Abstract
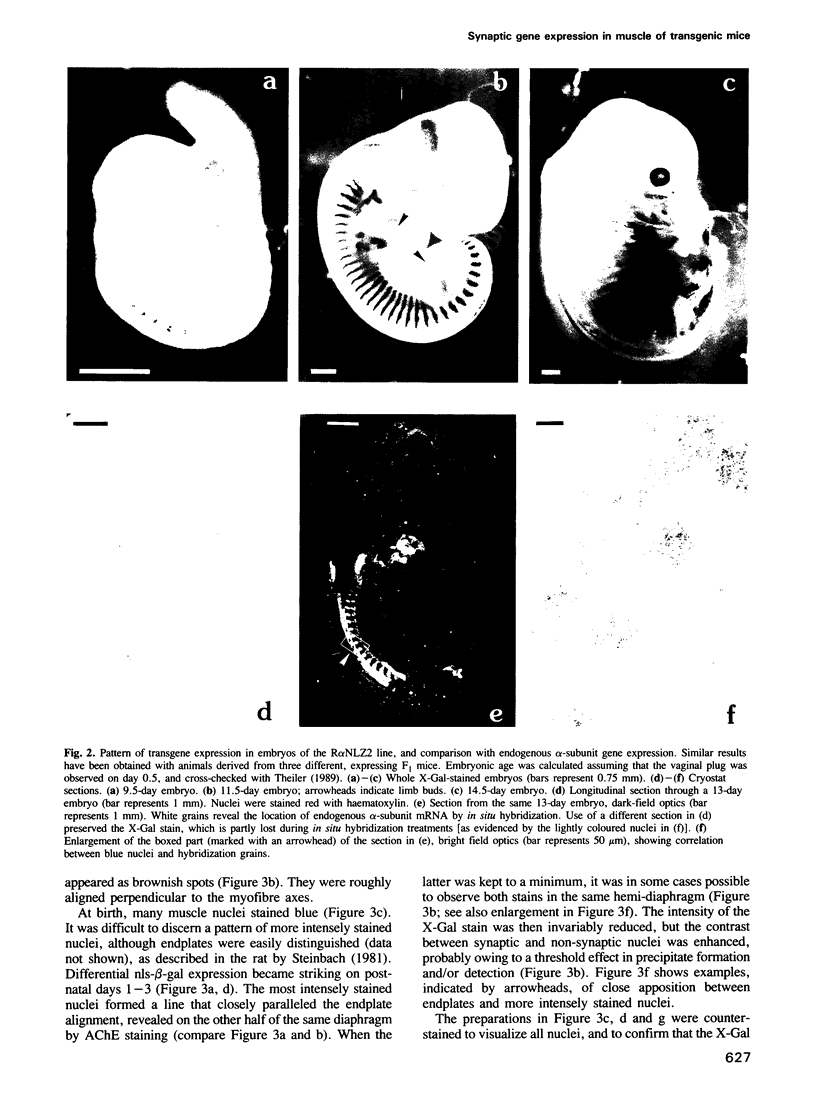
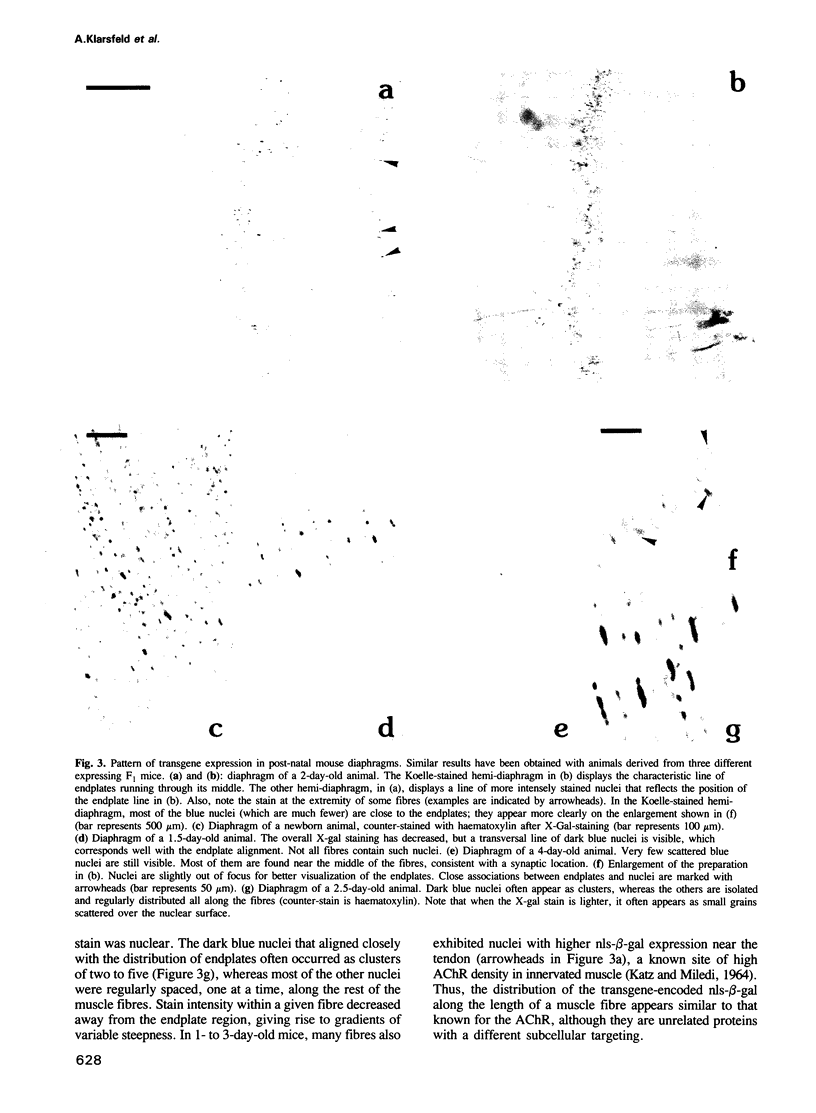
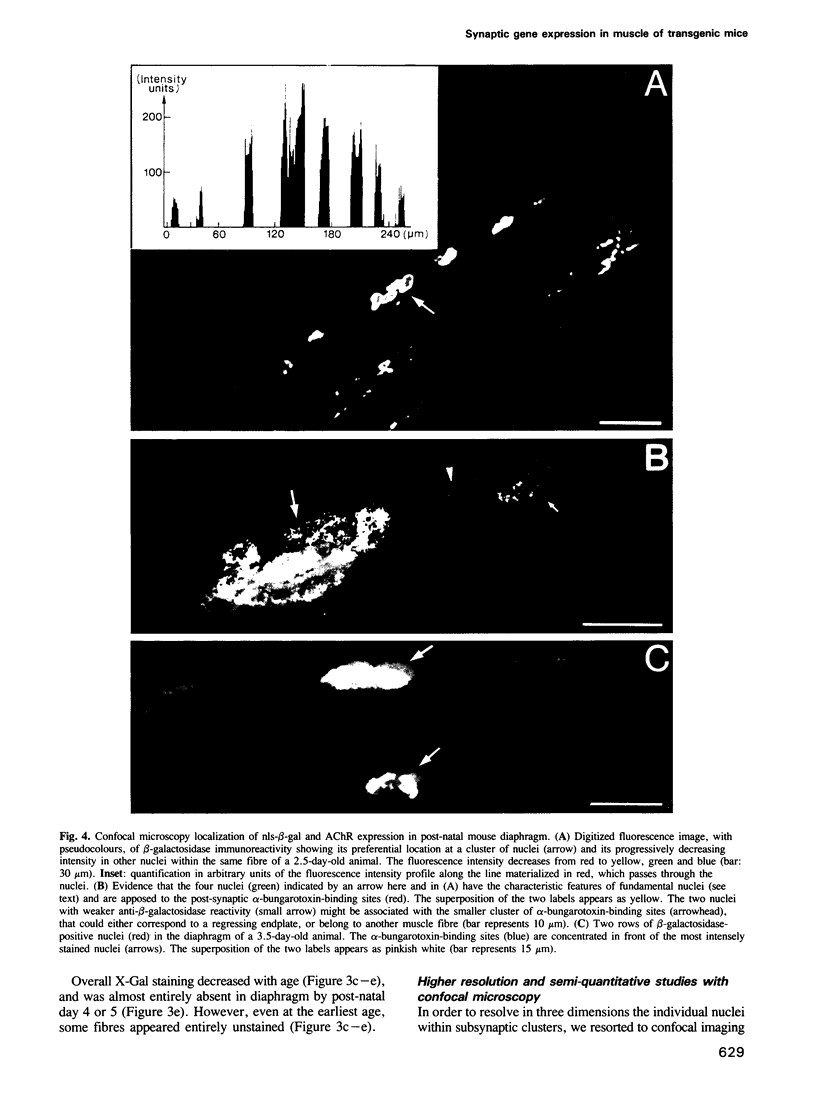
We have obtained transgenic mice expressing nuclearly targeted beta-galactosidase (nls-beta-gal) under the control of a chicken acetylcholine receptor alpha-subunit promoter. The expression of the transgene was detected in early somites, starting before embryonic day 9.5. In 13-day embryos, the expression pattern of the transgene closely paralleled that of the endogenous mouse alpha-subunit gene, assessed by in situ hybridization. Our results illustrate, with single-cell resolution, the tissue specificity of this alpha-subunit promoter during embryogenesis. After birth, the overall beta-galactosidase activity rapidly decreased with age. However, in diaphragms of newborn animals, beta-galactosidase activity selectively persisted in nuclei underlying the motor endplates. The latter were revealed by an acetylcholinesterase stain. Nls-beta-gal was also visualized by indirect immunofluorescence, while endplates were labelled with fluorescent alpha-bungarotoxin. Confocal microscopy unambiguously identified the more intensely stained nuclei as synaptic 'fundamental nuclei', and allowed estimates of relative staining levels. Thus an 842 bp acetylcholine receptor gene promoter confers preferential synaptic expression to a reporter gene within myofibres in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessereau J. L., Fontaine B., Changeux J. P. Denervation of mouse skeletal muscle differentially affects the expression of the jun and fos proto-oncogenes. New Biol. 1990 Apr;2(4):375–383. [PubMed] [Google Scholar]
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S., Steinbach J. H. The distribution of alpha-bungarotoxin binding sites of mammalian skeletal muscle developing in vivo. J Physiol. 1977 May;267(1):195–213. doi: 10.1113/jphysiol.1977.sp011808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H. R., Witzemann V., Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990 Apr 5;344(6266):544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden S. Development of the neuromuscular junction in the chick embryo: the number, distribution, and stability of acetylcholine receptors. Dev Biol. 1977 Jun;57(2):317–329. doi: 10.1016/0012-1606(77)90218-4. [DOI] [PubMed] [Google Scholar]
- Covault J., Sanes J. R. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986 Mar;102(3):716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Changeux J. P. Localization of nicotinic acetylcholine receptor alpha-subunit transcripts during myogenesis and motor endplate development in the chick. J Cell Biol. 1989 Mar;108(3):1025–1037. doi: 10.1083/jcb.108.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine B., Sassoon D., Buckingham M., Changeux J. P. Detection of the nicotinic acetylcholine receptor alpha-subunit mRNA by in situ hybridization at neuromuscular junctions of 15-day-old chick striated muscles. EMBO J. 1988 Mar;7(3):603–609. doi: 10.1002/j.1460-2075.1988.tb02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman D., Staple J. Spatial and temporal expression of acetylcholine receptor RNAs in innervated and denervated rat soleus muscle. Neuron. 1989 Aug;3(2):219–228. doi: 10.1016/0896-6273(89)90035-4. [DOI] [PubMed] [Google Scholar]
- Herbomel P., Bourachot B., Yaniv M. Two distinct enhancers with different cell specificities coexist in the regulatory region of polyoma. Cell. 1984 Dec;39(3 Pt 2):653–662. doi: 10.1016/0092-8674(84)90472-0. [DOI] [PubMed] [Google Scholar]
- Hunter D. D., Shah V., Merlie J. P., Sanes J. R. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989 Mar 16;338(6212):229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Isenberg K. E., Mudd J., Shah V., Merlie J. P. Nucleotide sequence of the mouse muscle nicotinic acetylcholine receptor alpha subunit. Nucleic Acids Res. 1986 Jun 25;14(12):5111–5111. doi: 10.1093/nar/14.12.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin B. J., Cartaud J., Bornens M., Changeux J. P. Golgi apparatus in chick skeletal muscle: changes in its distribution during end plate development and after denervation. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7218–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin B. J., Changeux J. P., Cartaud J. Compartmentalization of cold-stable and acetylated microtubules in the subsynaptic domain of chick skeletal muscle fibre. Nature. 1990 Apr 12;344(6267):673–675. doi: 10.1038/344673a0. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. FURTHER OBSERVATIONS ON THE DISTRIBUTION OF ACTYLCHOLINE-REACTIVE SITES IN SKELETAL MUSCLE. J Physiol. 1964 Mar;170:379–388. doi: 10.1113/jphysiol.1964.sp007338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Klarsfeld A., Daubas P., Bourachot B., Changeux J. P. A 5'-flanking region of the chicken acetylcholine receptor alpha-subunit gene confers tissue specificity and developmental control of expression in transfected cells. Mol Cell Biol. 1987 Feb;7(2):951–955. doi: 10.1128/mcb.7.2.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer R., Changeux J. P. Activity-dependent regulation of gene expression in muscle and neuronal cells. Mol Neurobiol. 1989 Spring-Summer;3(1-2):1–53. doi: 10.1007/BF02935587. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Kornhauser J. M. Neural regulation of gene expression by an acetylcholine receptor promoter in muscle of transgenic mice. Neuron. 1989 Apr;2(4):1295–1300. doi: 10.1016/0896-6273(89)90067-6. [DOI] [PubMed] [Google Scholar]
- Merlie J. P., Sanes J. R. Concentration of acetylcholine receptor mRNA in synaptic regions of adult muscle fibres. Nature. 1985 Sep 5;317(6032):66–68. doi: 10.1038/317066a0. [DOI] [PubMed] [Google Scholar]
- Pavlath G. K., Rich K., Webster S. G., Blau H. M. Localization of muscle gene products in nuclear domains. Nature. 1989 Feb 9;337(6207):570–573. doi: 10.1038/337570a0. [DOI] [PubMed] [Google Scholar]
- Piette J., Bessereau J. L., Huchet M., Changeux J. P. Two adjacent MyoD1-binding sites regulate expression of the acetylcholine receptor alpha-subunit gene. Nature. 1990 May 24;345(6273):353–355. doi: 10.1038/345353a0. [DOI] [PubMed] [Google Scholar]
- Piette J., Klarsfeld A., Changeux J. P. Interaction of nuclear factors with the upstream region of the alpha-subunit gene of chicken muscle acetylcholine receptor: variations with muscle differentiation and denervation. EMBO J. 1989 Mar;8(3):687–694. doi: 10.1002/j.1460-2075.1989.tb03427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E., Hall Z. W. Intracellular and surface distribution of a membrane protein (CD8) derived from a single nucleus in multinucleated myotubes. J Cell Biol. 1989 Nov;109(5):2345–2352. doi: 10.1083/jcb.109.5.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E., Hall Z. W. Transfer of a protein encoded by a single nucleus to nearby nuclei in multinucleated myotubes. Science. 1989 Jun 2;244(4908):1066–1069. doi: 10.1126/science.2543074. [DOI] [PubMed] [Google Scholar]
- Rotundo R. L. Nucleus-specific translation and assembly of acetylcholinesterase in multinucleated muscle cells. J Cell Biol. 1990 Mar;110(3):715–719. doi: 10.1083/jcb.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzler S., Brenner H. R. Metabolic stabilization of acetylcholine receptors in vertebrate neuromuscular junction by muscle activity. J Cell Biol. 1990 Aug;111(2):655–661. doi: 10.1083/jcb.111.2.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Cooper D. L., Levitt-Gilmour T. Degradation rates of acetylcholine receptors can be modified in the postjunctional plasma membrane of the vertebrate neuromuscular junction. J Cell Biol. 1986 Oct;103(4):1399–1403. doi: 10.1083/jcb.103.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salpeter M. M., Loring R. H. Nicotinic acetylcholine receptors in vertebrate muscle: properties, distribution and neural control. Prog Neurobiol. 1985;25(4):297–325. doi: 10.1016/0301-0082(85)90018-8. [DOI] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Tapscott S. J., Davis R. L., Thayer M. J., Cheng P. F., Weintraub H., Lassar A. B. MyoD1: a nuclear phosphoprotein requiring a Myc homology region to convert fibroblasts to myoblasts. Science. 1988 Oct 21;242(4877):405–411. doi: 10.1126/science.3175662. [DOI] [PubMed] [Google Scholar]
- Wang Y., Xu H. P., Wang X. M., Ballivet M., Schmidt J. A cell type-specific enhancer drives expression of the chick muscle acetylcholine receptor alpha-subunit gene. Neuron. 1988 Aug;1(6):527–534. doi: 10.1016/0896-6273(88)90183-3. [DOI] [PubMed] [Google Scholar]
- Witzemann V., Barg B., Criado M., Stein E., Sakmann B. Developmental regulation of five subunit specific mRNAs encoding acetylcholine receptor subtypes in rat muscle. FEBS Lett. 1989 Jan 2;242(2):419–424. doi: 10.1016/0014-5793(89)80514-9. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]