Abstract
Background and objectives
Cardiovascular disease is the most common cause of death in patients on hemodialysis (HD). HD-associated cardiomyopathy is appreciated to be driven by exposure to recurrent and cumulative ischemic insults resulting from hemodynamic instability of conventionally performed intermittent HD treatment itself. Cooled dialysate reduces HD-induced recurrent ischemic injury, but whether this confers long-term protection of the heart in terms of cardiac structure and function is not known.
Design, setting, participants, & measurements
Between September 2009 and January 2013, 73 incident HD patients were randomly assigned to a dialysate temperature of 37°C (control) or individualized cooling at 0.5°C below body temperature (intervention) for 12 months. Cardiac structure, function, and aortic distensibility were assessed by cardiac magnetic resonance imaging. Mean between-group difference in delivered dialysate temperature was 1.2°C±0.3°C. Treatment effects were determined by the interaction of treatment group with time in linear mixed models.
Results
There was no between-group difference in the primary outcome of left ventricular ejection fraction (1.5%; 95% confidence interval, –4.3% to 7.3%). However, left ventricular function assessed by peak systolic strain was preserved by the intervention (–3.3%; 95% confidence interval, –6.5% to –0.2%) as was diastolic function (measured as peak diastolic strain rate, 0.18 s−1; 95% confidence interval, 0.02 to 0.34 s−1). Reduction of left ventricular dilation was demonstrated by significant reduction in left ventricular end-diastolic volume (–23.8 ml; 95% confidence interval, –44.7 to –2.9 ml). The intervention was associated with reduced left ventricular mass (–15.6 g; 95% confidence interval, –29.4 to –1.9 g). Aortic distensibility was preserved in the intervention group (1.8 mmHg−1×10−3; 95% confidence interval, 0.1 to 3.6 mmHg−1×10−3). There were no intervention-related withdrawals or adverse events.
Conclusions
In patients new to HD, individualized cooled dialysate did not alter the primary outcome but was well tolerated and slowed the progression of HD-associated cardiomyopathy. Because cooler dialysate is universally applicable at no cost, the intervention warrants wider adoption or confirmation of these findings in a larger trial.
Keywords: heart disease, hemodialysis, clinical trial
Introduction
Over 2 million people worldwide require on-going dialysis to sustain life. Most patients endure unpleasant dialysis-related symptoms and generally have a poor quality of life. Patients on dialysis continue to have a lower life expectancy than many patients with cancer, with cardiovascular disease being the leading cause of death. Several cardiovascular disease therapies effective in the general population have been entirely ineffective in patients on hemodialysis (HD). However, cardiovascular disease in patients on dialysis is not solely caused by traditional risk factors or the uremic milieu (1). HD itself causes recurrent and cumulative ischemic injury to the heart, brain, and other organs (2). Recurrent HD-induced myocardial stunning results in long-term contractile dysfunction, abnormal ventricular morphology, and increased mortality (3–5).
The reduction in intradialytic hypotension by cooling the dialysate was first demonstrated by the original pioneering work of Maggiore et al. (6). However, this therapy remains greatly underused. In the first half of 2014, 24% of in-center HD treatments had dialysate temperature of <36.5°C in a sample of a large dialysis organization in the United States (Len Usvyat, Fresenius Medical Care, personal communication). We have demonstrated in several small short-term pilot studies that improvement in hemodynamic tolerability of HD is associated with marked reduction in myocardial stunning (7–9). The use of extremely low temperature dialysate (34°C–35°C) is effective but limited by cold symptomatic intolerance. The cardioprotective effect and cold symptom tolerability can be optimized by an individualized approach to dialysate temperature prescription (10).
We recently reported that an individualized dialysate cooling strategy is effective in reducing HD-associated progressive white matter brain injury (11). This trial aimed to test whether dialysate cooling could provide long-term cardiac protection and abrogate progressive morphologic and functional changes characteristic of HD-associated cardiomyopathy in patients new to conventional thrice-weekly HD.
Materials and Methods
Study Design and Treatment Regimen
The trial was conducted in accordance with the Declaration of Helsinki of 1975 and as revised in 1983. The trial design was a prospectively registered (ISRCTN00206012, October 15, 2009), multicenter, randomized, controlled, open-label, blinded end point study, and the protocol has been published (12). Nottingham Ethics Committee approved the protocol, and all patients gave written informed consent.
Participants
Participants were recruited from the HD centers of four university teaching hospitals in the United Kingdom. Inclusion criteria were ≥16 years of age, being within 180 days of commencing in-center HD treatment three times per week, and capacity to consent for the trial. Exclusion criteria were inability to tolerate cardiac magnetic resonance imaging (CMR) because of claustrophobia, contraindications for CMR, pregnancy or lactating, and New York Heart Association grade IV heart failure.
Randomization and Blinding
Patients were randomized 1:1 by a computer-generated sequence placed into sealed envelopes by an independent statistician. Because the HD monitor was iteratively programmed per treatment session with potentially visible settings, it was not pragmatic to reliably maintain blinding for 12 months. Per the prospectively registered, multicenter, randomized, controlled, open-label, blinded end point design, all cardiac imaging analyses were centrally conducted and blinded to patient details or treatment group allocations.
Dialysis Intervention
The control group used a dialysate temperature of 37°C for 12 months. The intervention group used an individualized cooled dialysate temperature for 12 months. The latter was set at 0.5°C less than the patient’s, determined from the mean of six prior treatment sessions with a tympanic thermometer, between a minimum of 35°C and a maximum of 36°C. The allocated dialysate temperature remained unchanged for the study period. This ensured a minimum between-group temperature separation of 1°C in the event that a participant had a mean temperature >36.5°C. Preliminary review of electronically recorded data indicated the mean allocated intervention group temperature was likely to be 1°C–1.5°C lower than the control group. Protocol adherence was regularly assessed and recorded by HD center staff.
Concurrent Treatments
Patients in both trial arms had standard management with dry weight prescribed by the clinical team according to local protocol, including thrice-weekly HD to achieve an equilibrated Kt/Vurea>1.1, a standardized Kt/Vurea>2.0, and a treatment time of between 3.5 and 4 hours. HD treatments used low-flux polysulfone dialyzers (1.8–2.0 m2; LOPS 18/20; Braun Medical, Sheffield, UK). Dialysate composition was sodium 313–317 mg/dl, potassium 3.9 mg/dl, calcium 5.0 mg/dl, magnesium 1.2 mg/dl, bicarbonate 32–38 mEq/L and glucose 101 mg/dl. Dialysate flow rate was 500 ml/min, and blood pump speed was 250–450 ml/min using anticoagulation by unfractionated heparin.
Data Collection
Imaging studies by CMR occurred on a midweek postdialysis day at baseline and 12 months to avoid the long interdialytic break after the weekend, when patients are most hypervolemic (13,14). Studies were conducted at two centers using a 1.5-T scanner (GE Signa HDxt; GE Healthcare, Milwaukee, WI) by validated methods (15). Acquisition parameters achieved a spatial resolution of <2 mm and a temporal resolution of 20–40 ms. Radiofrequency tagging used a spatial modulation of magnetization sequence applied at basal, midventricular, and apical levels. An oblique sagittal orientation was acquired to image the aorta in long axis, and a 5-mm–thick axial cine was acquired at the level of the pulmonary artery bifurcation.
Body Composition
Volume status was assessed by segmental multiple frequency bioelectric impedance measurements at baseline and 12 months, using tetrapolar 8-point tactile electrodes (InBody S20; Biospace, Seoul, South Korea) (16). Hypervolemia was estimated by the ratio of extracellular water to total body water (17).
Data Analysis
Planimetry, as previously described, was used to determine left ventricular (LV) mass, volumes, and ejection fraction (EF) with cvi42 software version 4.0.2 (Circle Cardiovascular Imaging, Calgary, AB, Canada) (15). Values were indexed to body surface area using the method described by Mosteller (18).
LV Strain Analysis
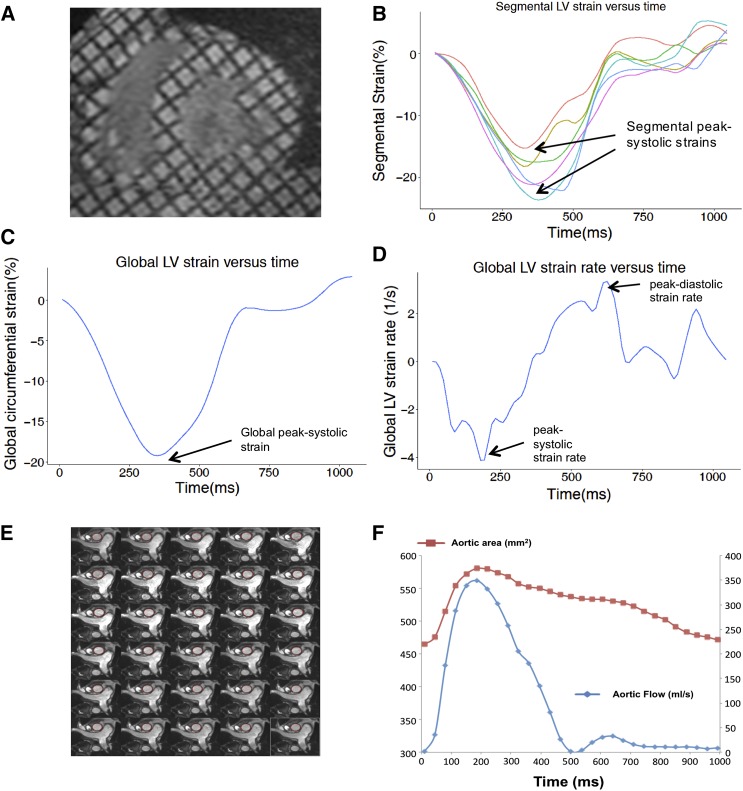
Direct assessment of regional motion within myocardial tissue by LV strain has inherently greater sensitivity and specificity to detect LV dysfunction than the EF, which infers function from the bulk effect of LV boundaries. Greater LV strain denotes better LV function. Reduced LV strain precedes LV dilation, LV hypertrophy, and reduced EF in a range of cardiomyopathies (19). Tagged CMR is a highly reproducible, reference standard technique to quantify regional LV strain (19). This involves changing magnetization during CMR image acquisition to tag the left ventricle with a grid pattern. Software then tracks the motion of these tags to quantify the magnitude (strain) and velocity (strain rate) of tissue motion. Midwall circumferential strain was measured in tagged LV short-axis images using validated software (HARP 3.0; Diagnosoft, Palo Alto, CA) as depicted in Figure 1, A–D (20). Values were mapped to the 16-segment model of the left ventricle recommended by the American Heart Association (21) then averaged to derive global peak systolic strain (%), peak systolic strain rate (s−1), and peak diastolic strain rate (s−1).
Figure 1.
Cardiac magnetic resonance imaging analysis in a typical study participant. Tagged LV short-axis images (A) and derived LV segmental strain curves (B). These were averaged to determine the global LV strain (C) and strain-rate (D) curves from which peak systolic and diastolic values were used as study outcomes (arrows). Aortic distensibility was determined by semiautomated tracing of the ascending aorta in 30 phases per cardiac cycle (E) to derive the aortic area (F, red line, left y axis) and aortic blood flow (F, blue line, right y axis). LV, left ventricular.
Aortic Distensibility
Aortic distensibility analysis is depicted in Figure 1, E and F as previously described (22). The ascending thoracic aortic area was manually traced using Jim version 6 (Xinapse software, UK) and graphically represented against time. Aortic distensibility was determined by the following validated formula:
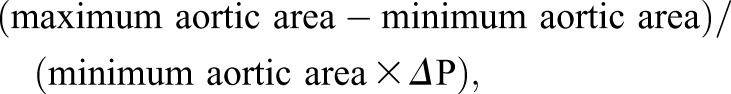 |
where ΔP is the mean of three brachial pulse pressure readings performed during CMR (23).
Statistical Analyses
Descriptive statistics for continuous variables were tested for normality and summarized using mean±SD or median (interquartile range; 75th percentile–25th percentile). Discrete variables were summarized by proportions. Treatment outcomes were estimated by the interaction of treatment group with time in linear mixed-effects models. Patients were specified as random intercepts adjusted for study center, age, sex, and diabetes status, and the covariance matrix was unstructured. Treatment effect estimates for the segmental strain also accounted for correlations of LV segments within patients. Because outcomes were assessed at a single follow-up time, these models produced essentially identical results to the analysis of covariance. All prespecified analyses used a two-sided significance at P<0.05, with the per-comparison error rate controlled using the correction described by Šidák (24). To minimize bias in treatment outcome estimates, analyses were performed by the intention-to-treat principle with multiple imputation of missing follow-up CMR data, as detailed in the Supplemental Material (25). Post hoc explanatory analyses of interdialytic weight gain and predialysis BP were done without data imputation using a two-sided significance level of P<0.001. SPSS version 21.0 was used for all analyses.
Primary Study Outcome
The prespecified primary outcome was the change in the resting EF by CMR at 12 months compared with baseline between the intervention and control groups.
Secondary Study Outcomes
The prespecified secondary outcomes were LV mass and LV volumes, global peak systolic strain, global peak systolic strain rate, global peak diastolic strain rate, and aortic distensibility.
Rationale for Study Outcomes
No applicable strain-based data on patients on HD were available before this study. We used data from a previous natural history study of patients well established on HD, using the observed 13% reduction in the EF by echocardiography over 12 months seen in patients experiencing HD-induced LV wall motion abnormalities versus those who did not (3). Prior data supported the EF as a predictor of cardiovascular mortality, with a change of 5% approaching the minimum clinically important difference (26). CMR is the reference technique to determine the EF (27). Because some clinical therapy trials for heart failure reported mortality reductions with an unaltered EF, we augmented assessment of the EF with LV mass, LV strain, and aortic distensibility, characterizing potentially subclinical cardiomyopathy. Several studies in patients on HD showed an independent association of changes in LV mass with cardiovascular mortality (28,29). Recent studies determined that systolic strain and diastolic dysfunction predicted all-cause mortality or cardiovascular events in patients on HD with a normal EF (30,31). Both HD and non-HD population studies show that aortic distensibility independently predicts future cardiovascular events (22,32).
Sample Size Estimation
Assuming a mean±SD EF of 67%±6% in the control arm, a study of 64 participants would resolve a 5% difference in the EF between groups with 90% power at 5%, two-sided significance level (15). The trial would require 52 participants to retain 80% power for a 5% difference in the EF under the same assumptions. Allowing for study attrition of 10%, target recruitment was set at 72 participants.
Monitoring for Adverse Events
Treatment-related adverse events were summarized for each treatment group.
Results
Patient Characteristics
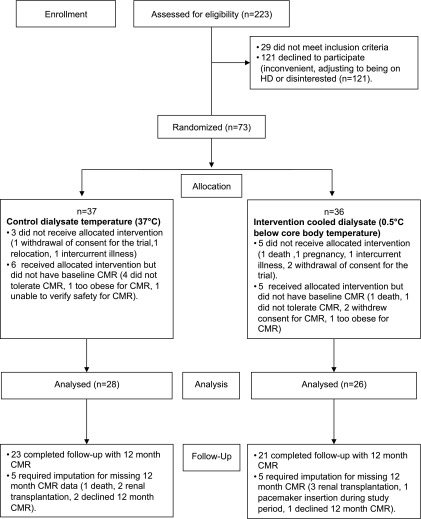
Seventy-three patients were enrolled into the trial and randomized; 54 patients were analyzed (28 control, 26 intervention; age, 60±24 years). The Consolidated Standards of Reporting Trials flowchart is provided in Figure 2. Baseline characteristics were similar between the two randomized groups (Table 1).
Figure 2.
Consolidated Standards of Reporting Trials flowchart. CMR, cardiac magnetic resonance imaging; HD, hemodialysis.
Table 1.
Baseline patient characteristics
| Characteristic | Control Dialysate Temperature 37°C (n=28) | Individualized Cooled Dialysate Temperature (n=26) |
|---|---|---|
| Age (y) | 60 (25) | 60 (26) |
| Female (%) | 8 (36) | 7 (33) |
| BMI (kg/m2) | 28±6 | 28±6 |
| BSA (m2) | 1.9±0.2 | 1.9±0.2 |
| Systolic BP (mmHg) | 142 (28) | 140 (28) |
| Diastolic BP (mmHg) | 76±13 | 75±11 |
| Pulse pressure (mmHg) | 62 (29) | 59 (30) |
| Medical history | ||
| HD vintage (d) | 135±69 | 122±69 |
| Tunneled catheter (%) | 8 (29) | 5 (19) |
| Arteriovenous fistula (%) | 20 (71) | 21 (81) |
| Ischemic heart disease (%) | 4 (14) | 3 (12) |
| Current/ex-smoker (%) | 8 (36) | 13 (59) |
| Peripheral vascular disease (%) | 2 (9) | 5 (24) |
| Primary renal disease | ||
| Diabetes mellitus (%) | 8 (29) | 6 (21) |
| GN (%) | 4 (14) | 3 (11) |
| Interstitial nephritis (%) | 2 (7) | 4 (14) |
| Vasculitis (%) | 3 (11) | 1 (4) |
| Polycystic kidney disease (%) | 2 (7) | 1 (4) |
| Renovascular disease/malignant hypertension (%) | 2 (7) | 4 (14) |
| Plasma cell dyscrasias (%) | 1 (4) | 3 (11) |
| Unknown/others (%) | 6 (21) | 4 (14) |
| Medication | ||
| Treated hypertension (%) | 21 (75) | 21 (81) |
| RAAS antagonist (%) | 8 (29) | 6 (23) |
| β-Blocker (%) | 9 (32) | 10 (39) |
| Statin use (%) | 12 (43) | 11 (42) |
| Phosphate binder | ||
| Calcium containing (%) | 11 (39) | 4 (15) |
| Noncalcium (%) | 9 (32) | 5 (19) |
| Erythropoiesis-stimulating agent (%) | 21 (75) | 26 (69) |
| Vitamin D analog (%) | 16 (57) | 15 (58) |
| Laboratory values | ||
| Hemoglobin (g/dl) | 10.9±1.4 | 11.1±1.6 |
| Calcium (mg/dl) | 9.2±0.8 | 9.2±0.4 |
| Phosphate (mg/dl) | 5.0 (1.2) | 4.7 (2.8) |
| Albumin (g/dl) | 3.6±0.4 | 3.6±0.3 |
| Total cholesterol (mg/dl) | 127.6 (23.2) | 139.2 (30.9) |
| Ultrafiltration per session (L) | 2.0±0.9 | 2.0±0.8 |
Continuous data were tested for normality and summarized using mean±SD or median (interquartile range). Categorical data were expressed as counts (percentages). BMI, body mass Index; BSA, body surface area; HD, hemodialysis; RAAS, renin-angiotensin aldosterone system.
Adverse Events and Protocol Adherence to Treatment Allocation
There were no intervention-related withdrawals in the intervention group. Two patients in the control group did not tolerate a dialysate temperature of 37°C and had dialysate temperature lowered to 36°C by the clinical team independent of the investigators. They were included in all analyses by their original treatment allocation. The mean delivered dialysate temperature in the intervention group was 35.8°C±0.3°C achieving a 1.2°C±0.3°C between-group separation.
The prespecified treatment outcomes are summarized in Table 2 and additionally represented as standardized effect sizes in Figure 3.
Table 2.
Trial outcomes
| End Pointa | Treatment | Baseline | 12 mo | Treatment Difference Between Groupsf |
|---|---|---|---|---|
| EF (%) | Control | 58.7±8.5 | 57.6±8.5 | 1.5 (−4.3 to 7.3) |
| Intervention | 57.4±15.3 | 61.0±18.4 | ||
| LV mass (g) | Control | 140.3±48.7 | 141.3±48.7 | −15.6 (−29.4 to −1.9) |
| Intervention | 157.9±50.5 | 145.6±50.5 | ||
| LV mass indexed to BSA (g/m2) | Control | 72.4±21.7 | 73.5±31.7 | −8.1 (−15.5 to −0.8) |
| Intervention | 80.7±17.8 | 75.0±26.5 | ||
| Global peak systolic strain (%)b | Control | −16.3±3.7 | −13.0±6.9 | −3.3 (−6.5 to −0.2) |
| Intervention | −15.9±4.6 | −15.9±7.6 | ||
| Global peak systolic strain rate (s−1)c | Control | −0.97±0.2 | −0.87±0.21 | −0.2 (−0.3 to −0.08) |
| Intervention | −1.02±0.3 | −1.03±0.25 | ||
| Global peak diastolic strain rate (s−1)d | Control | 1.05±0.3 | 0.90±0.42 | 0.18 (0.02 to 0.34) |
| Intervention | 1.12±0.4 | 1.00±0.41 | ||
| LV end-diastolic volume (ml) | Control | 150.3±37 | 156.1±37 | −23.8 (−44.7 to −2.9) |
| Intervention | 162.9±39.8 | 144.5±39.8 | ||
| LV end-diastolic volume indexed to BSA (ml/m2) | Control | 78.8±21.2 | 75.6±31.2 | −12.4 (−23.2 to −1.5) |
| Intervention | 83.5±27 | 73.3±29.1 | ||
| Aortic distensibility (mmHg−1×10−3)e | Control | 4.4±2.6 | 2.1±2.6 | 1.8 (0.1 to 3.6) |
| Intervention | 3.4±2.5 | 3.1±2.5 |
EF, left ventricular ejection fraction; LV, left ventricle; BSA, body surface area.
Mean±SD of observed values were adjusted using a linear mixed-effects model for study center, age, sex, and diabetes status.
Systolic strain values are conventionally expressed on a negative scale as a percentage change in LV length from baseline, with more negative changes denoting better systolic function.
Systolic strain rate values are expressed on a negative scale, with more negative changes denoting better systolic function.
Diastolic strain rate values are a measure of diastolic function, with greater values denoting better function.
Aortic distensibility data were skewed and therefore logarithmically transformed values were used, with coefficients back-transformed for presentation.
Treatment effects are expressed as mean (95% CI) and were determined by the interaction of treatment group with time.
Figure 3.

Trial outcomes expressed as standardized effect sizes with 95% confidence intervals. The mean changes from Table 2 are divided by the pooled SD of the variable at baseline. LV, left ventricular; LVEF, left ventricular ejection fraction.
Primary Outcome
There was no statistically significant change in EF between control and intervention groups (1.5%; 95% confidence interval [95% CI], –4.3% to 7.3%).
Secondary Outcomes
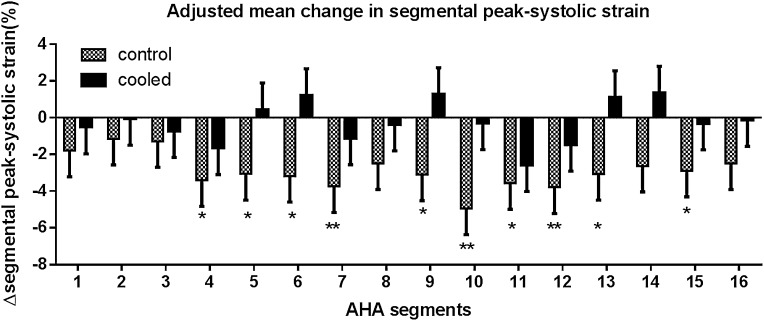
The intervention was associated with significant reductions in LV mass (–15.6 g; 95% CI, –29.4 to –1.9g) (Table 2). The intervention was also associated with a significant reduction in LV end-diastolic volumes compared with no change in the control group (–23.8 ml; 95% CI, –44.7 to –2.9 ml) (Table 2). Global LV systolic function by peak systolic strain was preserved in the intervention group and significantly reduced in the control group (within-group change 0%±7.6% in intervention group versus 3.3%±6.9% decrease in control group; difference, –3.3%; 95% CI, –6.5% to –0.2%). Similarly, the intervention preserved the peak systolic strain rate (–0.2 s−1; 95% CI, –0.3 to –0.08 s−1). There was also preservation of diastolic function in the intervention group relative to the control group (0.18 s−1; 95% CI, 0.02 to 0.34 s−1). Regional LV analysis showed significant segmental strain reduction in 10 of 16 LV segments in the control group compared with no significant change in the intervention group (Figure 4). Aortic distensibility was preserved in the intervention group and significantly decreased in the control group (–0.3±2.6 mmHg−1×10−3 intervention group versus –2.3±0.6 mmHg−1×10−3 control group; difference, 1.8; 95% CI, 0.1 to 3.6 mmHg−1×10−3).
Figure 4.
Within-group comparisons of adjusted mean±SEM of absolute change in peak systolic strain (%) from baseline to 12 months using the American Heart Association 16-segment model of the left ventricle. Strain values are conventionally expressed on a negative scale as a percentage change in length from baseline, with larger changes denoting better function. P values are for the interaction of treatment group with time in a linear mixed-effects model. *P<0.05; **P<0.01. AHA, American Heart Association.
Post Hoc Explanatory Analyses
Analyses of volume status by bioimpedance, interdialytic weight gain, and predialysis mean arterial BP showed no significant differences between groups over 12 months and no differential effects of baseline LV mass on treatment effects (as detailed in the Supplemental Material).
Discussion
This randomized controlled trial demonstrates that the use of individualized cooler dialysate results in preservation, or improvements, in important structural and functional cardiovascular abnormalities in patients on HD. The intervention was operationally simple to deliver, fitting well within an existing model of care for patients on HD and providing a substantial differential in delivered temperature. The existence of this differential reinforces the considerable variation in core temperature encountered within patients on dialysis. Although a significant difference in the primary outcome of EF was not evident, it should be emphasized that the intervention was associated with preservation or improvement in an otherwise complete range of prespecified and clinically relevant secondary outcomes. This comprehensive assessment was made possible by the application of advanced CMR, to directly quantify included measures of LV systolic and diastolic function, aortic distensibility, and LV mass. The observed effect sizes are of a magnitude previously associated with changes in cardiovascular mortality in patients on HD (28,33).
The more recent application of strain analysis has confirmed the prognostic value when the EF is preserved, supporting that the EF may not have been the optimal primary cardiac imaging–based outcome (30,31). In this trial there was a clear and consistent signal of both preservation of function directly measured by LV strain and inferred from reductions in LV mass and LV dilation that the cooling intervention was effective at protecting the heart.
LV hypertrophy is an established predictor of cardiovascular mortality in patients on HD, and reducing LV mass is recognized as an important therapeutic goal (28). Conventional approaches to achieve this have largely relied on the control of pressure and volume overload. Daily dialysis is an effective approach in this respect, and the Frequent Hemodialysis Network trials reported that HD six times weekly led to an adjusted mean reduction in LV mass (−13.8 g; 95% CI, −21.8 to −5.8g) and LV end-diastolic volume (−11.0%; 95% CI, −16% to −6%), with no change in EF over 12 months compared with conventional thrice-weekly HD (34,35). This trial reports a similar reduction in LV mass (−15.6 g; 95% CI, −29.4 to −1.9 g) and LV end-diastolic volume (−23.8 ml; 95% CI, −44.7 to −2.9 ml), with no change in EF over 12 months. In keeping with the Frequent Hemodialysis Network trials, the magnitude of the LV mass reduction was partially dependent on baseline LV mass (36). It is of particular interest in our trial that the observed reductions of LV mass cannot be explained by differences in predialysis BP, fluid status, or interdialytic weight gain. However, the myocardium is capable of responding to a wide variety of other remodeling signals (37,38). Repetitive cardiac ischemia leads to cardiac hypertrophy and fibrosis; reduced HD induced ischemia by cooler dialysate may exert a potent influence (39,40). Directly protective benefits of lower temperatures on cardiac myocytes have also been described (41). Furthermore, there are important interactions between the left ventricle and aortic function (so-called ventriculovascular coupling). Prior data showed reduced aortic distensibility or LV hypertrophy increased myocardial oxygen demand or reduced diastolic coronary artery perfusion (42). Therefore, patients with lesser aortic distensibility or increasing LV mass are more vulnerable to repetitive HD-induced cardiac ischemia (43,44). The preserved aortic distensibility over 12 months in the intervention group compared with significant reductions in the control group may have contributed to the observed cardiac effects (22,32). The interactions between intradialytic events and fluid/volume status are complex, and additional investigations are warranted to understand the mechanisms by which dialysate cooling might attenuate the progression of cardiomyopathy in patients on HD. Future trials might be predicated on clinical outcomes and run as registry-based, cluster randomized controlled trials. On the basis of current event rates, 3-year follow-up, and average facility size of 121 patients, we estimate a study would require 14 clusters per arm to detect a 20% reduction in a primary composite end point of all-cause mortality or hospitalizations with a major cardiovascular event. However, because dialysate cooling is universally applicable without cost and well tolerated, with no apparent harm and probable benefits to both the heart and brain (11), dialysis clinicians and patients may alternatively consider that these data approach proof beyond a reasonable doubt and adopt wider use without further trials.
Limitations
This first randomized trial of dialysate cooling has important limitations. The trial was not designed to reliably estimate hard clinical outcomes such as hospitalization rates or mortality. Data on nursing interventions and intradialytic hypotensive events were beyond the scope and resources of the trial but may have better explained the mechanisms of the reported benefits. Data to reliably inform the likely effect size were unavailable. With multiple imputation, the sample size retained 80% power to determine differences in the primary outcome of EF compared with the original target of 90%. The sample size also retained 90% power to resolve a 10% difference in LV peak systolic strain (45); however, data to inform estimations for this more sensitive outcome were unavailable when the trial was designed. There was greater than anticipated attrition before the first CMR assessment that was not treatment related. Similar attrition from CMR assessment has been reported in recent clinical trials in patients on HD using CMR (35). Such attrition might in part be caused by recruiting from an incident population, who were adjusting to starting on HD, and wide inclusion criteria allowing enrollment of patients with significant comorbidities who were less likely to undergo CMR.
Although the primary outcome of EF was unchanged, this study is the first to demonstrate that an intervention delivered within the context of conventional thrice-weekly HD attenuates the progression of cardiomyopathy by reducing LV mass and dilation and preserving LV strain and aortic distensibility without adverse events. Dialysate cooling was operationally simple to deliver, well tolerated, universally applicable, and cost free. This strongly suggests that attention to the hemodynamic tolerability of dialysis is important to improve long-term outcomes for patients on HD and warrants further definitive study or wider adoption.
Disclosures
None.
Supplementary Material
Acknowledgments
We acknowledge the statistical support of Mr. Apostolos Fakis. We acknowledge the use of software and analytic assistance of Dr. Mark Horsfield. We thank Prof. Simon Davies, Dr. Veena Reddy, Dr. Indranil Dasgupta, study participants, and nursing and allied health care professionals for their time. We thank the magnetic resonance radiographers led by Mrs. Kathryn Appleyard at the Nuffield Derby Hospital and Mr. David Capener at the University of Sheffield. We acknowledge useful discussions with Dr. Robert Foley and the assistance of Dr. James Fotheringham with data parsing. We acknowledge the helpful comments of the anonymous peer reviewers.
This study was funded by a National Institute for Health Research Research for Patient Benefit Grant (PB-PG-0408-16195). Dr. Odudu acknowledges the support of a British Heart Foundation Clinical Research Training Fellowship Grant (FS/11/10/28564) and a National Institute for Health Research Clinical Lectureship. Dr. McCann is supported by a National Institute for Health Research Post-Doctoral Research Fellowship (PDF-2011-04-51). The study was sponsored by Derby Hospitals National Health Service Foundation Trust.
Parts of the study outcomes were presented in preliminary form at the American Society of Nephrology conference in November 2013.
The funder had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Lower Dialysate Temperature in Hemodialysis: Is It a Cool Idea?,” on pages 1318–1320.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.00200115/-/DCSupplemental.
References
- 1.Gross ML, Ritz E: Hypertrophy and fibrosis in the cardiomyopathy of uremia—beyond coronary heart disease. Semin Dial 21: 308–318, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Breidthardt T, McIntyre CW: Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med 12: 13–20, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced repetitive myocardial injury results in global and segmental reduction in systolic cardiac function. Clin J Am Soc Nephrol 4: 1925–1931, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton JO, Jefferies HJ, Selby NM, McIntyre CW: Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 4: 914–920, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, Franssen CF: Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol 7: 1615–1623, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maggiore Q, Pizzarelli F, Zoccali C, Sisca S, Nicolò F, Parlongo S: Effect of extracorporeal blood cooling on dialytic arterial hypotension. Proc Eur Dial Transplant Assoc 18: 597–602, 1981 [PubMed] [Google Scholar]
- 7.Selby NM, McIntyre CW: A systematic review of the clinical effects of reducing dialysate fluid temperature. Nephrol Dial Transplant 21: 1883–1898, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW: Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 6: 1326–1332, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chesterton LJ, Selby NM, Burton JO, McIntyre CW: Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int 13: 189–196, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Jefferies HJ, Burton JO, McIntyre CW: Individualised dialysate temperature improves intradialytic haemodynamics and abrogates haemodialysis-induced myocardial stunning, without compromising tolerability. Blood Purif 32: 63–68, 2011 [DOI] [PubMed] [Google Scholar]
- 11.Eldehni MT, Odudu A, McIntyre CW: Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 26: 957–965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Odudu A, Eldehni MT, Fakis A, McIntyre CW: Rationale and design of a multi-centre randomised controlled trial of individualised cooled dialysate to prevent left ventricular systolic dysfunction in haemodialysis patients. BMC Nephrol 13: 45, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Movilli E, Cancarini GC, Cassamali S, Camerini C, Brunori G, Maffei C, Maiorca R: Inter-dialytic variations in blood volume and total body water in uraemic patients treated by dialysis. Nephrol Dial Transplant 19: 185–189, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Sheen V, Bhalla V, Tulua-Tata A, Bhalla MA, Weiss D, Chiu A, Abdeen O, Mullaney S, Maisel A: The use of B-type natriuretic peptide to assess volume status in patients with end-stage renal disease. Am Heart J 153: e1–e5, e5, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hudsmith LE, Petersen SE, Francis JM, Robson MD, Neubauer S: Normal human left and right ventricular and left atrial dimensions using steady state free precession magnetic resonance imaging. J Cardiovasc Magn Reson 7: 775–782, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Fürstenberg A, Davenport A: Comparison of multifrequency bioelectrical impedance analysis and dual-energy X-ray absorptiometry assessments in outpatient hemodialysis patients. Am J Kidney Dis 57: 123–129, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, Heitmann BL, Kent-Smith L, Melchior JC, Pirlich M, Scharfetter H, Schols AM, Pichard C, Composition of the ESPEN Working Group : Bioelectrical impedance analysis—part I: review of principles and methods. Clin Nutr 23: 1226–1243, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Mosteller RD: Simplified calculation of body-surface area. N Engl J Med 317: 1098, 1987 [DOI] [PubMed] [Google Scholar]
- 19.Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA: Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reson 11: 55, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garot J, Bluemke DA, Osman NF, Rochitte CE, McVeigh ER, Zerhouni EA, Prince JL, Lima JA: Fast determination of regional myocardial strain fields from tagged cardiac images using harmonic phase MRI. Circulation 101: 981–988, 2000 [DOI] [PubMed] [Google Scholar]
- 21.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, Pennell DJ, Rumberger JA, Ryan T, Verani MS, American Heart Association Writing Group on Myocardial Segmentation and Registration for Cardiac Imaging : Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation 105: 539–542, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Maroules CD, Khera A, Ayers C, Goel A, Peshock RM, Abbara S, King KS: Cardiovascular outcome associations among cardiovascular magnetic resonance measures of arterial stiffness: the Dallas heart study. J Cardiovasc Magn Reson 16: 33, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bramwell JC, Hill AV: Velocity of transmission of the pulse wave and elasticity of arteries. Lancet 1: 891–892, 1922 [Google Scholar]
- 24.Šidák Z: Rectangular confidence regions for the means of multivariate normal distributions. J Am Stat Assoc 62: 626–633, 1967 [Google Scholar]
- 25.White IR, Carlin JB: Bias and efficiency of multiple imputation compared with complete-case analysis for missing covariate values. Stat Med 29: 2920–2931, 2010 [DOI] [PubMed] [Google Scholar]
- 26.Komajda M, Carson PE, Hetzel S, McKelvie R, McMurray J, Ptaszynska A, Zile MR, Demets D, Massie BM: Factors associated with outcome in heart failure with preserved ejection fraction: findings from the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-PRESERVE). Circ Heart Fail 4: 27–35, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Bellenger NG, Davies LC, Francis JM, Coats AJ, Pennell DJ: Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2: 271–278, 2000 [DOI] [PubMed] [Google Scholar]
- 28.London GM, Pannier B, Guerin AP, Blacher J, Marchais SJ, Darne B, Metivier F, Adda H, Safar ME: Alterations of left ventricular hypertrophy in and survival of patients receiving hemodialysis: follow-up of an interventional study. J Am Soc Nephrol 12: 2759–2767, 2001 [DOI] [PubMed] [Google Scholar]
- 29.Zoccali C, Benedetto FA, Mallamaci F, Tripepi G, Giacone G, Stancanelli B, Cataliotti A, Malatino LS: Left ventricular mass monitoring in the follow-up of dialysis patients: prognostic value of left ventricular hypertrophy progression. Kidney Int 65: 1492–1498, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Liu YW, Su CT, Sung JM, Wang SP, Su YR, Yang CS, Tsai LM, Chen JH, Tsai WC: Association of left ventricular longitudinal strain with mortality among stable hemodialysis patients with preserved left ventricular ejection fraction. Clin J Am Soc Nephrol 8: 1564–1574, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iwabuchi Y, Ogawa T, Inoue T, Otsuka K, Nitta K: Elevated E/E’ predicts cardiovascular events in hemodialysis patients with preserved systolic function. Intern Med 51: 155–160, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Guerin AP, Blacher J, Pannier B, Marchais SJ, Safar ME, London GM: Impact of aortic stiffness attenuation on survival of patients in end-stage renal failure. Circulation 103: 987–992, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, Russo PE, Golino P, Calabrò R: Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol 41: 1438–1444, 2003 [DOI] [PubMed] [Google Scholar]
- 34.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Yang PC, Rajagopalan S, Frequent Hemodialysis Network Trial Group : Effects of frequent hemodialysis on ventricular volumes and left ventricular remodeling. Clin J Am Soc Nephrol 8: 2106–2116, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, Gorodetskaya I, Greene T, James S, Larive B, Lindsay RM, Mehta RL, Miller B, Ornt DB, Rajagopalan S, Rastogi A, Rocco MV, Schiller B, Sergeyeva O, Schulman G, Ting GO, Unruh ML, Star RA, Kliger AS, FHN Trial Group : In-center hemodialysis six times per week versus three times per week. N Engl J Med 363: 2287–2300, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chan CT, Greene T, Chertow GM, Kliger AS, Stokes JB, Beck GJ, Daugirdas JT, Kotanko P, Larive B, Levin NW, Mehta RL, Rocco M, Sanz J, Schiller BM, Yang PC, Rajagopalan S, Frequent Hemodialysis Network (FHN) Trial Group : Determinants of left ventricular mass in patients on hemodialysis: Frequent Hemodialysis Network (FHN) Trials. Circ Cardiovasc Imaging 5: 251–261, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kehat I, Molkentin JD: Molecular pathways underlying cardiac remodeling during pathophysiological stimulation. Circulation 122: 2727–2735, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glassock RJ, Pecoits-Filho R, Barberato SH: Left ventricular mass in chronic kidney disease and ESRD. Clin J Am Soc Nephrol 4[Suppl 1]: S79–S91, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Shivalkar B, Flameng W, Szilard M, Pislaru S, Borgers M, Vanhaecke J: Repeated stunning precedes myocardial hibernation in progressive multiple coronary artery obstruction. J Am Coll Cardiol 34: 2126–2136, 1999 [DOI] [PubMed] [Google Scholar]
- 40.Selby NM, Burton JO, Chesterton LJ, McIntyre CW: Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 1: 1216–1225, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Tissier R, Ghaleh B, Cohen MV, Downey JM, Berdeaux A: Myocardial protection with mild hypothermia. Cardiovasc Res 94: 217–225, 2012 [DOI] [PubMed] [Google Scholar]
- 42.Watanabe H, Ohtsuka S, Kakihana M, Sugishita Y: Coronary circulation in dogs with an experimental decrease in aortic compliance. J Am Coll Cardiol 21: 1497–1506, 1993 [DOI] [PubMed] [Google Scholar]
- 43.Niizuma S, Takiuchi S, Okada S, Horio T, Kamide K, Nakata H, Yoshihara F, Nakamura S, Kawano Y, Nakahama H, Iwanaga Y, Nakatani S: Decreased coronary flow reserve in haemodialysis patients. Nephrol Dial Transplant 23: 2324–2328, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Bouthier JD, De Luca N, Safar ME, Simon AC: Cardiac hypertrophy and arterial distensibility in essential hypertension. Am Heart J 109: 1345–1352, 1985 [DOI] [PubMed] [Google Scholar]
- 45.Swoboda PP, Larghat A, Zaman A, Fairbairn TA, Motwani M, Greenwood JP, Plein S: Reproducibility of myocardial strain and left ventricular twist measured using complementary spatial modulation of magnetization. J Magn Reson Imaging 39: 887–894, 2014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.