Abstract
We compared the antibacterial and residual antimicrobial activities of five root canal irrigants (17% EDTA,2% chlorhexidine,0.2% cetrimide, MTAD, and QMix) in a model of Enterococcus faecalis biofilm formation. Sixty dentin blocks with 3-week E. faecalis biofilm were divided into six equal groups and flushed with irrigant for 2 min or left untreated. A blank control group was also established. Antibacterial activities of the irrigants were evaluated by counting colony forming units. To test residual antimicrobial activities, 280 dentin blocks were divided into seven equal groups and flushed with irrigant for 2 min or left untreated and then incubated with E. faecalis suspension for 48 h, or used as a blank. No bacteria were observed in the blank control group. The number of viable E. faecalis was significantly fewer in the irrigant-treated groups compared with the untreated control (P < 0.05). Among the five irrigants, QMix had the strongest antibacterial activity. Residual antimicrobial activities of CHX were significantly higher at 12 h, 24 h and 36 h compared to untreated control (P < 0.05). All five root canal irrigants were effective to some extent against E. faecalis, but QMix and CHX had the strongest, and CHX the longest (up to 36 h), antimicrobial activity.
The root canal biofilm is the main reason for root canal treatment failure1. Many studies have shown that Enterococcus faecalis is the predominant bacterial species in teeth with persistent periapical periodontitis2,3,4. Enterococcus faecalis is detected in 24%–77% of the filled root canals with persistent periapical lesions and usually exists in the infected root canals in the form of a biofilm5. This species is also present in most peripheral parts of the root-canal system, such as fins, anastomoses, apical canals, lateral canals, and dentinal canals6,7. The smear layer, which is formed only in instrumented areas, enhances the adhesion of E. faecalis and provides a matrix for deeper bacterial infection, thus influencing the effect of root canal irrigants on bacterial removal8. Therefore, E. faecalis biofilm has strong viability and drug resistance.
Several root canal irrigants have antimicrobial activities when contacting microorganisms directly, but few can eliminate E. faecalis absolutely9. Researchers have proved that 17% EDTA, 2% chlorhexidine (CHX), and 0.2% cetrimide (CTR) can eliminate E. faecalis at some extent10,11. However, the effects of CHX on E. faecalis are weaker than those of MTAD and Tetraclean12. MTAD (a mixture of tetracycline isomer, citric acid, and detergent) can sterilize E. faecalis rapidly, as can 0.2% CHX13,14. CTR is also a cationic surfactant with excellent antibacterial activity15. However, the residual antimicrobial activity of CHX could last longer than that of CTR16. QMix, a recent root canal irrigant that was first introduced in the marked in 201217, has comparable scavenging effects on smear layer to EDTA and its antibacterial effect is better than that of CHX18.
Currently, most studies focus on the antibacterial activities of different irrigating solutions. However, there are few studies on the residual antimicrobial activities of root canal irrigants. Therefore, this study aimed to compare the antibacterial and residual antimicrobial activities of five root canal irrigants (17% EDTA, 2% CHX, 0.2% CTR, MTAD, and QMix) in a model of E. faecalis biofilm infection of dentin blocks.
Material and Methods
Preparation of the dentin blocks
A total of 200 freshly extracted, intact maxillary and mandibular molars without preoperative treatment, root caries, and cracks were collected from the maxillofacial department of Tianjin Medical University Dental Hospital. The study was approved by the Medical Ethics Committee of Tianjin Medical University and the methods were carried out in accordance with the Declaration of Helsinki (2008). All patients have obtained the informed consent.
The dental calculus and soft tissue remnants of all molars were mechanically cleaned with a periodontal curette, and then stored in a 0.1% thymol solution at 4 °C until required. Next, a total of 350 dentin blocks (2 mm × 2 mm × 1 mm [width × length × height]) were prepared by selecting the coronal one-third of the roots (from the cementoenamel junction); then, the pulpal side of the dentin blocks was polished with 600-grit silicon carbide abrasive paper (Buehler, IL, USA). The dentin blocks were immersed into 3% NaOCl (Septodont, Saint-Maur, France) for 1 min, and then transferred into 17% EDTA (META, Chungbuk, Republic of Korea) for 2 min to remove the smear layer. Next, the dentin blocks were steam autoclaved for 30 min under 15 psi pressure at 121 °C to ensure that no bacteria remained. All samples were preserved in 37 °C brain heart infusion (BHI; Qingdao Haibo Biotechnology Co., Ltd., Qingdao, China) for 24 h to test for the presence of bacteria. After confirming complete sterilization, all dentin blocks were put into sterile saline.
Preparation of the bacterial suspension
After the standard strains of E. faecalis preserved at −80 °C were thawed, they were inoculated into freshly prepared BHI and cultivated under anaerobic condition at 37 °C for 24 h. Then, a bacterial suspension with 1 × 107/ml E. faecalis was obtained by dilution with BHI broth.
Root canal irrigants
Five root canal irrigants were used in the present study: 17% EDTA (Sigma-Aldrich, St Louis, USA), 2% CHX (Sigma-Aldrich), 0.2% CTR (Sigma-Aldrich), MTAD (Dentsply Tulsa, Tulsa, OK), and QMix (Dentsply Tulsa, Tulsa, OK).
Antibacterial activity test
A total of 60 dentin blocks were randomly selected from the initial 350 blocks and put into 200 μl tubes (one block per tube). Then, 180 μl E. faecalis suspension was added to each tube. After sealing with Parafilm®, the tubes were incubated under aerobic condition at 37 °C for 3 weeks. During this period, BHI broth was changed every other day to maintain normal growth of E. faecalis. Before each exchange, two samples of broth culture were collected randomly. A portion from each sample was inoculated into BHI, and further cultivated at 37 °C for 24 h. Finally, some of the bacterial colonies were dyed with Gram stain. Finally, the E. faecalis-infected models were established.
The 60 infected dentin blocks were flushed with 180 μl sterile saline for 2 min, and put into 96-well plates (one block per well). The dentin blocks were randomly divided into six groups: EDTA, CHX, CTR, MTAD, QMix, and untreated control groups (n = 10 per group); ten sterile dentin blocks were used as the blank control group. The five different root canal irrigants or BHI broth were added into the corresponding wells (100 μl per well). After 2-min incubation, the dentin blocks were removed and dried with aseptic absorbent paper. Next, the dentin blocks were centrifuged in 200 μl BHI for 2 s and vortexed (Vortex-6, Shanghai Pure One Biotechnology Co., Ltd., Shangai, China) for 1 min. The supernatants in the centrifuge tubes were removed by pipette and diluted ten times. The diluted supernatants were inoculated into BHI medium and cultivated under anaerobic conditions at 37 °C for 24 h. Finally, the colony-forming units (CFUs) were counted.
Residual antimicrobial activity test
The remaining 280 sterile dentin blocks were randomly divided into seven groups: EDTA, CHX, CTR, MTAD, QMix, untreated control, and blank control groups (n = 40 per group). The dentin blocks were put into 96-well plates (one block per well). The five different root canal irrigants or BHI broth were added into the corresponding wells (100 μl per well). All blocks were removed after 2 min and put into 200-μl tubes. Enterococcus faecalis suspension (180 μl) was added to the treated and untreated control groups, while an equal volume of BHI broth was added to the blank control group.
All samples were incubated at 37 °C and cultivated under anaerobic conditions for 48 h. Every 12 h, ten samples were collected from each group to count the CFUs using the same method as described above.
Statistical analysis
After the values of CFUs were converted into lgCFUs, data expressed as mean ± standard deviation were analyzed by SPSS version 17.0 statistical software (Chicago, IL, USA). The differences in the antibacterial and residual antimicrobial activities were analyzed by one-way and multivariate ANOVA. A P-value of less than 0.05 was considered to be statistically significant.
Results
Antibacterial activity
No bacteria were observed in the blank control group. Compared with the untreated control group, E. faecalis IgCFUs in dentin blocks treated with EDTA, CHX, CTR, MTAD, and QMix were significantly reduced (P < 0.05) (Table 1, Fig. 1). The QMix group had the lowest lgCFU value (2.31 ± 0.32), followed by the CHX group (2.41 ± 0.21), the MTAD group (3.26 ± 0.23), the CTR group (3.71 ± 0.72), and the EDTA group (4.37 ± 0.31). The antibacterial activities of the six groups were significantly different (P < 0.05), except between CHX and QMix groups (P > 0.05).
Table 1. Antibacterial and residual antimicrobial activities (lgCFUs) of the five root canal irrigants (EDTA, CHX, CTR, MTAD, and QMix) and untreated control groups.
| Groups | Antibacterial activity | Residual antimicrobial activity | |||
|---|---|---|---|---|---|
| 12 h | 24 h | 36 h | 48 h | ||
| EDTA | 4.37 ± 0.31* | −0.98 ± 3.78 | 7.00 ± 0.18 | 7.21 ± 0.06 | 0.10 ± 4.58 |
| CHX | 2.41 ± 0.21* | −0.92 ± 2.92 | 0.86 ± 6.51* | 0.31 ± 4.72* | 1.41 ± 2.80 |
| CTR | 3.71 ± 0.72* | −3.85 ± 2.89* | −2.33 ± 5.50* | 4.41 ± 0.23 | 4.24 ± 0.25 |
| MTAD | 3.26 ± 0.23* | −1.06 ± 3.72 | 8.55 ± 0.03 | 6.33 ± 0.07 | 3.86 ± 0.42 |
| QMix | 2.31 ± 0.32* | −6.00 ± 0.00* | 8.34 ± 0.03 | 2.94 ± 5.08 | −1.89 ± 4.88 |
| Untreated | 5.09 ± 0.26 | 1.89 ± 0.14 | 8.62 ± 0.02 | 8.15 ± 0.09 | 0.66 ± 6.08 |
CFUs: colony-forming units; EDTA: ethylene diamine tetraacetic acid; CHX: chlorhexidine; CTR: cetrimide; MTAD: a mixture of tetracycline isomer, citric acid and detergent.
*P < 0.05 compared with the untreated control group.
Figure 1. Antibacterial activities (lgCFUs) of the five root canal irrigants (EDTA, CHX, CTR, MTAD, and QMix) and untreated control groups.
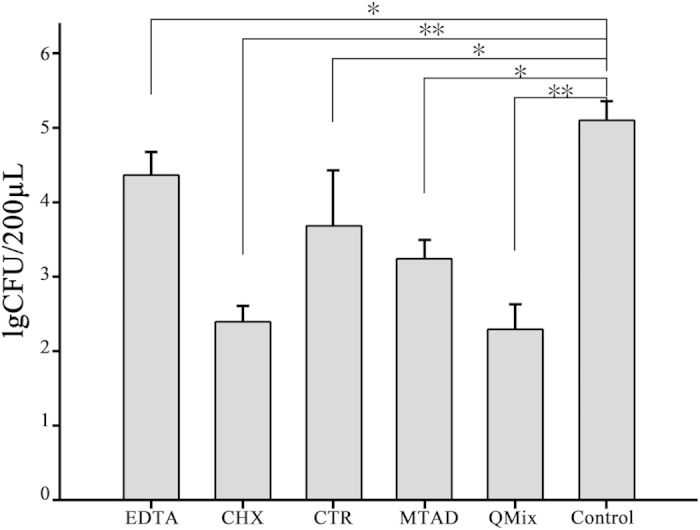
*P < 0.05, **P < 0.01 compared with the untreated control group.
Residual antimicrobial activity
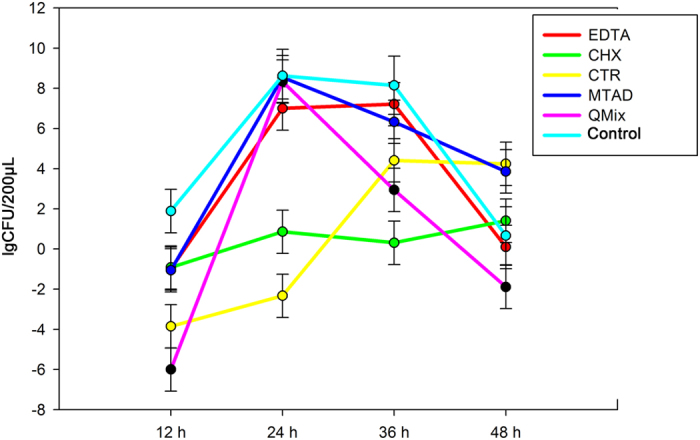
Differences in the residual antimicrobial activities among the six groups at 12 h, 24 h, 36 h, and 48 h are shown in Table 1 and Fig. 2. Compared with the untreated control group, the residual antimicrobial activities of the EDTA, CHX, and MTAD groups were not significantly different (P > 0.05). On the other hand, the lgCFUs of the CTR and QMix groups were significantly lower than those of the untreated control group (−3.85 ± 2.89 [CTR] and −6.00 ± 0.00 [QMix] vs. 1.89 ± 0.14 [untreated control]; P < 0.05) at 12 h. There was no significant difference in the residual antimicrobial activities among the EDTA, MTAD, QMix, and the untreated control groups at 24 h (P > 0.05). However, the lgCFUs of the CHX and CTR groups were significantly lower than those of the untreated control group (0.86 ± 6.51 and −2.33 ± 5.50 vs. 8.62 ± 0.02; P < 0.05) at 24 h. At 36 h, only the lgCFUs of the CHX group was significantly lower than those of the untreated control group (0.31 ± 4.72 vs. 8.15 ± 0.09; P < 0.05). There was no significant difference in the residual antimicrobial activities among the treated and untreated control groups at 48 h (P > 0.05).
Figure 2. Residual antimicrobial activities (lgCFUs) of the five root canal irrigants (EDTA, CHX, CTR, MTAD, and QMix) and untreated control groups at 12 h, 24 h, 36 h, and 48 h.
Discussion
Enterococcus faecalis, which usually exists in the form of a biofilm, is considered one of the most intractable bacteria. Kishen et al. have determined the chemical composition of E. faecalis biofilms and observed apatite deposition on mature biofilm19. This microstructure enhanced E. faecalis adhesion and drug resistance, making it more difficult to remove. This study was designed to identify the most effective root canal irrigant against E. faecalis, in the hopes of improving root canal treatment outcomes.
We discovered that 2% CHX had similar antibacterial activity to QMix. It has been reported that 2% CHX can sterilize 99.93% of the bacteria in E. faecalis biofilm10. QMix is composed of various ingredients, including EDTA, CHX, and detergents. As the amounts of QMix and 2% CHX were the same in this study, QMix contained relatively less CHX. However, CHX in QMix might produce synergistic antibacterial activity with EDTA, and the detergents in QMix might increase wettability and permeability, which might altogether enhance the antibacterial activity of QMix. Therefore, the antibacterial activities of 2% CHX and QMix were similar.
EDTA has not shown an obvious antibacterial effect against E. faecalis in many studies10,20. This study indicated that the antibacterial activity of 17% EDTA against E. faecalis was weaker than that of the other four irrigants. As a chelating agent removing the inorganic components in the smear layer, EDTA could change the permeability of cell membrane; this is the mechanism for its antibacterial activity21. Nevertheless, 17% EDTA has large surface tension and small permeability, which made it difficult to penetrate into the dentine tubules to kill the E. faecalis exhaustively.
Previous studies have shown that 0.1% CTR and 2% CHX could sterilize similar amount of E. faecalis on the dentin13,22. In this study, the antibacterial effect of 0.2% CTR was weaker than that of 2% CHX, which might be due to the fact that CTR had a stronger ability of reducing bacterial adhesion, so more viable bacteria could be counted.
Residual antimicrobial activities of the five irrigants were also examined to evaluate their effects on inhibiting E. faecalis biofilm formation. At 12 h, the fewest viable bacteria were detected in the QMix group, followed by the CTR group. However, at 24 h, the number of viable bacteria in QMix group increased significantly, suggesting that residual antimicrobial activity of QMix was strong, yet of short duration. Although there were more viable bacteria at 24 h than at 12 h, the CTR group had the lowest CFUs at 24 h, and residual antimicrobial activity of CTR remained until at least 36 h. Therefore, residual antimicrobial activity of CTR was more stable and durable.
Residual antimicrobial activities of irrigants weaken gradually and finally disappear23, as confirmed in this study except for CHX. CHX did not have residual antimicrobial activity at 12 h, while its residual antimicrobial activity became significant at 24 h and 36 h, probably because there were few E. faecalis at 12 h or CHX had a weak residual antimicrobial activity. Since the number E. faecalis increased gradually, but the residual antimicrobial activity of CHX did not change over time, the effect of CHX became gradually decreased. Therefore, compared with CTR and QMix, CHX had the weakest yet the most stable residual antimicrobial activity. Baca et al. have tested the residual antimicrobial activities of CHX and CTR with a fresh bacteria suspension and found that CHX and CTR can last for more than 40 days16. In this study, the residual antimicrobial activities of the five irrigants disappeared at 48 h. In our study, bacteria were grown in a closed system (also known as a batch culture) to which no nutrients were added or removed, thus maintaining a constant culture volume. The growth curve of a typical batch culture can be divided into four phases: lag phase, log phase, stationary phase, and decline phase. Eventually, the death rate of E. faecalis gradually increases as nutrients are depleted and toxic waste products accumulate, and E. faecalis ultimately enter the decline phase. Residual antimicrobial activities of EDTA and MTAD may not have been detected because of this experimental limitation. In addition, a relatively large volume of bacterial suspension was added, and the small amount of irrigating solution remaining on the dentin blocks was diluted, which would decrease its residual antimicrobial activity.
In conclusion, EDTA, CHX, CTR, MTAD, and QMix can kill E. faecalis in the matured biofilm at different levels. Their antibacterial activity from strong to weak was CHX and QMix, MTAD, CTR, and EDTA. However, only CHX, CTR, and QMix had residual antimicrobial activities that lasted for at least 36 h, 24 h, and 12 h, respectively.
Additional Information
How to cite this article: Zhang, R. et al. Antibacterial and residual antimicrobial activities against Enterococcus faecalis biofilm: A comparison between EDTA, chlorhexidine, cetrimide, MTAD and QMix. Sci. Rep. 5, 12944; doi: 10.1038/srep12944 (2015).
Footnotes
Author Contributions Rui Zhang and Min Chen performed the experiments, analyzed and interpreted the data, and wrote the manuscript. Yan Lu and Xiangjun Guo interpreted the data and helped to prepare the manuscript. Feng Qiao performed the statistical analysis. Ligeng Wu designed the study, interpreted the data, and reviewed the manuscript. All authors have read and approved the final version of the manuscript.
References
- Vivacqua-Gomes N. et al. Recovery of Enterococcus faecalis after single- or multiple-visit root canal treatments carried out in infected teeth ex vivo. Int Endod J 38, 697–704 (2005). [DOI] [PubMed] [Google Scholar]
- Hancock H. H. 3rd, Sigurdsson A., Trope M. & Moiseiwitsch J. Bacteria isolated after unsuccessful endodontic treatment in a North American population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91, 579–586 (2001). [DOI] [PubMed] [Google Scholar]
- Peciuliene V., Reynaud A. H., Balciuniene I. & Haapasalo M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int Endod J 34, 429–434 (2001). [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Flannagan S. E. & Sedgley C. M. Coaggregation interactions between oral and endodontic Enterococcus faecalis and bacterial species isolated from persistent apical periodontitis. J Endod 32, 946–950 (2006). [DOI] [PubMed] [Google Scholar]
- Siqueira J. F. Jr. & Rocas I. N. Exploiting molecular methods to explore endodontic infections: Part 2–Redefining the endodontic microbiota. J Endod 31, 488–498 (2005). [DOI] [PubMed] [Google Scholar]
- Gomes B. P. et al. Microbiological examination of infected dental root canals. Oral Microbiol Immunol 19, 71–76 (2004). [DOI] [PubMed] [Google Scholar]
- Pinheiro E. T. et al. Microorganisms from canals of root-filled teeth with periapical lesions. Int Endod J 36, 1–11 (2003). [DOI] [PubMed] [Google Scholar]
- Yang S. E. et al. Effect of smear layer and chlorhexidine treatment on the adhesion of Enterococcus faecalis to bovine dentin. J Endod 32, 663–667 (2006). [DOI] [PubMed] [Google Scholar]
- Chavez de Paz L. E., Bergenholtz G. & Svensater G. The effects of antimicrobials on endodontic biofilm bacteria. J Endod 36, 70–77 (2010). [DOI] [PubMed] [Google Scholar]
- Baca P., Junco P., Arias-Moliz M. T., Gonzalez-Rodriguez M. P. & Ferrer-Luque C. M. Residual and antimicrobial activity of final irrigation protocols on Enterococcus faecalis biofilm in dentin. J Endod 37, 363–366 (2011). [DOI] [PubMed] [Google Scholar]
- Williamson A. E., Cardon J. W. & Drake D. R. Antimicrobial susceptibility of monoculture biofilms of a clinical isolate of Enterococcus faecalis. J Endod 35, 95–97 (2009). [DOI] [PubMed] [Google Scholar]
- Giardino L. et al. Antimicrobial effect of MTAD, Tetraclean, Cloreximid, and sodium hypochlorite on three common endodontic pathogens. Indian J Dent Res 20, 391 (2009). [DOI] [PubMed] [Google Scholar]
- Portenier I., Waltimo T., Orstavik D. & Haapasalo M. Killing of Enterococcus faecalis by MTAD and chlorhexidine digluconate with or without cetrimide in the presence or absence of dentine powder or BSA. J Endod 32, 138–141 (2006). [DOI] [PubMed] [Google Scholar]
- Krause T. A., Liewehr F. R. & Hahn C. L. The antimicrobial effect of MTAD, sodium hypochlorite, doxycycline, and citric acid on Enterococcus faecalis. J Endod 33, 28–30 (2007). [DOI] [PubMed] [Google Scholar]
- Wang Z., Shen Y., Ma J. & Haapasalo M. The effect of detergents on the antibacterial activity of disinfecting solutions in dentin. J Endod 38, 948–953 (2012). [DOI] [PubMed] [Google Scholar]
- Baca P. et al. Antimicrobial substantivity over time of chlorhexidine and cetrimide. J Endod 38, 927–930 (2012). [DOI] [PubMed] [Google Scholar]
- Ma J., Wang Z., Shen Y. & Haapasalo M. A new noninvasive model to study the effectiveness of dentin disinfection by using confocal laser scanning microscopy. J Endod 37, 1380–1385 (2011). [DOI] [PubMed] [Google Scholar]
- Dai L. et al. The effect of QMix, an experimental antibacterial root canal irrigant, on removal of canal wall smear layer and debris. J Endod 37, 80–84 (2011). [DOI] [PubMed] [Google Scholar]
- Kishen A., George S. & Kumar R. Enterococcus faecalis-mediated biomineralized biofilm formation on root canal dentine in vitro. J Biomed Mater Res A 77, 406–415 (2006). [DOI] [PubMed] [Google Scholar]
- Ferrer-Luque C. M., Arias-Moliz M. T., Gonzalez-Rodriguez M. P. & Baca P. Antimicrobial activity of maleic acid and combinations of cetrimide with chelating agents against Enterococcus faecalis biofilm. J Endod 36, 1673–1675 (2010). [DOI] [PubMed] [Google Scholar]
- Arias-Moliz M. T., Ferrer-Luque C. M., Espigares-Garcia M. & Baca P. Enterococcus faecalis biofilms eradication by root canal irrigants. J Endod 35, 711–714 (2009). [DOI] [PubMed] [Google Scholar]
- Giardino L., Ambu E., Becce C., Rimondini L. & Morra M. Surface tension comparison of four common root canal irrigants and two new irrigants containing antibiotic. J Endod 32, 1091–1093 (2006). [DOI] [PubMed] [Google Scholar]
- Mohammadi Z. & Abbott P. V. Antimicrobial substantivity of root canal irrigants and medicaments: a review. Aust Endod J 35, 131–139 (2009). [DOI] [PubMed] [Google Scholar]


