic acid or (E)-2-dodecenal reduced the attractiveness significantly relative to the complete synthetic mixture (Fig. 3). When diacetin was removed from the mixture (together with geranic acid, see material and methods) bees were attracted only to the complete mixture (Fig. 3).
Discussion
Our data demonstrate that flowers of most of the studied oil species around the world emit the fatty acid derivative diacetin. This compound elicits strong antennal responses in oil bees from different floristic regions and continents, but it does not elicit antennal responses in related non-oil bees. This suggests an olfactory adaptation in oil bees to this uncommon compound. Diacetin is a key signal in the Lysimachia-Macropis pollination system, but other compounds can also add to the attractiveness of a scent blend. Overall, our data suggest that diacetin is a private communication channel and honest signal in the oil flower/oil bee pollination system.
Diacetin, only recently described as a floral compound39, occurs as a floral scent constituent in most (82%) of the oil plant species tested, regardless of floristic region (Holarctic, Neotropical, Afrotemperate region) or plant lineage (Asparagales, Malpighiales, Ericales, Lamiales). These findings strongly suggest, therefore, that the production of diacetin in oil flowers has evolved independently several times, in accordance with the independent evolution of oil secretion in these flowers18,40.
In contrast to the widespread occurrence of diacetin in oil plants, we did not find diacetin in related non-oil species with one exception. In the non-oil secreting Lysimachia thyrsiflora, the sister species of oil secreting and diacetin emitting L. vulgaris40, this compound was detected in flower extracts and more recently also in headspace samples. The presence of diacetin in this non-oil species may be the result of a recent switch away from pollination by oil bees yet with retention of the ability to produce small quantities of floral oil and diacetin due to relaxed selection against its production41,42.
Diacetin has not been found in dynamic headspace collections from several oil species25,43, even though we identified it in solvent extracts of flowers of these same species. This suggests that diacetin is present only in small and hard to detect amounts in floral headspace samples (see also below). Interestingly, diacetin has not been identified in studies focusing on the chemistry of the floral oils15,31. We attribute this to its smaller size and higher volatility compared to the target non-volatile oils, and a methodology that did not allow its detection. The amount of diacetin available in the samples was quite small compared to the oils and this small amount may have been lost in the process of evaporating the “oil samples” to dryness.
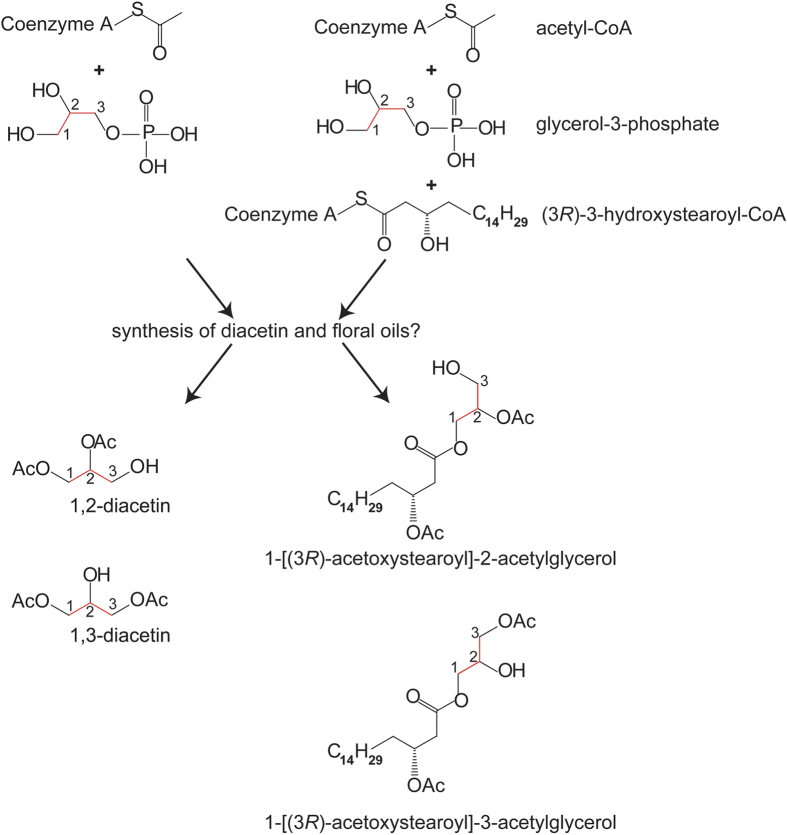
The basic structure of floral oils (i.e. acylglycerols) is similar for the oil species found around the world and resembles that of the volatile compound diacetin as well as some lipids in plant tissues31. As exemplified by L. punctata, major compounds in the floral oil are 1-[(3R)-acetoxystearoyl]-2-acetylglycerol and 1-[(3R)-acetoxystearoyl]-3-acetylglycerol and both of these compounds are composed of a glycerol esterified with one acetic acid and with one substituted long-chain fatty acid (Fig. 4). Structural similarities of these two compounds with 1,2- and 1,3-diacetin are evident. It can be assumed that metabolic pathways or enzymes utilized, such as those involved in ester formation of glycerol with fatty acids44 (specifically 3-hydroxy/3-acetoxy fatty acids) or acetic acid, are to some extent identical for this group of lipids and for diacetin production (Fig. 4).
Figure 4. Diacetin is likely a derivative of the fatty floral oil biosynthesis.
Schematic overview of proposed biosynthesis of diacetin and abundant floral oil components found in Lysimachia punctata.
Since acetylation of glycerol or the backbone of the hydroxylated long chain fatty acids is almost universal in “non-volatile” floral oils30,31, it can be hypothesized that diacetin might be present in all oils of this type, whereas it may not be present in oils made up of other types of lipids (e.g. free fatty acids, classical triglycerides or wax esters, terpenoids). Indeed, we found diacetin in all plants having oils congruent with these criteria with the exception of Momordica (Cucurbitaceae) and Bunchosia (Malpighiaceae) species. Diacetin was also missing from Nierembergia (Solanaceae) species, but their oils do not consist of acetylated glycerols (Table S2). The common occurrence of diacetin with ‘acetylated’ floral oils supports the idea that these compounds are derived from the same metabolic pathway or, at least partially, rely on the same enzymatic endowment. Even if diacetin evolved initially as a by-product of oil synthesis and was subsequently co-opted by oil bees as an “index signal”28,29, it still represents a reliable cue for bees looking for floral oils. Parallels can be drawn to a communication system between male and female rattlebox moths (Utetheisa ornatrix). Here, the males use a volatile derivative of a larger defence compound, to indicate to the female the quality of the sequestered defence compounds that they pass along to the female during mating45.
Our data show that diacetin is widespread among oil species and a good candidate for use by oil bees around the world as a reliable cue for locating oil rewards. They also indicate that diacetin represents a private communication channel between oil plants and oil bees. In our electrophysiological measurements, diacetin elicited antennal responses in melittid bees from both Europe (M. fulvipes) and South Africa (R. neliana). It also elicits responses in another European Macropis species, M. europaea Warncke, and two additional South African Rediviva species, R. brunnea Whitehead & Steiner, and R. pallidula Whitehead & Steiner (Dötterl and Steiner, unpublished data). Diacetin did not, however, elicit significant antennal responses in the closely related non-oil melittid bee (M. haemorrhoidalis) or the honey bee (A. mellifera, Apidae). This difference in antennal response to diacetin between oil and non-oil bees demonstrates that the oil bees have specific olfactory adaptations in the periphery of the olfactory circuit to detect diacetin. This adaptation functions most likely at the level of the olfactory receptors or the olfactory binding proteins11,46,47, but additional adaptation in the brain (e.g. processing) cannot be excluded. Such adaptations towards volatile signals of host plants have not been described for any other pollinators and our next step will be to test whether oil bees belonging to the Apidae exhibit a similar positive response to diacetin.
Our bioassays with M. fulvipes and the EAD-active scent compounds of its host plant L. punctata point towards a key function of diacetin in host plant location. The presence of diacetin alone was sufficient to attract Macropis bees. Two other EAD-active compounds (heptanoic acid, (E)-2-dodecenal) were also behaviourally active. However, a mixture containing these two and two additional EAD-active compounds (2-tridecanone, geranic acid) but lacking diacetin did not attract bees when tested against a synthetic mixture that contained all compounds (Fig. 3).
Trace amounts of diacetin were found as a contaminant in our synthetic geranic acid sample and, therefore, we had to exclude geranic acid from our mixture in order to obtain a diacetin-free sample for the choice tests. These trace amounts proved sufficient to elicit behavioural responses in Macropis, because a synthetic mixture without diacetin but with geranic acid attracted Macropis bees (Schäffler, unpublished data). When removing only geranic acid from the complete synthetic mixture the bees did not discriminate between the depleted and the complete mixture, demonstrating not only that geranic acid has no influence on bee behaviour, but also that the absence of trace amounts of diacetin (when higher amounts are still present) did not influence the choice of bees. Overall, we conclude that diacetin and not geranic acid was responsible for the loss of attractiveness relative to the complete scent mixture when excluding both substances from the complete mixture. This confirms that diacetin is a key compound in attracting Macropis.
In addition to diacetin, heptanoic acid and (E)-2-dodecenal are used by Macropis bees for locating oil flowers. Heptanoic acid was detected in about 20 oil species in three floristic regions, and recently in a few oil and non-oil species43,48. The only other reported instance of a biological function for the compound is as a kairomone in an insect host-parasite communication system49. Even rarer is the floral scent compound (E)-2-dodecenal that was found in three floral oil secreting Lysimachia species. Until now, this compound was known from only a few South African oil orchids43 and from two species without floral oils50,51, and from a millipede where it acts as an insect deterrent52. In contrast, the EAD-active compound 2-tridecanone is very widespread among our oil species studied, among a large number of oil orchids of South Africa43, and among several non-oil species48,50,51, yet it did not influence the attractiveness of the synthetic mixture. This compound, known as a repellent for insects53 including generalized bee pollinators54, could act as a floral filter39,43 at least in the Macropis-Lysimachia pollination system to reduce visitation rates from inappropriate visitors that might remove pollen without providing adequate pollination.
Interestingly, while diacetin is very widespread among oil plants, the plants emit additional scent compounds, several of which are not widespread and do not occur in more than one or a few of the species studied39,43,51. There is a high overall variation in floral scent among oil plants, which is true for species within floristic regions and even for species pollinated by the same oil bee species (Holarctic:39, South Africa:43) as well as among floristic regions. These findings lead us to believe that diacetin is a reliable volatile marker for ‘non-volatile’ fatty oils throughout the world, whereas the emission of other compounds, like geranic acid, 3,5-dimethoxytoluene, or (E)-2-dodecenal, may be important for allowing bees to discriminate among co-blooming species. Scents distinguishable among plant species are known to promote effective pollen transfer within species and species integrity through flower constancy of pollinators55. However, we did not find diacetin in all of the floral oil species suggesting that they may emit diacetin in amounts too low for detection or that diacetin is not used as a signal by these plants. If the latter is true, compounds other than diacetin may occasionally be important as pollinator attractants. Since oil production has evolved independently in several families and some plants produce floral oils structurally dissimilar to diacetin31, it would not be altogether surprising if some oil species used different signals.
Conclusion
Diacetin occurs in several floral oil plants around the world and is detected by oil bees from at least two continents. It allows the Holarctic Macropis oil bee, and probably other oil bees, to rapidly and efficiently locate their oil secreting host plants. Our data for Macropis and Lysimachia suggest that diacetin represents the first demonstrated private communication channel between a pollinator and its host plant. Notably, diacetin satisfies the two requirements of a private communication channel: 1) it is an uncommon compound and 2) it can be detected by its specialized and specific pollinators, but apparently not by other potential pollinators in the environment. We cannot rule out the possibility that one or more of the thousands of non-oil bees that we didn’t test may be able to detect diacetin, yet, there seems little selective value in evolving or retaining such an ability outside of an oil flower/oil bee relationship. Dated phylogenies show that Lysimachia and Macropis are of similar age making it plausible that they coevolved from the onset18. Thus, the described fine-tuned adaptation towards diacetin in the sensory apparatus of the bees and the chemical profile of the host plants may be the result of coevolutionary processes. The obvious sharing of the biosynthetic production by diacetin and floral oils, at least those with acetylation, make diacetin an ideal and reliable cue for oil bees.
Materials and Methods
Bee study species
The oil bee Macropis fulvipes (Fab.) (Melittidae, Melittinae) is distributed in Europe and, like other Macropis species, is specialized on the oil secreting flowers of Lysimachia species (Primulaceae)15,56. Fatty floral oils and pollen of these plants are the only food collected by adult females for the offspring. Adult males and females feed on pollen of Lysimachia and females use the oil to line the brood cells15,19. Individuals used for behavioural tests were from a flight cage population19 (see below) and Lysimachia-naive, while those used for electrophysiological measurements (see below) were from a natural population in the Ecological Botanical Garden of the University of Bayreuth (EBG) and likely Lysimachia-experienced.
Rediviva (Melittidae, Melittinae) oil bees are closely related to Macropis, occur in Southern Africa, and also collect floral oils as food for the offspring21. Rediviva neliana Cock. is widespread in the summer rainfall area57. Specimens for electrophysiological measurements (see below) were collected in the Witsieshoek region of the Drakensberg while visiting oil or nectar/pollen plants.
Melitta bees which occur in the Holarctic and in Africa are from the same subfamily as Macropis and Rediviva, i.e. Melittinae, but species do not collect floral oils. Melitta haemorrhoidalis (Fab.) is distributed in Europe and is specialized on pollen of Campanula species56. Specimens for electrophysiological measurements were collected from natural populations in the EBG and served as a phylogenetic control.
The non-oil honey bee, Apis mellifera L., originally native to Europe and Africa, now occurs throughout the world and, in contrast to the other bee species used, belongs to the Apidae. It is among the most generalist bees and therefore is expected to have the capacity to detect a large array of scent compounds. Individuals used for electroantennographic measurements were collected in the EBG from established hives.
Plant material and volatile collection
Floral scents for chemical analyses were collected from 58 plant species (50 oil and 8 non-oil) from different geographic regions and phylogenetically disparate plant families and genera (supplementary data, Table S1). Samples of four of these oil species were additionally used for electrophysiological analyses, and samples of L. punctata were additionally used for bioassays. Samples were either collected from plants growing in the natural habitat or from material collected in different greenhouses (supplementary data, Table S1). Flowers were removed from the plants using clean forceps and extracted for one minute in 2–3 ml pentane (p.a., 99%, Grüssing, Germany). The 106 obtained samples were subsequently filtered with silanized glass wool (Supelco) to remove particles and concentrated by evaporation under a gentle stream of nitrogen to a volume of 0.5 ml. The solvent extracts of leaves were used as negative controls.
Gas Chromatography with Electroantennographic Detection (GC-EAD)
Both sexes of M. fulvipes were used because we did not want to use too many females from the small populations of Macropis and did not find differences in antennal responses between sexes in previous analyses26. Such potential differences were not expected to occur as both sexes visit Lysimachia flowers. Similar to females, males feed on pollen of the flowers after hatching, and throughout their life search for females on the flowers19,20. Antennae were tested using scent samples of four different oil species from three different plant orders (Ericales, Lamiales, Asparagales) and two different continents (Europe, Africa). By using this approach, compounds could be identified that are widespread among oil plants (phylogenetically independent) and potentially important in the oil flower/oil bee pollination system. Five Lysimachia punctata flower extracts (from different plants) were tested on antennae of 7 male and 6 female bees (one antenna per bee). Additionally, one flower extract of L. congestiflora Hemsl. and one of Diascia integerrima E.Mey. ex Benth. were tested on the antennae from two different males (one antenna per bee), and the flower extract of Corycium dracomontanum Parkman & Schelpe was tested on one male antenna.
Electroantennography (EAG)
For the EAG tests we used five antennae from M. fulvipes (all female), six antennae from R. neliana (five males, one female), five antennae from Melitta haemorrhoidalis (all female), and nine antennae from honey bee workers as described above to measure dose-response curves for diacetin (diluted in acetone to four concentrations, 10−2, 10−3, 10−4, and 10−5; v/v). Antennae of M. haemorrhoidalis were only tested on the two highest concentrations. Both female and male antennae of Rediviva were used, because we found in GC-EAD analyses (unpublished data) that both sexes responded similarly to diacetin.
As a positive control we used linalool (10−2 in acetone), a compound widespread among plants pollinated by bees32, and as negative control we used acetone.
To test whether different bee species responded differently to the dilution series of diacetin, data were analysed using a repeated measurement ANOVA (STATISTICA v. 7.1.; www.statsoft.com) with individual bees as subject for repeated measures and the different dilutions and bee species as categorical factors. Tukey was used as post hoc test. Responses of Melitta were excluded from these analyses as only two of the four diacetin dilutions were tested in this species. Instead, we tested for a dilution effect in Melitta using a paired t-test (STATISTICA). A paired t-test was also used to test for differences in responses to acetone and the 10−2 dilution of diacetin in each species. For more detailed information, see Supplemental Experimental Procedures.
Chemical Analyses
To identify the EAD-active compounds in the four species used for GC-EAD measurements, 1 μl of the flower extracts was analysed on a Varian Saturn 2000 mass spectrometer coupled to a Varian 3800 gas chromatograph fitted with a 1079 injector (Varian Inc., Palo Alto, CA, USA). Additionally, we analysed samples of the 50 oil and eight non-oil species available by GC-MS for the presence of the EAD-active compounds (for further information, see Supplemental Experimental Procedures).
Preparation of synthetic scent mixture
For testing the attractiveness of complete and partially depleted synthetic mixtures of EAD-active substances from L. punctata to M. fulvipes bees, we prepared dilutions of the synthetic substances in acetone (99.9%, AnalaR NORMAPUR, VWR): diacetin (after purification, see Supplemental Experimental Procedures), geranic acid (98%, ABCR), heptanoic acid (99%, Aldrich), (E)-2-dodecenal (93%, Aldrich) and 2-tridecanone (98%, ABCR). Though EAD-active, we didn’t include 1-hydroxy-1-phenyl-2-propanone in our behavioural experiments because it failed to attract bees in previous tests26.
The absolute amount of synthetic compounds in the 10 μl extract offered to the bees during the bioassay was equivalent to the quantity of compounds found in extracts of 100 flowers (few flowering stems) of L. punctata (2 μg heptanoic acid, 4 μg geranic acid, 2 μg (E)-2-dodecenal, 4 μg 2-tridecanone, and 0.3 μg diacetin).
Bioassays
Behavioural assays were needed in this study, because electroantennographically active substances do not necessarily elicit behavioural responses in insects58. Two-choice bioassays in the flight cage tested the importance of EAD-active floral volatiles of L. punctata for host plant location by Lysimachia-naïve M. fulvipes females. Lysimachia-naïve bees were used to study the innate basis of host plant location. Naïve bees were not trained before the experiments, did not know the test scenario before the testing started, and were not rewarded when responding (see also Supplemental Experimental Procedures). Diacetin was tested against an acetone negative control and against a natural flower extract of L. punctata (positive control; from 100 flowers). We further tested a natural extract against the completely synthetic (5 EAD-active compounds) mixture, as well as the complete synthetic mixture against incomplete synthetic mixtures from which one of the components was omitted. To obtain a mixture without diacetin, we additionally had to eliminate geranic acid as GC-MS analyses revealed trace amounts of diacetin (0.24 ng in 4 μg geranic acid) as a contaminant in synthetic geranic acid.
Additional Information
How to cite this article: Schäffler, I. et al. Diacetin, a reliable cue and private communication channel in a specialized pollination system. Sci. Rep. 5, 12779; doi: 10.1038/srep12779 (2015).
Supplementary Material
Acknowledgments
We thank J. Schmidt, C. Kuhnt, A. Werner, J. Mühlbradt, and A. Jürgens for technical support, K.H. Seifert for chemical support and comments on earlier versions of the manuscript, M. Kaib for providing a GC-FID (HP 5890), and G. Aas for making a greenhouse available. This work was supported by the German Research Foundation (DO 1250/3-1).
Footnotes
Author Contributions S.D. conceived the project, and I.S. and S.D. designed the study. I.S. performed bioassays. S.D.J. made electrophysiological equipment available, and S.D. and I.S. performed the electrophysiological measurements. I.S., S.D., G.G. and K.E.S. collected scent samples. M.H., S.S.v.B. and L.W. purified diacetin. I.S. and S.D. analysed the data. I.S., S.D. and L.W. wrote the first draft of the manuscript and all authors contributed to interpretation of the findings and edited and approved the manuscript.
References
- Ollerton J., Winfree R. & Tarrant S. How many flowering plants are pollinated by animals? Oikos 120, 321–326 (2011). [Google Scholar]
- Johnson S. D. & Steiner K. E. Generalization versus specialization in plant pollination systems. Trends Ecol. Evol. 15, 140–143 (2000). [DOI] [PubMed] [Google Scholar]
- Fenster C. B., Armbruster W. S., Wilson P., Dudash M. R. & Thomson J. D. Pollination syndromes and floral specialization. Annu. Rev. Ecol. Evol. Syst. 35, 375–403 (2004). [Google Scholar]
- Willmer P. Pollination and floral ecology (Princeton University Press, Princeton, New Jersey, 2011). [Google Scholar]
- Sedivy C., Müller A. & Dorn S. Closely related pollen generalist bees differ in their ability to develop on the same pollen diet: evidence for physiological adaptations to digest pollen. Funct. Ecol. 25, 718–725 (2011). [Google Scholar]
- Raguso R. A. Wake up and smell the roses: the ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 39, 549–569 (2008). [Google Scholar]
- Dötterl S., Milchreit K. & Schäffler I. Behavioural plasticity and sex differences in host finding of a specialized bee species. J. Comp.Physiol. A 197, 1119–1126 (2011). [DOI] [PubMed] [Google Scholar]
- Schiestl F. P. et al. The chemistry of sexual deception in an orchid-wasp pollination system. Science 302, 437–438 (2003). [DOI] [PubMed] [Google Scholar]
- Bohman B. et al. Discovery of pyrazines as pollinator sex pheromones and orchid semiochemicals: implications for the evolution of sexual deception. New Phytol. 203, 939–952 (2014). [DOI] [PubMed] [Google Scholar]
- Schiestl F. P. et al. Orchid pollination by sexual swindle. Nature 399, 421–421 (1999). [Google Scholar]
- Eltz T. et al. An olfactory shift is associated with male perfume differentiation and species divergence in orchid bees. Curr. Biol. 18, 1844–1848 (2008). [DOI] [PubMed] [Google Scholar]
- Dekker T., Ibba I., Siju K. P., Stensmyr M. C. & Hansson B. S. Olfactory shifts parallel superspecialism for toxic fruit in Drosophila melanogaster sibling, D. sechellia. Curr. Biol. 16, 101–109 (2006). [DOI] [PubMed] [Google Scholar]
- Stökl J. et al. A deceptive pollination system targeting drosophilids through olfactory mimicry of yeast. Curr. Biol. 20, 1846–1852 (2010). [DOI] [PubMed] [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Vol. 7 (Akademie der Wissenschaft und der Literatur, Franz Steiner Verlag Wiesbaden GmbH, 1974). [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen, Zweite Folge: Lysimachia und Macropis. Vol. 54 (Akademie der Wissenschaft und der Literatur, Franz Steiner Verlag Wiesbaden GmbH, 1986). [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen, Dritte Folge: Momordica, Thladianthia und die Ctenoplectridae . Vol. 73 (Akademie der Wissenschaft und der Literatur Franz Steiner Verlag Wiesbaden GmbH, 1990). [Google Scholar]
- Buchmann S. L. The ecology of oil flowers and their bees. Annu. Rev. Ecol. Syst. 18, 343–369 (1987). [Google Scholar]
- Renner S. S. & Schaefer H. The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philos. Trans. R. Soc. B-Biol. Sci. 365, 423–435 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäffler I. & Dötterl S. A day in the life of an oil bee: phenology, nesting, and foraging behavior. Apidologie 42, 409–424 (2011). [Google Scholar]
- Cane J. H. Foraging, grooming, and mating behaviors of Macropis nuda (Hymenoptera: Melittidae) and use of Lysimachia ciliata (Primulaceae) oils in larval provisions and cell lining. Am. Midl. Nat. 110, 257–264 (1983). [Google Scholar]
- Michener C. D. The bees of the world . 2nd edn, (The John Hopkins University Press, 2007). [Google Scholar]
- Alves-dos-Santos I., Capelari Naxara S. R. & Patricio E. F. L. R. A. Notes on the morphology of Tetrapedia diversipes Klug 1810 (Tetrapediini, Apidae), an oil-collecting bee. Brazil J Morph Sci 23, 425–430 (2006). [Google Scholar]
- Machado I. C. Oil-collecting bees and related plants: a review of the studies in the last twenty years and case histories of plants occurring in NE Brazil in Solitary bees: conservation, rearing and management for pollination (eds Freitas Breno M. & Pereira Júlio Otávio P.) 255–280 (Imprensa Universitária, 2004).
- Neff J. L. & Simpson B. B. in Practical Pollination Biology (eds Dafni A., Kevan P. G. & Husband B. C.) Ch. 5.4, 314–328 (Enviroquest, Ltd., 2005). [Google Scholar]
- Dötterl S. & Schäffler I. Flower scent of oil-producing Lysimachia punctata as attractant for the oil-bee Macropis fulvipes. J. Chem. Ecol. 33, 441–445 (2007). [DOI] [PubMed] [Google Scholar]
- Dötterl S. & Vereecken N. J. The chemical ecology and evolution of bee-flower interactions: a review and perspectives. Can. J. Zool. 88, 668–697 (2010). [Google Scholar]
- Raguso R. A. Why are some floral nectars scented? Ecology 85, 1486–1494 (2004). [Google Scholar]
- Raguso R. A. et al. The raison d'être of chemical ecology. Ecology 96, 617–630 (2015). [DOI] [PubMed] [Google Scholar]
- Smith M. J. & Harper D. G. C. Animal Signals: Models and Terminology. J. Theor. Biol. 177, 305–311 (1995). [Google Scholar]
- Dumri K. et al. Non-volatile floral oils of Diascia spp. (Scrophulariaceae). Phytochemistry 69, 1372–1383 (2008). [DOI] [PubMed] [Google Scholar]
- Seipold L., Gerlach G. & Wessjohann L. A new type of floral oil from Malpighia coccigera (Malpighiaceae) and chemical considerations on the evolution of oil flowers. Chem. Biodivers. 1, 1519–1528 (2004). [DOI] [PubMed] [Google Scholar]
- Dobson H. E. M. in Biology of Floral Scent (eds Dudareva N. & Pichersky E.) 147–198 (CRC Press, 2006). [Google Scholar]
- Knauer A. C. & Schiestl F. P. Bees use honest floral signals as indicators of reward when visiting flowers. Ecol. Lett. 18, 135–143 (2015). [DOI] [PubMed] [Google Scholar]
- Teichert H., Dötterl S., Zimma B., Ayasse M. & Gottsberger G. Perfume-collecting male euglossine bees as pollinators of a basal angiosperm: the case of Unonopsis stipitata (Annonaceae). Plant Biol. 11, 29–37 (2009). [DOI] [PubMed] [Google Scholar]
- Tan K. H., Tan L. & Nishida R. Floral phenylpropanoid cocktail and architecture of Bulbophyllum vinaceum orchid in attracting fruit flies for pollination. J. Chem. Ecol. 32, 2429–2441 (2006). [DOI] [PubMed] [Google Scholar]
- Eltz T. & Lunau K. Antennal response to fragrance compounds in male orchid bees. Chemoecology 15, 135–138 (2005). [Google Scholar]
- Tan K. H. & Nishida R. Mutual reproductive benefits between a wild orchid, Bulbophyllum patens, and Bactrocera fruit flies via a floral synomone. J. Chem. Ecol. 26, 533–546 (2000). [Google Scholar]
- Tan K. H. & Nishida R. Synomone or Kairomone? Bulbophyllum apertum flower releases raspberry ketone to attract Bactrocera fruit flies. J. Chem. Ecol. 31, 497–507 (2005). [DOI] [PubMed] [Google Scholar]
- Schäffler I., Balao F. & Dötterl S. Floral and vegetative cues in oil-secreting and non-oil-secreting Lysimachia species. Ann. Bot. 110, 125–138 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg A. A., Manns U. & Källersjö M. Phylogeny and floral evolution of the Lysimachieae (Ericales, Myrsinaceae): evidence from ndhF sequence data. Willdenowia 37, 407-d (2007). [Google Scholar]
- Steiner K. E. The evolution of beetle pollination in a South African orchid. Am. J. Bot. 85, 1180 (1998). [PubMed] [Google Scholar]
- Lahti D. C. et al. Relaxed selection in the wild. Trends Ecol. Evol. 24, 487–496 (2009). [DOI] [PubMed] [Google Scholar]
- Steiner K. E., Kaiser R. & Dötterl S. Strong phylogenetic effects on floral scent variation of oil-secreting orchids in South Africa. Am. J. Bot. 98, 1663–1679 (2011). [DOI] [PubMed] [Google Scholar]
- Yu K., McCracken C. T. J. & Hildebrand D. F. in Current advances in the biochemistry and cell biology of plant lipids (eds Benning Christoph & Ohlrogge John) 6–10 (Aardvark Global Publishing Company, LLC, 2006). [Google Scholar]
- Dussourd D. E., Harvis C. A., Meinwald J. & Eisner T. Pheromonal advertisement of a nuptial gift by a male moth (Utetheisa ornatrix). P. Natl. Acad. Sci. 88, 9224–9227 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensmyr M. C., Dekker T. & Hansson B. S. Evolution of the olfactory code in the Drosophila melanogaster subgroup. Proc. R. Soc. Lond. B. Biol. Sci. 270, 2333–2340 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson Bill S. & Stensmyr, Marcus C. Evolution of insect olfaction. Neuron 72, 698–711 (2011). [DOI] [PubMed] [Google Scholar]
- Knudsen J. T., Eriksson R., Gershenzon J. & Ståhl B. Diversity and distribution of floral scent. Bot. Rev. 72, 1–120 (2006). [Google Scholar]
- Hendry L. B., Wichmann J. K., Hindenlang D. M., Weaver K. M. & Korzeniowski S. H. Plants—the origin of kairomones utilized by parasitoids of phytophagous insects? J. Chem. Ecol. 2, 271–283 (1976). [Google Scholar]
- Kaiser R. Meaningful scents around the world . (Wiley-VCH, 2006). [Google Scholar]
- Kaiser R. Scent of the vanishing flora . (Wiley-VCH, 2011). [Google Scholar]
- Wheeler J. W., Meinwald J., Eisner T. & Hurst J. J. trans-2-Dodecenal + 2-methyl-1,4-quinone produced by millipede. Science 144, 540–541 (1964). [DOI] [PubMed] [Google Scholar]
- Williams W. G., Kennedy G. G., Yamamoto R. T., Thacker J. D. & Bordner J. 2-Tridecanone: A naturally occurring insecticide from the wild tomato Lycopersicon hirsutum f. glabratum. Science 207, 888–889 (1980). [DOI] [PubMed] [Google Scholar]
- Dobson H. E. M., Danielson E. M. & Wesep I. D. V. Pollen odor chemicals as modulators of bumble bee foraging on Rosa rugosa Thunb. (Rosaceae). Plant Species Biol. 14, 153–166 (1999). [Google Scholar]
- Wright G. A. & Schiestl F. P. The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Funct. Ecol. 23, 841–851 (2009). [Google Scholar]
- Westrich P. Die Wildbienen Baden-Württembergs . Vol. I and II (Ulmer, 1989). [Google Scholar]
- Whitehead V. B., Steiner K. E. & Eardley C. D. Oil-collecting bees mostly of the summer rainfall area of Southern Africa (Hymenoptera: Melittidae: Rediviva). J. Kansas Entemol. Soc. 81, 122–141 (2008). [Google Scholar]
- Burger H., Dötterl S., Häberlein C., Schulz S. & Ayasse M. An arthropod deterrent attracts specialised bees to their host plants. Oecologia 168, 727–736 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.