Highlight
CLE19, as an embryo-expressed CLV3/ESR gene, regulates cotyledon establishment in embryos and nuclear proliferation and cellularization in the endosperm.
Key words: Antagonistic peptide, CLE19, cotyledon establishment, endosperm cellularization, embryo–endosperm interaction.
Abstract
Embryo and endosperm development are two well co-ordinated developmental processes in seed formation; however, signals involved in embryo and endosperm interactions remain poorly understood. It has been shown before that CLAVATA3/ESR-RELATED 19 (CLE19) peptide is able to trigger root meristem consumption in a CLV2-dependent manner. In this study, the role of CLE19 in Arabidopsis seed development was explored using antagonistic peptide technology. CLE19 is expressed in the epidermal layers of the cotyledon primordia, hypocotyl, and root cap in the embryo. Transgenic plants carrying an antagonistic CLE19 G6T construct expressed under the control of CLE19 regulatory elements exhibited a dominant seed abortion phenotype, with defective cotyledon establishment in embryos and delayed nuclear proliferation and cellularization in endosperms. Ectopic expression of CLE19 G6T in Arabidopsis under the control of an endosperm-specific ALE1 promoter led to a similar defect in cotyledon establishment in embryos but without an evident effect on endosperm development. We therefore propose that CLE19 may act as a mobile peptide co-ordinating embryo and endosperm development.
Introduction
Double fertilization in angiosperms, where two male gametes join with the female gametophyte, initiates two parallel developmental events to produce a diploid embryo and a triploid endosperm. Embryo and endosperm development are highly co-ordinated processes (Lafon-Placette and Kohler, 2014). It has been reported previously that several endosperm-expressed genes regulate embryo development: ABNORMAL LEAF SHAPE1 (ALE1) and ZHOUPI (ZOU), which are expressed in the endosperm, regulate the formation of the cuticle layer of the embryo (Tanaka et al., 2001; Yang et al., 2008); and EMBRYO SURROUNDING FACTOR 1 (ESF1) expressed in the endosperm promotes suspensor elongation (Costa et al., 2014). However, how embryo-derived factors regulate endosperm development remains elusive.
As a founding member of the CLAVATA3 (CLV3)/EMBRYO SURROUNDING REGION (ESR)-RELATED (CLE) family genes, CLV3 acts as 12 or 13 amino acid peptide to interact with several leucine-rich repeat (LRR) receptor kinases to regulate stem cell homeostasis in shoot apical meristems (SAMs) (Clark et al., 1997; Fletcher et al., 1999; Kondo et al., 2006; Ohyama et al., 2009; Shinohara and Matsubayashi, 2015). Thirty-two CLE genes with diverse expression patterns have been identified in the Arabidopsis genome (Cock and McCormick, 2001; Oelkers et al., 2008; Jun et al., 2010), and among them only three have been genetically characterized. Mutation or T-DNA insertion in CLV3 led to plants with an enlarged SAM and increased numbers of floral organs in Arabidopsis (Leyser and Furner, 1992). T-DNA insertion in CLE40 led to plants with a slightly reduced root length and delayed differentiation of columella stem cells in root meristems (Hobe et al., 2003; Stahl et al., 2009). CLE8 is a seed-specific CLE family member expressed in both embryo and endosperm in Arabidopsis (Sharma et al., 2003), and a homozygous cle8-1 mutant showed defective embryo and endosperm development in ~15% of the seeds produced (Fiume and Fletcher, 2012). Although CLE41 and CLE44 have been implicated in xylem differentiation (Ito et al., 2006), BnCLE19 in cotyledon development (Fiers et al., 2004), and ESR1 in the embryo–endosperm interaction (Opsahl-Ferstad et al., 1997), since no genetic data are available for these genes, their endogenous functions remain to be elucidated. Studies performed thus far suggest a high level of redundancy among CLE members since mutants carrying T-DNA insertions in CLE1, CLE7, CLE10, CLE16, CLE18, or CLE19 in Arabidopsis showed no visible phenotype (Fiers et al., 2004; Jun et al., 2010), and overexpression of a large group of CLE genes using the cauliflower mosaic virus (CaMV) 35S promoter exhibited a similar dwarf and short-root phenotype (Fiers et al., 2004; Strabala et al., 2006).
BnCLE19 is an embryo-expressed CLE gene that was first identified in microspore embryogenesis of Brassica napus (Fiers et al., 2004). Transgenic Arabidopsis carrying the BnCLE19 regulatory elements fused with β-glucuronidase (GUS) or green fluorescent protein (GFP) reporter genes revealed expression in cotyledon primordia in triangular-stage embryos, and the expression persisted in the epidermal layer of cotyledons during embryogenesis (Fiers et al., 2004). Overexpression of BnCLE19 or Arabidopsis CLE19 under the control of the CaMV 35S promoter, or treatment of Arabidopsis seedlings with synthetic 12–14 amino acid CLE19 peptides, led to a CLV2-dependent premature stem cell differentiation in root meristems (Fiers et al., 2004, 2005). Suppressor screening using RCH1-CLE19 transgenic plants led to the identification of two genetic loci, SOL1 and SOL2 (Casamitjana-Martínez et al., 2003). SOL1 encodes a Zn2+-dependent carboxypeptidase that functions to remove the C-terminal arginine residue in CLE19 processing (Casamitjana-Martínez et al., 2003; Tamaki et al., 2013), while SOL2 is allelic to CRN that encodes an extracellular domain-free receptor-like kinase (Miwa et al., 2008; Muller et al., 2008). However, since the T-DNA insertional CLE19 mutant showed no visible phenotype (Fiers et al., 2004), the function of endogenous CLE19 remains unclear.
In this study, detailed expression and functional analyses were performed in CLE19 to elucidate its role in Arabidopsis. Using the recently developed antagonistic peptide technology (Song et al., 2013), it was demonstrated that CLE19, as an embryo-specifically expressed gene, regulates both embryo and endosperm development. Transgenic plants carrying the antagonistic CLE19 G6T construct expressed under the control of the endogenous CLE19 regulatory elements exhibited a dominant seed abortion phenotype, with defective cotyledon establishment and delayed endosperm development. The phenotype of altered cotyledon establishment was mimicked when CLE19 G6T was expressed under the control of an endosperm-specific ALE1 promoter.
Materials and methods
Plant materials and growth conditions
Wild-type and transgenic Arabidopsis thaliana plants (Col-0) were grown either on agar plates or in soil as described previously (Song et al., 2012). For transformation, 4- to 5-week-old Arabidopsis plants were transformed with Agrobacterium tumefaciens using the floral dip method (Clough and Bent, 1998).
Molecular cloning
A CLE19 genomic fragment containing a 1782bp 5′ upstream region (pCLE19), 225bp coding region, and a 1205bp 3′ downstream region (tCLE19) was amplified and cloned into pDONR201 (Invitrogen) to construct the pDONR201-pCLE19:CLE19:tCLE19 entry clone. To generate a glycine to threonine substitution (G6T) at the sixth amino acid (G6) of the CLE motif, a Fast Mutagenesis Kit (TransGen, Beijing) was used to introduce point mutations into pDONR201-pCLE19:CLE19:tCLE19 to produce pDONR201-pCLE19:CLE19 G6T :tCLE19, which was then transferred to the pBGWFS7 binary vector to generate pCLE19:CLE19 G6T :tCLE19. pCLE19 and tCLE19 were cloned into the ligation-independent cloning vectors pPLV04 and pPLV15 (De Rybel et al., 2011) to generate pCLE19:SV40-3XGFP:tCLE19 and pCLE19:GUS:tCLE19, respectively. pWOX1:SV40-3XGFP, pWOX3:SV40-3XGFP, and pALE1:SV40-3XGFP were made in a similar manner using pPLV04 (De Rybel et al., 2011). The pALE1:CLE19 G6T construct was made by replacing the SV40-3XGFP cassette in pALE1:SV40-3XGFP with the CLE19 G6T coding sequence.
In vitro embryo culture
Siliques containing ovules with embryos at the heart-shape stage were surface-sterilized, and embryos were isolated and transferred to the top of Gamborg’s B5 basal medium (PhytoTechnology) containing 10% sucrose, 0.6% agar (type A, Sigma-Aldrich), 400 μg ml–1 glutamine, and 500 μg ml–1 inositol, and cultured as described (Liu et al., 1995; Hadfi et al., 1998).
Microscopy
Siliques were fixed in pre-cooled acetone at –20°C for 1h. GUS assays were performed on fixed tissue as previously described (Fiers et al., 2004). Dissected ovules were cleared in Hoyers’ solution (Berleth and Jürgens, 1993) for 2h to overnight depending on developmental stage, and were then observed under a microscope equipped with differential interference contrast (DIC). For fluorescence microscopy, ovules from transgenic plants carrying GFP fusion constructs were mounted with 5% glycerol and observed under a fluorescence microscope. Embryos for confocal laser scanning microscopy (CLSM) were dissected from ovules and transferred to a 9% glucose solution containing 20 μg ml–1 propidium iodide (PI) solution (Sigma-Aldrich) prior to imaging. ImageJ software (version 1.4.3) was used for image processing and analyses. For cytohistological analyses, periodic acid–Schiff’s reagent (PAS) staining (Baum, 2008) was performed on semi-thin sectioned ovules embedded in LR White resin (The London Resin Company).
RT–PCR and qRT-PCR analyses
RNAs were extracted from ~300 embryos or ~100 ovules using a Plant Total RNA Isolation Kit (GeneMark, Beijing) and reverse transcribed using a First Strand cDNA Synthesis Kit (Tiangen, Beijing). Reverse transcription–PCR (RT–PCR) was performed with the resultant cDNA for analysing EIF4A (used as an internal control) and CLE19 expression. Quantitative real-time PCR (qRT-PCR) was performed using a Rotor-Gene 3000 thermocycler (Corbett) with the SYBR Premix ExTaq II kit (TaKaRa, Dalian) to assess relative expression levels of WOX1, WOX3, MEA, FIS2, and AGL62. Expression data were normalized to EIF4A using the 2–ΔΔCT method (Livak and Schmittgen, 2001). All primers used are listed in Supplementary Table S1 available at JXB online.
Results
Transgenic plants carrying the pCLE19:CLE19 G6T :tCLE19 construct exhibited defective embryo and endosperm development
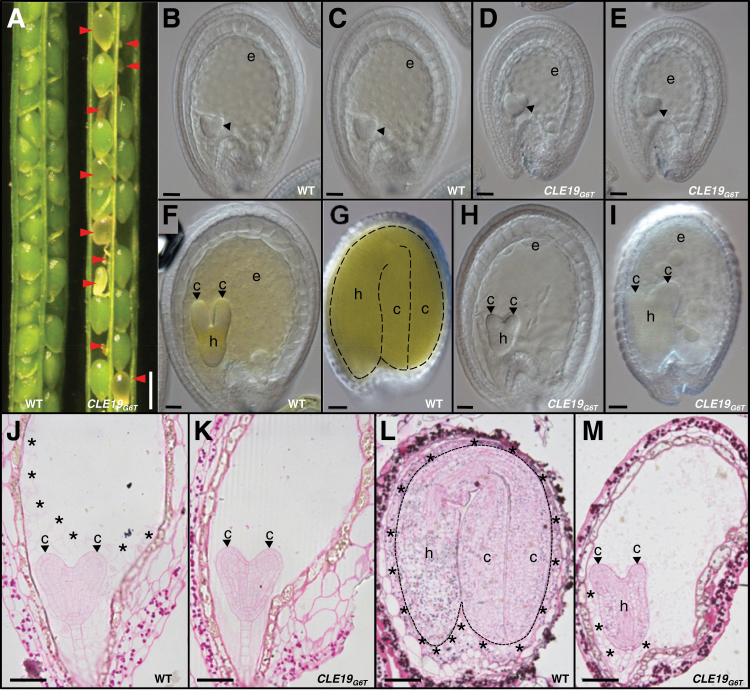
It has been shown previously that BnCLE19 is an embryo-specific gene expressed in cotyledon primordia in triangular-stage embryos, and in the epidermal layer of cotyledons in heart-shape and torpedo-stage embryos (Fiers et al., 2004). In this study, the antagonistic pCLE19:CLE19 G6T :tCLE19 construct (Song et al., 2013) was devised to elucidate the role of CLE19 in Arabidopsis. The construct consisted of a 1782bp 5′ upstream region (pCLE19), a 225bp coding region, and a 1205bp 3′ downstream sequence (tCLE19) of CLE19, with the conserved sixth glycine in the CLE motif substituted by threonine. The construct was transformed into Arabidopsis (Col-0) using the floral dip method (Clough and Bent, 1998). Among 54 independent T1 transgenic lines examined, 12 showed different percentages of seed abortions, and among these 12 lines, three (#1, #2, and #3) exhibited consistently high frequencies of a seed abortion phenotype (Fig. 1A). Examination of seeds at 12 days after pollination (DAP) under a dissection microscope revealed that 36.3, 33.8, and 33.4% of ovules in lines #1, #2, and #3, respectively, were aborted before maturation (Table 1). Progeny plants produced from these three lines showed a consistent seed abortion phenotype for four generations examined so far. The transgenic plants were pollinated with pollen from wild-type plants, and the resultant ovules showed similar frequencies of seed abortion, indicating that the abortion phenotype was a dominant trait. The phenotypes of these three transgenic lines were indistinguishable from one another, and transgenic line #1 was used in all subsequent studies.
Fig. 1.
Defective embryo and endosperm development in pCLE19:CLE19 G6T :tCLE19 transgenic plants. (A) Siliques from wild-type (WT) and pCLE19:CLE19 G6T :tCLE19 transgenic plants (CLE19 G6T), showing aborted ovules (indicated by arrowheads) at 12 DAP. (B–I) DIC microscopic observations of cleared ovules from wild-type (B, C, F, G) and pCLE19:CLE19 G6T :tCLE19 transgenic plants (D, E, H, I) at 5 (B–E), 7 (F, H), and 12 DAP (G, I). Note the reduced sizes of embryo sacs in (D), and decreased numbers of endosperm nuclei in (E), as compared with (B) and (C), while there is no obvious defect in the embryo (indicated by arrowheads) at this stage. Delayed cotyledon formation (indicated by arrowheads) in embryos from pCLE19:CLE19 G6T :tCLE19 transgenic plants at 7 (H) and 12 DAP (I), as compared with wild-type ovules at the same stages (F, G). (J–M) Cytohistological analyses of embryos and endosperms in the wild-type (J, L) and pCLE19:CLE19 G6T :tCLE19 transgenic plants (K, M) at 5 (J, K) and 12 DAP (L, M), to show the delayed endosperm cellularization (indicated by asterisks) and defective cotyledon establishment (indicated by arrowheads) in ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants (K, M), as compared with the wild type at the corresponding stages (J, L). c, cotyledon; h, hypocotyl; e, endosperm. Scale bars: in A=500 μm; in B–E=100 μm; in F and H=50 μm; in G and I=100 μm; and in J–M=50 μm.
Table 1.
Frequencies of seed abortions in pCLE19:CLE19G6T:tCLE19 and pALE1:CLE19G6T transgenic plants
| Line | Normal | Aborted | Abortion frequency (%) |
|---|---|---|---|
| pCLE19:CLE19 G6T :tCLE19 | |||
| #1 | 272 | 155 | 36.3 |
| #2 | 290 | 148 | 33.8 |
| #3 | 289 | 145 | 33.4 |
| pALE1:CLE19 G6T | |||
| #1 | 106 | 54 | 33.3 |
| #2 | 101 | 40 | 28.4 |
| #3 | 100 | 49 | 32.9 |
Ovules were examined under a DIC microscope after clearing using Hoyers’ solution. Embryos at the early heart-shaped stage (5 DAP) in ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants were morphologically indistinguishable from those in the wild type (Fig. 1B, D). However, the size of embryo sacs in some ovules from the transgenic plants was smaller than those in the wild type at the same stage (Fig. 1B, D). In addition, reduced numbers of endosperm nuclei were often observed in these small embryo sacs (Fig. 1C, E), suggesting that endosperm development was delayed in these transgenic plants. Additionally, evident abnormal embryo development was observed in some ovules from these transgenic plants from 7 DAP onwards, with defective cotyledon formation (Fig. 1F–I, indicated by arrowheads).
Cytohistological examinations of PAS-stained semi-thin sectioned ovules confirmed that at 5 DAP embryos in pCLE19:CLE19 G6T :tCLE19 transgenic plants were morphologically indistinguishable from those in the wild type (Fig. 1J, K). Strikingly, no cellularization was seen in endosperms from transgenic plants at this stage (Fig. 1K); in contrast, wild-type endosperms at the same stage showed complete cellularization in the embryo surrounding region, and partial cellularization at the periphery of the endosperm (Fig. 1J, indicated by asterisks). Even at 12 DAP, only partially cellularized endosperms were observed in aborted ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants (Fig. 1L, M; indicated by asterisks), while endosperms in the wild type were cellularized completely by 8 DAP. Moreover, although cotyledon primordia were formed in aborted embryos in the transgenic plants at 12 DAP, further growth of these primordia was arrested (Fig. 1L, M; indicated by arrowheads), suggesting a defect in cotyledon establishment. In wild-type embryos, starch grains (stained dark red by PAS) were accumulated in the whole embryos by 12 DAP (Fig. 1L), while those arrested embryos from the transgenic plants exhibited a substantially lower starch accumulation in cotyledon primordia (Fig. 1M, indicated by arrowheads), suggesting that cotyledon differentiation was delayed. It should be noted that no obvious defect was observed in the hypocotyl and the root tip regions (Fig. 1I, M). Thus, expression of CLE19 G6T under the control of CLE19 regulatory elements in Arabidopsis led to defective cotyledon establishment and delayed endosperm nuclear proliferation and cellularization.
Expression of CLE19 in seeds is confined to the embryo
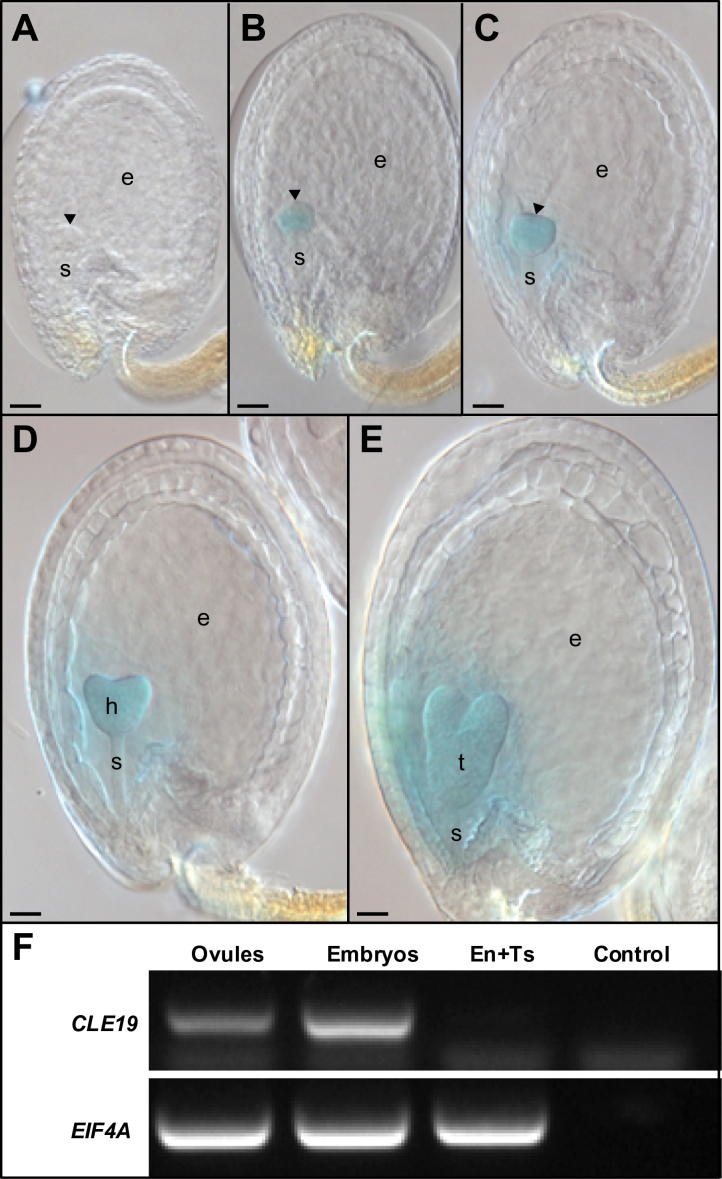
To investigate the expression of CLE19 in Arabidopsis, a pCLE19:GUS:tCLE19 reporter construct was made, with the coding region of pCLE19:CLE19 G6T :tCLE19 replaced by the GUS gene, and transformed into Arabidopsis (Col-0). In ovules from the transgenic plants obtained, GUS expression was first observed in embryos at the late globular stage (Fig. 2A, B), and persisted in the triangular (Fig. 2C), heart-shaped (Fig. 2D), and torpedo-stage (Fig. 2E) embryos. No GUS expression was observed in testa or suspensors.
Fig. 2.
Embryo-specific expression of CLE19 during seed development. (A–E) GUS expression in seeds excised from pCLE19:GUS:tCLE19 transgenic plants. Note that GUS staining was first detected in late globular-stage embryos (B), and persisted in the triangular (C), heart-shaped (D), and torpedo-stage embryos (E), while no GUS expression was observed in the early globular embryo (A). Scale bars=50 μm. Early embryos (A–C) are indicated by arrowheads; h, heart-shaped embryo; t, torpedo-stage embryo; e, endosperm; s, suspensor. (F) RT–PCR to show CLE19 expression in ovules and embryos, but not in mixed endosperm and testa tissues (En+Ts). EIF4A was used as an internal standard. Control, without cDNA.
A weak GUS signal was detected in the ESR (Fig. 2C–E) in pCLE19:GUS:tCLE19 transgenic plants. To examine if the signal is from expression or diffusion, RT–PCR was performed using RNA extracted from (i) whole ovules; (ii) isolated embryos; and (iii) the mixed endosperm and testa tissues when embryo development in these ovules was at the heart-shaped stage. Expression of the CLE19 transcript was detected in whole ovules and in embryos, but not in mixed endosperm and testa tissues (Fig. 2F), suggesting that the weak GUS staining signal detected in the ESR was likely to be diffused from the GUS-positive embryo.
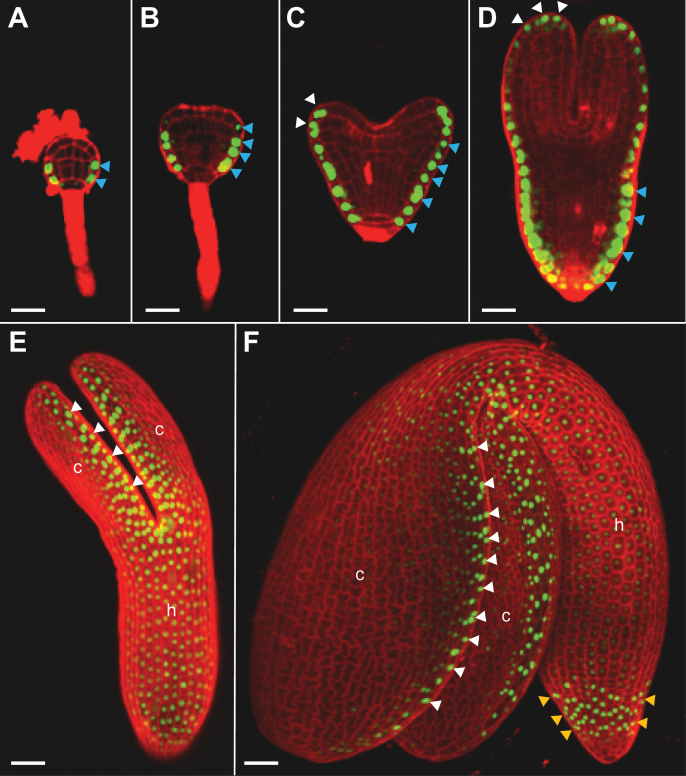
A pCLE19:SV40-3XGFP:tCLE19 reporter construct with a nuclear-localized triple GFP (SV40-3XGFP) was made and transformed into Arabidopsis (Col-0) to define further the expression pattern of CLE19 in developing embryos. CLSM was performed in embryos dissected from pCLE19:SV40-3XGFP:tCLE19 transgenic plants. GFP expression was first observed in epidermal cells located in the lower portion of the embryo at the late globular stage, and in their progeny cells in triangular-stage embryos (indicated by blue arrowheads; Fig. 3A, B). In heart-shaped and torpedo-stage embryos, additional GFP expression was observed in cotyledon primordia (indicated by white arrowheads; Fig. 3C, D). In walking-stick and cotyledonary-stage embryos, GFP expression was observed in cells located at the edges of the two cotyledons and cells in the root cap (Fig. 3E, F). This expression pattern differs slightly from that observed previously for BnCLE19 from Brassica napus, as no expression was observed in the hypocotyl and root cap regions when the BnCLE19 regulatory elements were examined (Fiers et al., 2004).
Fig. 3.
Dynamic expression of CLE19 during embryogenesis. As examined under a confocal microscope in embryos excised from pCLE19:SV40-3XGFP:tCLE19 transgenic plants, GFP expression was first observed in protodermal cells (indicated by blue arrowheads) at the lower portion of the 32-cell stage embryo (A), and persisted in their progeny cells in the triangular-stage embryo (B). Additional GFP expression was observed in epidermal cells at the tips and abaxial sides of the cotyledon (indicated by white arrowheads) in heart-shaped (C) and torpedo-stage embryos (D). In walking-stick (E) and cotyledonary-stage embryos (F), strong GFP expression was seen in epidermal cells located at the edges of cotyledons (c, indicated by white arrowheads) and in root caps (indicated by yellow arrowheads), weak GFP expression was observed in the hypocotyl (h). (E) and (F) were prepared by superimposing multiple scanned images. Scale bars=50 μm.
Cotyledon establishment, not initiation, is defective in pCLE19:CLE19 G6T :tCLE19 transgenic plants
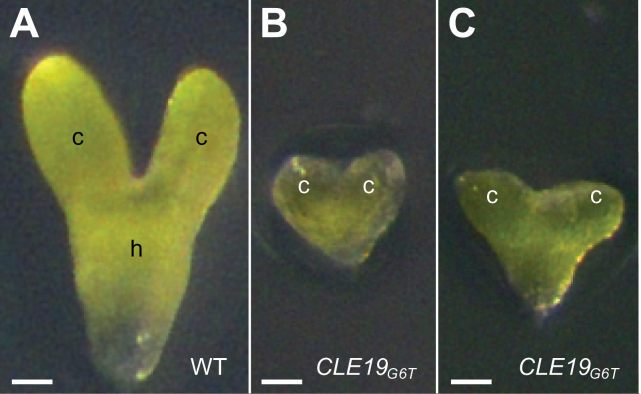
The major defect of pCLE19:CLE19 G6T :tCLE19 transgenic plants was observed in embryos after the heart-shaped stage. Therefore, in vitro embryo culture was used to examine whether the defective cotyledon development phenotype could be rescued. Early heart-shaped embryos were isolated from the wild-type and transgenic plants and cultured in vitro. After 5 d of cultivation, wild-type embryos had progressed to the cotyledonary stage and had formed two well-established cotyledons (Fig. 4A). However, embryos isolated from pCLE19:CLE19 G6T :tCLE19 transgenic plants exhibited a severe defect in cotyledon establishment (Fig. 4B–D), suggesting that the cotyledon arrest was intrinsic to the embryo.
Fig. 4.
Embryo culture in vitro did not rescue the defective cotyledon phenotype in embryos excised from pCLE19:CLE19 G6T :tCLE19 transgenic plants. Note the abnormal cotyledon establishment in embryos from pCLE19:CLE19 G6T :tCLE19 transgenic plants (B, C), as compared with the control embryo from the wild type (A). c, cotyledon; h, hypocotyl. Scale bars=50 μm.
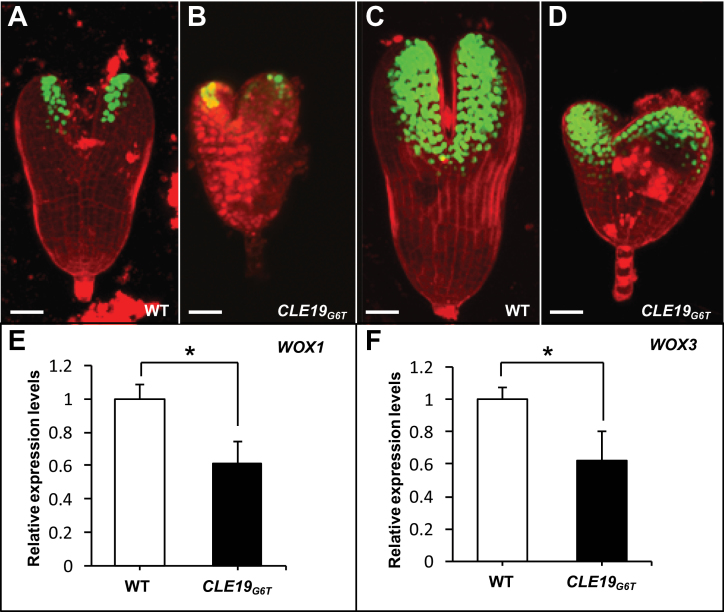
To elucidate further whether the defect in cotyledon development in pCLE19:CLE19 G6T :tCLE19 plants is attributed to failures in cotyledon initiation or cotyledon establishment, two cotyledon marker lines were developed based on published in situ hybridization results for WUSCHEL-RELATED HOMEOBOX 1 (WOX1) and WOX3 (Haecker et al., 2004). Upstream sequences from WOX1 and WOX3 (4454bp and 5028bp, respectively) were fused to the SV40-3XGFP reporter gene and transformed into the wild-type Arabidopsis (Col-0). Transgenic plants were examined and crossed to pCLE19:CLE19 G6T :tCLE19 plants, and progeny plants carrying pCLE19:CLE19 G6T :tCLE19 and homozygous pWOX1:SV40-3XGFP or pWOX3:SV40-3XGFP constructs were examined under CLSM. As shown in Fig. 5, GFP expression in torpedo-stage embryos from wild-type plants carrying pWOX1:SV40-3XGFP was restricted to cells located at the edges of two cotyledons (Fig. 5A). Embryos from plants carrying both the pCLE19:CLE19 G6T :tCLE19 and the pWOX1:SV40-3XGFP constructs exhibited a similar GFP expression pattern despite a severe delay in cotyledon development in these embryos (Fig. 5B). GFP expression in wild-type torpedo-stage embryos carrying the pWOX3:SV40-3XGFP construct was observed at the adaxial side of two cotyledons (Fig. 5C). Similar GFP expression was also observed in transgenic plants carrying both the pWOX3:SV40-3XGFP and pCLE19:CLE19 G6T :tCLE19 constructs (Fig. 5D). Real-time PCR analyses confirmed that both WOX1 and WOX3 were expressed in pCLE19:CLE19 G6T :tCLE19 plants, albeit with a 30–40% reduction observed in ovules carrying defective embryos compared with those from the wild type (Fig. 5E, F). These data suggest that cotyledon initiation was unaffected in pCLE19:CLE19 G6T :tCLE19 transgenic plants.
Fig. 5.
Expression of cotyledon-specific genes in arrested embryos from pCLE19:CLE19 G6T :tCLE19 transgenic plants. (A–D) Confocal microscopic examinations of embryos excised from wild-type plants (WT) carrying pWOX1:SV40-3XGFP (A) or pWOX3:SV40-3XGFP marker constructs (C), or from plants carrying pWOX1:SV40-3XGFP/pCLE19:CLE19 G6T :tCLE19 (B) or pWOX3:SV40-3XGFP/pCLE19:CLE19 G6T :tCLE19 double constructs (D). Note that similar GFP expression, though with a reduced level, was observed in cotyledon primordia of arrested embryos from pCLE19:CLE19 G6T :tCLE19 transgenic plants (CLE19 G6T), as compared with embryos from plants carrying only marker constructs (A, C). Scale bars=50 μm. (E, F) qRT-PCR showed reduced levels of WOX1 (E) and WOX3 expression (F) in ovules from the wild type (WT) and pCLE19:CLE19 G6T :tCLE19 transgenic plants (CLE19 G6T). Data represent the mean ±SD from three independently extracted RNA samples. Asterisks indicate significant differences from the wild type (P < 0.01 by Student’s t-test).
Endosperm development is delayed in pCLE19:CLE19 G6T :tCLE19 transgenic plants
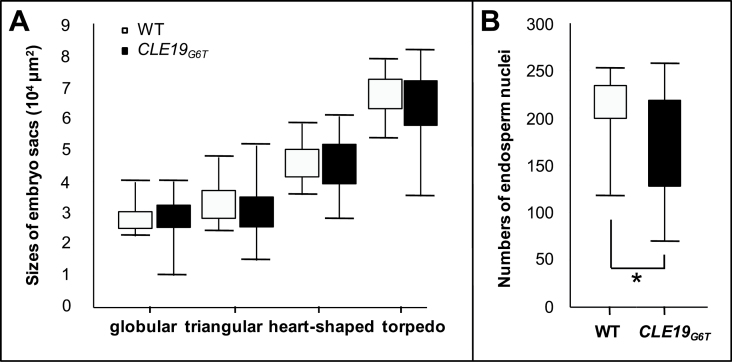
To examine further the endosperm defect in pCLE19:CLE19 G6T :tCLE19 transgenic plants, the size of the embryo sac (the total area occupied by the endosperm and the embryo) was measured in ovules when embryos were at the late globular, triangular, heart-shaped, or torpedo stages. Compared with those in the wild type, embryo sacs in ovules from siliques of pCLE19:CLE19 G6T :tCLE19 transgenic plants exhibited much greater variation in size. Many embryo sacs from the transgenic plant were smaller than those from the wild type at the same stage (Fig. 6A). The smallest embryo sac in pCLE19:CLE19 G6T :tCLE19 transgenic plants was only half the size of those from the wild type, while the largest embryo sac was not significantly bigger than those from the wild type (Fig. 6A). This result suggests that endosperm development is delayed in pCLE19:CLE19 G6T :tCLE19 transgenic plants.
Fig. 6.
Delayed embryo sac and endosperm development in pCLE19:CLE19 G6T :tCLE19 transgenic plants. (A) Smaller sizes of embryo sacs in pCLE19:CLE19 G6T :tCLE19 transgenic plants (CLE19 G6T) as compared with those in the wild type (WT). Box plots are used to show total embryo sac areas. The whole area occupied by the embryo and endosperm was measured. Upper and lower bars represent the largest and the smallest sizes of embryo sacs, respectively. Note that smaller embryo sac sizes were observed in transgenic plants at all four stages (globular, triangular, heart-shape, and torpedo) examined (n=30). (B) Lower numbers of endosperm nuclei in pCLE19:CLE19 G6T :tCLE19 transgenic plants (CLE19 G6T) compared with the wild type (WT) when counted at the triangular stage of embryo development (n=30). Significantly reduced endosperm nuclei numbers (P<0.05 by Welch’s t-test) were observed in transgenic plants (indicated by asterisks).
Next, the number of endosperm nuclei was counted in ovules from the wild-type and pCLE19:CLE19 G6T :tCLE19 transgenic plants. A highly variable number of nuclei was observed in ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants when embryos inside these ovules were at the triangular stage. In general, the number of endosperm nuclei in these transgenic plants was significantly lower than that in the wild type (Fig. 6B), which was consistent with the smaller embryo sac observed in transgenic plants.
Expression of early endosperm-specific genes is prolonged in ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants
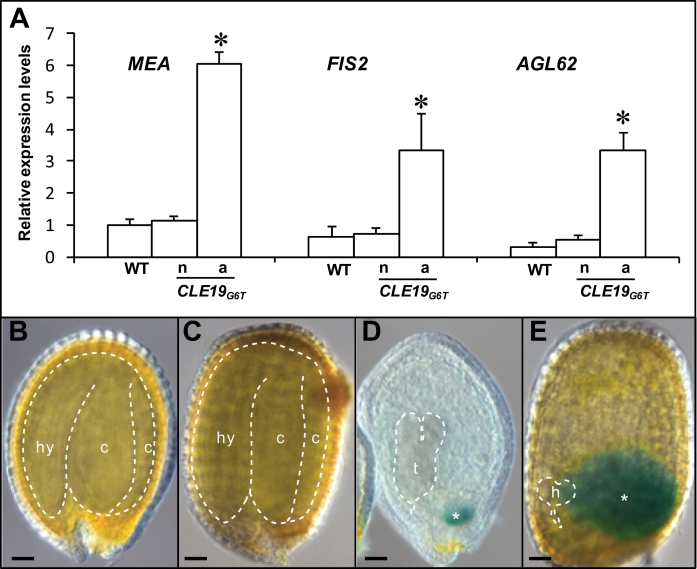
To determine whether expression of the early endosperm-specific genes was altered in pCLE19:CLE19 G6T :tCLE19 transgenic plants, expression of MEA, FIS2, and AGL62, which are expressed in the endosperm prior to cellularization (Luo et al., 2000; Kang et al., 2008), was analysed at 10–12 DAP using qRT-PCR. Compared with the wild-type, expression of all these three genes was elevated in those abnormal ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants (Fig. 7A). However, no significant difference in expression levels was seen between wild-type ovules and those normal-looking ovules from the transgenic plants (Fig. 7A).
Fig. 7.
Prolonged and elevated expression of early endosperm-specific genes in the endosperm of pCLE19:CLE19 G6T :tCLE19 transgenic plants. (A) qRT-PCR analyses showed elevated expression of MEA, FIS2, and AGL62 in those abnormal ovules (a) from a pCLE19:CLE19 G6T :tCLE19 transgenic plant (CLE19 G6T), as compared with those normal ovules (n) from the same plant, and ovules from the wild type (WT). All ovules were analysed at 12 DAP. Data represent the mean ±SD of three independently extracted RNA samples. (B–E) GUS expression in seeds from the wild type (B) and pCLE19:CLE19 G6T :tCLE19 transgenic plants carrying a pMEA:GUS reporter construct, examined at 12 DAP (C–E). Note that aborted seeds in (D) and (E) showed prolonged GUS expression in chalarzal endosperm (marked by asterisks), but not in the normal seed (C) in the same plant. Embryos are traced with dotted lines. c, cotyledon; hy, hypocotyl; h, heart-shaped stage embryo; t, torpedo-stage embryo. Asterisks indicate significant differences from the wild-type (P<0.01 by Student’s t-test). Scale bars in B–E=50 μm.
To access the MEA expression pattern in the pCLE19:CLE19 G6T :tCLE19 transgenic plants, a pMEA:GUS reporter construct (Luo et al., 2000) was introduced into pCLE19:CLE19 G6T :tCLE19 transgenic plants by crossing. Similar GUS expression was observed in endosperms from the wild type and pCLE19:CLE19 G6T :tCLE19 transgenic plants in the first 5 DAP, and no GUS expression was detected in those wild-type ovules or wild-type-appearing ovules from pCLE19:CLE19 G6T :tCLE19 transgenic plants at 12 DAP (Fig. 7B, C). However, in those aborted seeds from the transgenic plants, GUS expression was often observed in the chalarzal region of the endosperm (Fig. 7D, E), suggesting a prolonged MEA expression in chalarzal endosperms in aborted seeds. This observation provides further evidence for delayed endosperm development in pCLE19:CLE19 G6T :tCLE19 plants.
Expression of CLE19 G6T in the ESR of the endosperm mimics the defective cotyledon phenotype in pCLE19:CLE19 G6T :tCLE19 transgenic plants
The promoter from ALE1, expressed in the ESR of the endosperm in Arabidopsis (Tanaka et al., 2001), was used to examine if ectopic expression of CLE19 G6T in the endosperm can cause a defect in the embryo. To test the specificity of the ALE1 promoter, the ALE1 upstream region (1875bp) was fused to the SV40-3XGFP reporter gene to create pALE1:SV40-3XGFP and transformed into Arabidopsis (Col-0). As expected, GFP expression was observed specifically in the ESR of endosperms, and no GFP expression was observed in embryos (Supplementary Fig. S1 at JXB online).
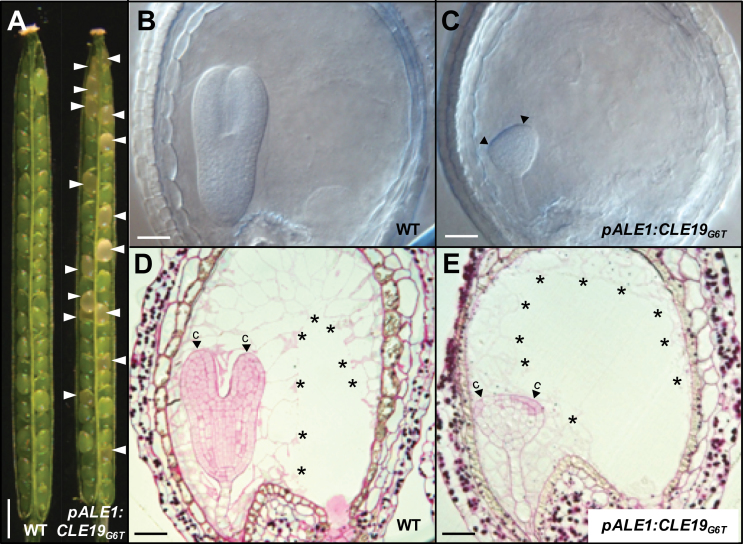
Next, a pALE1:CLE19 G6T construct was made and transformed into Arabidopsis (Col-0). Among the 35 individual transgenic lines obtained, three (#1, #2, and #3) showed high ratios of seed abortion (indicated by arrowheads; Fig. 8A), with abortion frequencies of 33.3, 28.4, and 32.9%, respectively (Table 1). The phenotypes of these lines were similar to one another, and line #1 was selected for further analyses. Examination under a DIC microscope showed that embryo development in the aborted seeds was mostly arrested at the triangular stage (Fig. 8B, C), slightly earlier than observed in pCLE19:CLE19 G6T :tCLE19 transgenic plants. These enlarged abnormal triangular embryos were often asymmetrical, most probably as a result of defective cotyledon development (indicated by arrowheads; Fig. 8C). Semi-thin sections in combination with PAS staining showed that endosperm cellularization occurred relatively normally in these aborted seeds (indicated by asterisks; Fig. 8D, E), while cotyledon development was severely arrested (indicated by arrowheads; Fig. 8C, E). The cell division pattern in the lower portion of the arrested embryos was normal, with a well-formed hypophysis and suspensor (Fig. 8E). It seems that expression of CLE19 G6T under the control of the ALE1 promoter led to an earlier defect in cotyledon establishment in embryos than that observed in pCLE19:CLE19 G6T :tCLE19 transgenic plants, and no evident defect in endosperm development. These data indicate that the CLE19 G6T expressed in the endosperm interferes with embryo development in a non-cell-autonomous manner.
Fig. 8.
Endosperm-specific expression of CLE19 G6T led to defective cotyledon establishment in embryos. (A) Seed abortions (indicated by arrowheads) observed in the pALE1:CLE19 G6T transgenic plant, as compared with the wild type (WT). (B and C) Defected cotyledon establishment (indicated by arrowheads) in embryos from pALE1:CLE19 G6T transgenic plants (C), as compared with the wild type (B) at the same stage (7 DAP). (D and E) Cytohistological examination of ovules from the wild type (D) and pALE1:CLE19 G6T transgenic plants (E), showing establishment of the defective cotyledon (E, indicated by arrowheads). Note the cellularized endosperms (indicated by asterisks). c, cotyledon primordia. Scale bars: in A=1mm; in B–E=100 μm.
Discussion
Genetic studies in Arabidopsis have identified a large number of embryo-defective (emb) mutants that exhibit a spectrum of embryo-lethal phenotypes, and endosperm development in these mutants is usually also defective (Liu and Meinke, 1998; McElver et al., 2001; Muralla et al., 2011), demonstrating indirectly that embryo and endosperm development are tightly linked. ALE1, ZOU, IKU2, and ESF1 are only expressed in endosperms, not in embryos; mutations or down-regulation of these genes cause defects in embryos (Tanaka et al., 2001; Garcia et al., 2003; Xing et al., 2013; Yang et al., 2008; Costa et al., 2014), suggesting that endosperm-expressed genes are involved in plant embryogenesis. In contrast, there was no direct evidence to show that embryo-expressed genes regulate endosperm development. In this study, it was shown that CLE19, as an embryo-expressed CLE gene, regulates both embryo and endosperm development in Arabidopsis.
In recent years it has been shown that CLE genes function as small extracellular peptides to regulate a number of developmental processes in a non-cell-autonomous manner (Fletcher et al., 1999; Fiers et al., 2005; Murphy et al., 2012). Two CLE genes are implicated in embryo development. CLE8, which is expressed in the embryo proper and the endosperm, regulates suspensor divisions via activation of WOX8 expression (Fiume and Fletcher, 2012). BnCLE19 was first identified in B. napus as a CLE gene expressed in cotyledon primordia during embryogenesis (Fiers et al., 2004). Overexpression of BnCLE19 and treatment of wild-type Arabidopsis seedlings with 14 amino acid CLE19 peptides led to CLV2-dependent premature stem cell differentiation in the root meristem (Fiers et al., 2004, 2005). Since the T-DNA insertional cle19 mutant in Arabidopsis showed no visible phenotype (Fiers et al., 2004), the function of the endogenous CLE19 remains unknown. Expression analyses performed in this study revealed that, in addition to those epidermal cells in cotyledon primordia as in BnCLE19 (Fiers et al., 2004), CLE19 in Arabidopsis was also expressed in epidermal cells of hypocotyls and root caps, while no expression was observed in the endosperm. Further studies showed that transgenic plants carrying the pCLE19:CLE19 G6T :tCLE19 construct exhibited a dominant seed abortion phenotype, with defective cotyledon establishment in embryos and compromised nuclear proliferation and cellularization in endosperms, suggesting a role for CLE19 in co-ordinating embryo and endosperm development. This hypothesis was further supported by the observation that the defective cotyledon establishment phenotype in pCLE19:CLE19 G6T :tCLE19 transgenic plants was mimicked when CLE19 G6T was expressed under the control of an endosperm-specific ALE1 promoter. It has been reported before that synthetic 14 amino acid CLE19 peptide applied to seedlings in vitro promotes stem cell differentiation in root meristems (Fiers et al., 2005; Tamaki et al., 2013). Most probably CLE19 peptides produced in embryos act as a differentiation-promoting signal to regulate cotyledon establishment in embryos, and to promote nuclear division and cellularization in endosperms. Further studies are needed to identify receptors involved in perceiving the CLE19 peptide.
The formation of cotyledons is a milestone event in embryo development, marking the initiation of a major organogenesis phase in the embryo. Auxin biosynthesis and transport are involved in cotyledon initiation, determining the emergence and the positions of cotyledon primordia (Liu et al., 1993; Furutani et al., 2004; Cheng et al., 2007). In addition, several WOX family transcription factors are expressed during cotyledon formation: WOX1 and WOX3 are expressed at positions where the two cotyledon primordia are formed (Haecker et al., 2004), while WOX2 is expressed in the apical region of the globular stage embryos (Haecker et al., 2004). Although the wox1/wox3 double mutant exhibited no defect in cotyledon formation, wox1/wox2/wox3 triple mutations enhanced the wox2 defect in cotyledon formation (Breuninger et al., 2008), suggesting a synergic interaction among these transcription factors. CUP-SHAPED COTYLEDON 1 (CUC1) and CUC2 are expressed in the boundary of two cotyledons and regulate the final patterning of cotyledons (Aida et al., 1997, 1999; Vroemen et al., 2003), while ASYMMETRIC LEAVES 1 (AS1) and AS2 regulate the adaxial–abaxial pattern of the cotyledons (Byrne et al., 2000; Lin et al., 2003). Data from this study showed that the primary defect of pCLE19:CLE19 G6T :tCLE19 transgenic plants is the establishment, not the initiation, of these cotyledons, suggesting a multilayered regulation of cotyledon formation.
It is also interesting to note that only a fraction of seeds produced in pCLE19:CLE19 G6T :tCLE19 and pALE1:CLE19 G6T transgenic plants showed defects in embryo development; the remaining embryos are able to form normal seeds. Such an incomplete penetration of phenotypes has been reported in overexpression or mutations of several CLE genes. For example, when BnCLE19 was overexpressed under the control of the CaMV 35S promoter, only 17% of the transgenic plants obtained exhibited the short-root and pin-shaped pistil phenotypes (Fiers et al., 2004). In the homozygous cle8-1 mutant (with a point mutation in the CLE motif), only 15% of embryos showed defects in embryogenesis (Fiume and Fletcher, 2012). It is believed that dosage effects, regulated most probably at the post-transcriptional peptide processing levels, are very important for peptide hormones.
There is no doubt that the antagonistic peptide technology provides a powerful in vivo tool to study the function of CLE genes (Song et al., 2013). The technology has already been used to elucidate the role of CLE22 in root development (Song et al., 2013), and CLE45 in phloem differentiation (Rodriguez-Villalon et al., 2014). The phenotypic and genetic analyses of the CLE19 gene in Arabidopsis by expression of the CLE19 G6T construct under control of the endogenous CLE19 promoter or the endosperm-specific ALE1 promoter in this work allowed the roles of CLE19 in co-ordinating embryo and endosperm development to be defined. When endogenous regulatory elements are used in this type of study, it is expected that the antagonistic peptides produced may act in situ to interfere with the signal transduction pathways involved. One study published in this issue showed that synthetic CLE peptides with a G6T substitution are less effective than wild-type peptides in seedling treatments in vitro (Czyzewicz et al., 2015). This is expected since it has been shown in CLV3 that the G6 residue is critical for the CLV3 function: (i) a clv3-1 mutant with a severe defect in SAM homeostasis was caused by a substitution in G6 (Fletcher et al., 1999); (ii) pCLV3:CLV3 G6A :tCLV3 is one of the least effective constructs in complementing the clv3-2 mutant (Song et al., 2012); and (iii) no transgenic plants carrying the pCLV3:CLV3 G6T :tCLV3 construct showed complete complementation of the clv3-2 phenotype (Song et al., 2013). Although it has been shown that treatment of wild-type Arabidopsis seedlings with CLV3G6T peptide in vitro, with peptides being refreshed every day, caused a slightly enlarged SAM phenotype (a weak clv3-like phenotype; Song et al., 2013), whether the antagonistic peptide technology could be used effectively in vitro remains to be evaluated further.
In summary, the results obtained in this study suggest that the CLE19 expressed in cotyledon primordia of Arabidopsis may act as a peptide ligand that constitutes a diffusible signal to regulate cotyledon establishment in embryos and to promote the nuclear proliferation and cellularization in endosperms.
Supplementary data
Supplementary data are available at JXB online
Figure S1. ALE1 promoter activity in seed development.
Table S1. Primers used in this study.
Acknowledgements
We thank Professor Ming Luo from the CSIRO, Australia, for providing pMEA:GUS transgenic seeds, and Professor Dolf Weijers from Wageningen University, The Netherlands, for providing pPLV vectors. This work was supported by projects from The National Basic Research Program of China (2013CB967301; 2014CB943400) and the National Natural Science Foundation of China (31161130531). The authors declare no conflict of interests.
References
- Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M. 1997. Genes involved in organ separation in Arabidopsis: an analysis of the cup-shaped cotyledon mutant. The Plant Cell 9, 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Tasaka M. 1999. Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126, 1563–1570. [DOI] [PubMed] [Google Scholar]
- Baum S. 2008. The PAS reaction for staining cell walls. CSH Protocols 3, 1–3. [DOI] [PubMed] [Google Scholar]
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118, 575–587. [Google Scholar]
- Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. 2008. Differential expression of WOX genes mediates apical–basal axis formation in the Arabidopsis embryo. Developmental Cell 14, 867–876. [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. 2000. Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis . Nature 408, 967–971. [DOI] [PubMed] [Google Scholar]
- Casamitjana-Martínez E, Hofhuis HF, Xu J, Liu CM, Heidstra R, Scheres B. 2003. Root-specific CLE19 overexpression and the sol1/2 suppressors implicate a CLV-like pathway in the control of Arabidopsis root meristem. Current Biology 13, 1435–1441. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Qin G, Dai X, Zhao Y. 2007. NPY1, a BTB-NPH3-like protein, plays a critical role in auxin-regulated organogenesis in Arabidopsis . Proceedings of the National Academy of Sciences, USA 104, 18825–18829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Williams RW, Meyerowitz EM. 1997. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis . Cell 89, 575–585. [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana . The Plant Journal 16, 735–743. [DOI] [PubMed] [Google Scholar]
- Cock JM, McCormick S. 2001. A large family of genes that share homology with CLAVATA3 . Plant Physiology 126, 939–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LM, Marshall E, Tesfaye M, et al. 2014. Central cell-derived peptides regulate early embryo patterning in flowering plants. Science 344, 168–172. [DOI] [PubMed] [Google Scholar]
- Czyzewicz N Wildhagen M Cattaneo P Stahl Y Gustavo Pinto K, Aalen RB Butenko MA Simon R Hardtke C De Smet I. 2015. Antagonistic peptide technology for functional dissection of CLE peptides revisited. Journal of Experimental Botany 66, 5367–5374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rybel B, van den Berg W, Lokerse A, Liao CY, van Mourik H, Moller B, Peris CL, Weijers D. 2011. A versatile set of ligation-independent cloning vectors for functional studies in plants. Plant Physiology 156, 1292–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers M, Golemiec E, Xu J, van der Geest L, Heidstra R, Stiekema W, Liu CM. 2005. The 14-amino acid CLV3, CLE19, and CLE40 peptides trigger consumption of the root meristem in Arabidopsis through a CLAVATA2-dependent pathway. The Plant Cell 17, 2542–2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers M, Hause G, Boutilier K, Casamitjana-Martinez E, Weijers D, Offringa R, van der Geest L, van Lookeren Campagne M, Liu CM. 2004. Mis-expression of the CLV3/ESR-like gene CLE19 in Arabidopsis leads to a consumption of root meristem. Gene 327, 37–49. [DOI] [PubMed] [Google Scholar]
- Fiume E, Fletcher JC. 2012. Regulation of Arabidopsis embryo and endosperm development by the polypeptide signaling molecule CLE8. The Plant Cell 24, 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC, Brand U, Running MP, Simon R, Meyerowitz EM. 1999. Signaling of cell fate decisions by CLAVATA3 in Arabidopsis shoot meristems. Science 283, 1911–1914. [DOI] [PubMed] [Google Scholar]
- Furutani M, Vernoux T, Traas J, Kato T, Tasaka M, Aida M. 2004. PIN-FORMED1 and PINOID regulate boundary formation and cotyledon development in Arabidopsis embryogenesis. Development 131, 5021–5030. [DOI] [PubMed] [Google Scholar]
- Garcia D, Saingery V, Chambrier P, Mayer U, Jurgens G, Berger F. 2003. Arabidopsis haiku mutants reveal new controls of seed size by endosperm. Plant Physiology 131, 1661–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadfi K, Speth V, Neuhaus G. 1998. Auxin-induced developmental patterns in Brassica juncea embryos. Development 125, 879–887. [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. 2004. Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131, 657–668. [DOI] [PubMed] [Google Scholar]
- Hobe M, Muller R, Grunewald M, Brand U, Simon R. 2003. Loss of CLE40, a protein functionally equivalent to the stem cell restricting signal CLV3, enhances root waving in Arabidopsis . Development Genes and Evolution 213, 371–381. [DOI] [PubMed] [Google Scholar]
- Ito Y, Nakanomyo I, Motose H, Iwamoto K, Sawa S, Dohmae N, Fukuda H. 2006. Dodeca-CLE peptides as suppressors of plant stem cell differentiation. Science 313, 842–845. [DOI] [PubMed] [Google Scholar]
- Jun J, Fiume E, Roeder AH, et al. 2010. Comprehensive analysis of CLE polypeptide signaling gene expression and overexpression activity in Arabidopsis . Plant Physiology 154, 1721–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang IH, Steffen JG, Portereiko MF, Lloyd A, Drews GN. 2008. The AGL62 MADS domain protein regulates cellularization during endosperm development in Arabidopsis . The Plant Cell 20, 635–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inze D, Depicker A. 2002. GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends in Plant Science 7, 193–195. [DOI] [PubMed] [Google Scholar]
- Kondo T, Sawa S, Kinoshita A, Mizuno S, Kakimoto T, Fukuda H, Sakagami Y. 2006. A plant peptide encoded by CLV3 identified by in situ MALDI-TOF MS analysis. Science 313, 845–848. [DOI] [PubMed] [Google Scholar]
- Lafon-Placette C, Kohler C. 2014. Embryo and endosperm, partners in seed development. Current Opinion in Plant Biology 17, 64–69. [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Furner IJ. 1992. Characterization of three shoot apical meristem mutants of Arabidopsis thaliana . Development 116, 397–403. [Google Scholar]
- Lin WC, Shuai B, Springer PS. 2003. The Arabidopsis LATERAL ORGAN BOUNDARIES-domain gene ASYMMETRIC LEAVES2 functions in the repression of KNOX gene expression and in adaxial–abaxial patterning. The Plant Cell 15, 2241–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Meinke DW. 1998. The titan mutants of Arabidopsis are disrupted in mitosis and cell cycle control during seed development. The Plant Journal 16, 21–31. [DOI] [PubMed] [Google Scholar]
- Liu CM, Xu Z, Chua NH. 1993. Auxin polar transport is essential for the establishment of bilateral symmetry during early plant embryogenesis. The Plant Cell 5, 621–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CM, Xu ZH, Chua NH. 1995. In vitro culture of Brassica juncea zygotic proembryo. Plant Tissue Culture Manual E5, 1–20. [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Luo M, Bilodeau P, Dennis ES, Peacock WJ, Chaudhury A. 2000. Expression and parent-of-origin effects for FIS2, MEA, and FIE in the endosperm and embryo of developing Arabidopsis seeds. Proceedings of the National Academy of Sciences, USA 97, 10637–10642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElver J, Tzafrir I, Aux G, et al. 2001. Insertional mutagenesis of genes required for seed development in Arabidopsis thaliana . Genetics 159, 1751–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwa H, Betsuyaku S, Iwamoto K, Kinoshita A, Fukuda H, Sawa S. 2008. The receptor-like kinase SOL2 mediates CLE signaling in Arabidopsis . Plant and Cell Physiology 49, 1752–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller R, Bleckmann A, Simon R. 2008. The receptor kinase CORYNE of Arabidopsis transmits the stem cell-limiting signal CLAVATA3 independently of CLAVATA1. The Plant Cell 20, 934–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muralla R, Lloyd J, Meinke D. 2011. Molecular foundations of reproductive lethality in Arabidopsis thaliana . PLoS One 6, e28398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Smith S, De Smet I. 2012. Small signaling peptides in Arabidopsis development: how cells communicate over a short distance. The Plant Cell 24, 3198–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelkers K, Goffard N, Weiller GF, Gresshoff PM, Mathesius U, Frickey T. 2008. Bioinformatic analysis of the CLE signaling peptide family. BMC Plant Biology 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohyama K, Shinohara H, Ogawa-Ohnishi M, Matsubayashi Y. 2009. A glycopeptide regulating stem cell fate in Arabidopsis thaliana . Nature Chemical Biology 5, 578–580. [DOI] [PubMed] [Google Scholar]
- Opsahl-Ferstad HG, Le Deunff E, Dumas C, Rogowsky PM. 1997. ZmEsr, a novel endosperm-specific gene expressed in a restricted region around the maize embryo. The Plant Journal 12, 235–246. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Villalon A, Gujas B, Kang YH, Breda AS, Cattaneo P, Depuydt S, Hardtke CS. 2014. Molecular genetic framework for protophloem formation. Proceedings of the National Academy of Sciences, USA 111, 11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma VK, Ramirez J, Fletcher JC. 2003. The Arabidopsis CLV3-like (CLE) genes are expressed in diverse tissues and encode secreted proteins. Plant Molecular Biology 51, 415–425. [DOI] [PubMed] [Google Scholar]
- Shinohara H, Matsubayashi Y. 2015. Reevaluation of the CLV3–receptor interaction in the shoot apical meristem: dissection of the CLV3 signaling pathway from a direct ligand-binding point of view. The Plant Journal 82, 328–336 [DOI] [PubMed] [Google Scholar]
- Song XF, Guo P, Ren SC, Xu TT, Liu CM. 2013. Antagonistic peptide technology for functional dissection of CLV3/ESR genes in Arabidopsis . Plant Physiology 161, 1076–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song XF, Yu DL, Xu TT, Ren SC, Guo P, Liu CM. 2012. Contributions of individual amino acid residues to the endogenous CLV3 function in shoot apical meristem maintenance in Arabidopsis . Molecular Plant 5, 515–523. [DOI] [PubMed] [Google Scholar]
- Stahl Y, Wink RH, Ingram GC, Simon R. 2009. A signaling module controlling the stem cell niche in Arabidopsis root meristems. Current Biology 19, 909–914. [DOI] [PubMed] [Google Scholar]
- Strabala TJ, O’Donnell PJ, Smit AM, Ampomah-Dwamena C, Martin EJ, Netzler N, Nieuwenhuizen NJ, Quinn BD, Foote HC, Hudson KR. 2006. Gain-of-function phenotypes of many CLAVATA3/ESR genes, including four new family members, correlate with tandem variations in the conserved CLAVATA3/ESR domain. Plant Physiology 140, 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki T, Betsuyaku S, Fujiwara M, Fukao Y, Fukuda H, Sawa S. 2013. SUPPRESSOR OF LLP1 1-mediated C-terminal processing is critical for CLE19 peptide activity. The Plant Journal 76, 970–981. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Onouchi H, Kondo M, Hara-Nishimura I, Nishimura M, Machida C, Machida Y. 2001. A subtilisin-like serine protease is required for epidermal surface formation in Arabidopsis embryos and juvenile plants. Development 128, 4681–4689. [DOI] [PubMed] [Google Scholar]
- Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MA, de Vries SC. 2003. The CUP-SHAPED COTYLEDON 3 gene is required for boundary and shoot meristem formation in Arabidopsis . The Plant Cell 15, 1563–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Q, Creff A, Waters A, Tanaka H, Goodrich J, Ingram GC. 2013. ZHOUPI controls embryonic cuticle formation via a signalling pathway involving the subtilisin protease ABNORMAL LEAF-SHAPE1 and the receptor kinases GASSHO1 and GASSHO2. Development 140, 770–779. [DOI] [PubMed] [Google Scholar]
- Yang S, Johnston N, Talideh E, Mitchell S, Jeffree C, Goodrich J, Ingram G. 2008. The endosperm-specific ZHOUPI gene of Arabidopsis thaliana regulates endosperm breakdown and embryonic epidermal development. Development 135, 3501–3509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.