Abstract
Background and objectives
AKI is frequent and is associated with poor outcomes. There is limited information on the epidemiology of AKI worldwide. This study compared patients with AKI in emerging and developed countries to determine the association of clinical factors and processes of care with outcomes.
Design, setting, participants, & measurements
This prospective observational study was conducted among intensive care unit patients from nine centers in developed countries and five centers in emerging countries. AKI was defined as an increase in creatinine of ≥0.3 mg/dl within 48 hours.
Results
Between 2008 and 2012, 6647 patients were screened, of whom 1275 (19.2%) developed AKI. A total of 745 (58% of those with AKI) agreed to participate and had complete data. Patients in developed countries had more sepsis (52.1% versus 38.0%) and higher Acute Physiology and Chronic Health Evaluation (APACHE) scores (mean±SD, 61.1±27.5 versus 51.1±25.2); those from emerging countries had more CKD (54.3% versus 38.3%), GN (6.3% versus 0.9%), and interstitial nephritis (7.0% versus 0.6%) (all P<0.05). Patients from developed countries were less often treated with dialysis (15.5% versus 30.2%; P<0.001) and started dialysis later after AKI diagnosis (2.0 [interquartile range, 0.75–5.0] days versus 0 [interquartile range, 0–5.0] days; P=0.02). Hospital mortality was 22.0%, and 13.3% of survivors were dialysis dependent at discharge. Independent risk factors associated with hospital mortality included older age, residence in an emerging country, use of vasopressors (emerging countries only), dialysis and mechanical ventilation, and higher APACHE score and cumulative fluid balance (developed countries only). A lower probability of renal recovery was associated with residence in an emerging country, higher APACHE score (emerging countries only) and dialysis, while mechanical ventilation was associated with renal recovery (developed countries only).
Conclusions
This study contrasts the clinical features and management of AKI and demonstrates worse outcomes in emerging than in developed countries. Differences in variations in care may explain these findings and should be considered in future trials.
Keywords: acute renal failure, epidemiology and outcomes, nephrology
Introduction
AKI occurs in up to 25% of critically ill patients (1–3) and is associated with higher mortality, especially in patients receiving RRT (2–5). AKI also prolongs lengths of stay, increases costs (4,6,7) and affects long-term kidney function (3,8). In patients who survive a hospitalization with RRT, 5%–20% will continue receiving RRT at discharge (9). Following AKI, the risk for CKD and ESRD increases by 8-fold and 3-fold, respectively (8,10,11).
Even the most sensitive definition of AKI by the AKI Network (AKIN) (12) has been associated with higher morbidity, mortality, and CKD (4,8,13). However, there is limited information on the epidemiology of the entire spectrum of AKI severity in patients worldwide (14). Most prior studies have been performed on a single continent (15) or in one country, such as Program to Improve Outcomes in Acute Renal Disease (PICARD) (16–18). Most studies have focused on severe AKI (15–17). However, a recent multicenter Italian prospective observational study of 576 critically ill patients demonstrated that 54.1% had RIFLE stage R at AKI diagnosis (18). Only one large study (Beginning and Ending Supportive Therapy for the Kidney [BEST]) included patients from various continents; however, most required RRT (2). PICARD and BEST were performed a decade ago and may not reflect current practice patterns.
Better representation of mild to severe AKI worldwide is needed to understand the differences between populations and facilitate resource allocations and research. Studies from developing countries have shown that AKI incidence varies (14,19–23) and that AKI is often community acquired and preventable (i.e., due to gastroenteritis and infections) (23–27). However, a formal comparison between developed and emerging countries is lacking (14,28). We tested the hypothesis that the cause, risk factors, and course of AKI are different in patients from emerging versus developed countries and that those variables influence resource utilization, renal recovery, and survival.
Materials and Methods
Study Design
In 2008, we initiated a multicenter prospective observational study on AKI in critically ill patients from emerging and developed countries, as defined by the World Bank (data.worldbank.org). We screened patients for AKI within 7 days from intensive care unit (ICU) admission and defined AKI with a modified AKIN creatinine criterion (≥0.3 mg/dl within 48 hours) (12). Patients were eligible if they were age 18 years or older; they were excluded if they were receiving RRT, had a kidney transplant, or had no creatinine measurement within 2 days after ICU admission. The study database has an automatic system to screen for missing or out-of-range values. We recorded baseline data on all screened patients and reported results for patients screened and enrolled between August 2008 and April 2012.
Data Collection
In enrolled patients, data on demographic characteristics and medical history were collected once. We determined a baseline creatinine value (>3–12 months before hospital admission) to establish CKD status and a reference creatinine (lowest creatinine level within 2 days before ICU admission or, if unavailable, first creatinine after ICU admission) to determine the AKI starting point. CKD status was defined as a GFR<60 ml/min per 1.73 m2 and was computed with the Modification of Diet in Renal Disease equation (29) using the most recent baseline creatinine. When a baseline creatinine level was unavailable, the most recent level before hospitalization was considered the baseline; if that was unavailable, we used the reference creatinine. We stratified patients as having de novo AKI or AKI-on-CKD. Data on laboratory test results and drugs were collected twice daily for a week. AKI risk factors, sepsis status (30), Acute Physiology and Chronic Health Evaluation (APACHE) III scores (31), and Sequential Organ Failure Assessment scores (32) were assessed daily for a week. Cumulative fluid balance (CFB) was the sum of daily fluid balances. We reported nonexclusive categories for AKI causes, as documented in patients’ charts. Outcomes included RRT requirement at any time, renal recovery, length of stay and survival in the ICU, and hospital discharge. Renal recovery occurred when the difference between creatinine at hospital discharge and reference creatinine was <0.3 mg/dl in patients independent from RRT.
Each center’s institutional review board approved the study. In eight centers, written informed consent was required from participants or their surrogates. Patients with surrogate consents were reconsented if they regained decision-making capacity.
Statistical Analyses
Continuous variables are presented as the mean±SD or median and interquartile range (IQR) and were compared using a t test or Mann–Whitney U test, where appropriate. Categorical variables are presented as proportions and were compared using a chi-squared test. We determined independent predictors of mortality and absence of renal recovery at hospital discharge using logistic regression, considering the following variables: age, race, sex, developed versus emerging country status, coronary artery disease, congestive heart failure, CKD, GFR, surgical diagnosis at ICU admission, Sequential Organ Failure Assessment and APACHE III scores, sepsis, vasopressors, mechanical ventilation, starches, RRT, diuretics, CFB, and maximal AKIN stage. Collinearity between variables was tested before modeling. Variables with a P value <0.10 by univariate analysis were included in the multivariate model. Discrimination of the model was assessed by the c-statistic. Calibration was assessed by the Hosmer–Lemeshow goodness-of-fit test. Because mechanical ventilation was found to be associated with better renal recovery, we performed sensitivity analyses with duration of mechanical ventilation, an alternate definition of absence of renal recovery and adjusted for ICU and hospital length of stay. We also tested interactions of emerging/developed country with the significant variables in the multivariate model one interaction at a time. We then repeated the analysis with all initial variables included in the multivariate model and significant interaction terms (P<0.1). Statistical tests were two sided and P<0.05 was considered to represent a statistically significant difference. Appropriate adjustments for the Bonferroni correction were mentioned in tables and figures with multiple P value comparisons. Statistical analyses were performed with SPSS software, version 19.0 (IBM, Armonk, NY).
Results
Demographic Characteristics of Screened and Enrolled Patients
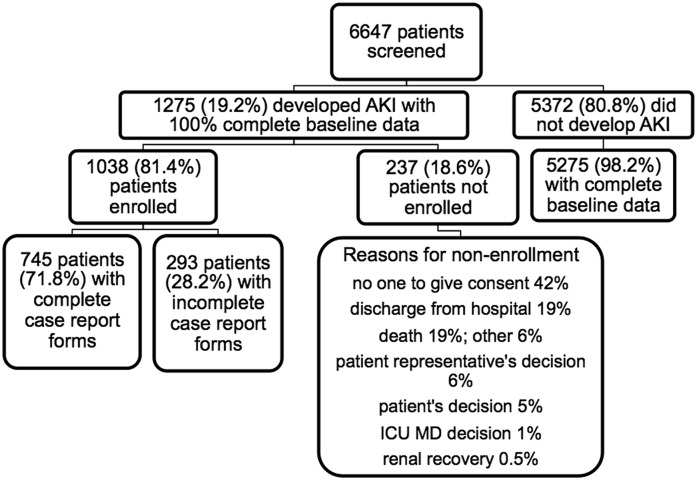
We screened 6647 patients from 14 centers (Figure 1). Among these, nine were in developed (United States [six centers; 3057 patients], Canada [one center; 355 patients], Ireland [one center; nine patients], and Greece [one center; three patients]) and five were in emerging countries (Brazil [one center; 1702 patients], India [one center; 345 patients], and China [three centers; 1176 patients]). All but two were university-affiliated hospitals, with number of beds ranging from 365 to 4800. Most units were closed, had intensivists as treating physicians, and had a large variety of specialized units. RRT management was usually shared between intensivists and nephrologists. Enrolled patients with AKI were similar to nonenrolled patients with AKI except for race, diabetes, and diagnosis at ICU admission (Supplemental Table 1).
Figure 1.
Enrollment of study patients. ICU, intensive care unit.
Among enrolled patients with AKI (Table 1), those from developed countries were heavier, had higher severity-of-illness scores, required mechanical ventilation and vasopressors more often, and had sepsis more often than those from emerging countries. CKD was more prevalent in emerging countries. The use of starches (10.0% in developed countries versus 7.4% in emerging countries; P=0.28) and diuretics (59.2% in developed countries versus 64.1% in emerging countries; P=0.17) during the 7-day period was similar between groups. CFB over 7 days was 1.44 (IQR, −0.91 to 5.10) L and was similar between developed and emerging countries (1.65 [IQR, −0.83 to 6.28] L versus 1.25 [IQR, −0.93 to 4.18] L; P=0.08).
Table 1.
Baseline characteristics of enrolled patients with AKI in developed and emerging countries at intensive care unit admission
| Characteristic | Total (n=745) | Developed Countries (n=316) | Emerging Countries (n=429) | P Valuea |
|---|---|---|---|---|
| Age (yr) | 60.5±18.4 | 62.2±16.9 | 59.2±19.4 | 0.004 |
| Men (%) | 62.2 | 61.7 | 62.7 | 0.92 |
| White (%) | 43.0 | 61.0 | 28.7 | <0.001a |
| Body weight (kg) | 75.7±21.6 | 84.1±26.9 | 69.6±13.6 | <0.001a |
| CKD (%) | 47.5 | 38.3 | 54.3 | <0.001a |
| eGFR (ml/min per 1.73 m2) | 64.8±41.0 | 73.6±38.1 | 58.2±41.9 | 0.001a |
| Hypertension (%) | 60.2 | 61.3 | 59.3 | 0.77 |
| Diabetes (%) | 35.3 | 33.5 | 36.6 | 0.67 |
| Coronary artery disease (%) | 32.4 | 35.1 | 30.3 | 0.28 |
| Heart failure (%) | 25.6 | 23.3 | 27.5 | 0.20 |
| Chronic obstructive pulmonary disease (%) | 14.3 | 17.6 | 11.7 | 0.04 |
| Peripheral artery disease (%) | 12.3 | 11.8 | 12.7 | 0.82 |
| Cirrhosis (%) | 8.8 | 11.8 | 6.4 | 0.01 |
| Underwent surgery (%) | 0.001a | |||
| Urgent surgery | 12.6 | 17.9 | 8.4 | |
| Elective surgery | 17.0 | 17.3 | 16.8 | |
| Cardiac surgery | 6.2 | 8.2 | 4.7 | 0.05 |
| Creatinine at ICU admission (mg/dl) | 1.7±1.7 | 1.6±1.3 | 1.7±1.8 | 0.001a |
| Main diagnosis (%) | <0.001a | |||
| Septic shock | 8.9 | 8.9 | 8.9 | |
| Cardiologic | 20.4 | 18.7 | 21.7 | |
| Respiratory | 10.6 | 16.2 | 6.5 | |
| Nephrologic | 13.0 | 2.5 | 20.7 | |
| On mechanical ventilation (%) | 37.2 | 47.5 | 29.6 | <0.001a |
| On vasopressors (%) | 23.5 | 33.2 | 16.3 | <0.001a |
| On diuretics (%) | 38.7 | 29.4 | 45.5 | <0.001a |
| Oliguria (%) | 28.5 | 31.7 | 26.1 | 0.11 |
| Sepsis (%) | 44.3 | 52.1 | 38.0 | <0.001a |
| APACHE III score | 55.5±26.7 | 61.1±27.5 | 51.1±25.2 | <0.01 |
| SOFA score | 6.2±4.0 | 6.8±4.1 | 5.7±3.9 | <0.001a |
eGFR was based on Modification of Diet in Renal Disease estimating equation. Values expressed with a plus/minus sign are the mean±SD. ICU, intensive care unit; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment.
P≤0.002 represented significant difference, with the Bonferroni correction.
AKI Characteristics
In the screened population, 1275 patients had AKI (19.2% [95% confidence interval (95% CI), 18.3% to 20.2%]) and the incidence was similar between developed and emerging countries (19.1% [95% CI, 17.8% to 20.5%] versus 19.9% [95% CI, 18.6% to 21.3%]; P=0.38).
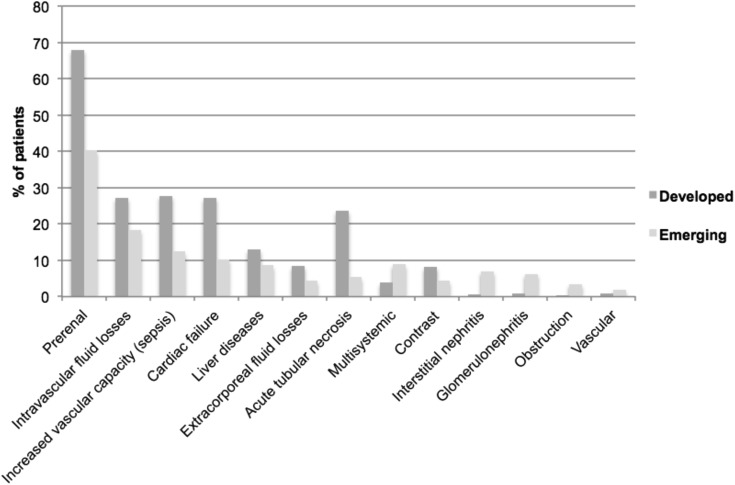
In enrolled patients, AKI was diagnosed 1.0 (IQR, 0–2.0) day after ICU admission (Table 2). Fifty-three percent of patients had de novo AKI (61.7% in developed countries versus 45.7% in emerging countries; P<0.001) (Table 1). Causes of AKI differed, with more GN and acute interstitial nephritis (AIN) in emerging countries and more prerenal AKI, sepsis, and acute tubular necrosis (ATN) in developed countries (Figure 2). No kidney biopsy was done in developed countries, while 5.2% (n=22) of patients from emerging countries had biopsy (for GN [n=8], AIN [n=6], ATN [n=5], vasculitis [n=2], and amyloidosis [n=1]).
Table 2.
Timing of AKI diagnosis, stages of AKI, and use of RRT
| Variable | Total (n=745) | Developed Countries (n=316) | Emerging Countries (n=429) | P Valuea |
|---|---|---|---|---|
| Timing of AKI diagnosis after ICU admission (%) | 0.24 | |||
| 0–2 d | 82.7 | 86.1 | 80.2 | |
| 3–4 d | 10.9 | 9.6 | 12.0 | |
| 5–7 d | 6.3 | 4.2 | 7.8 | |
| Maximal AKI stage (%) | <0.001a | |||
| 1 | 71.7 | 78.8 | 66.4 | |
| 2 | 3.5 | 3.8 | 3.3 | |
| 3 | 24.8 | 17.4 | 30.3 | |
| RRT (%) | 23.7 | 15.5 | 30.2 | <0.001a |
| Criteria for commencing RRT (%) | ||||
| Hyperkalemia | 9.3 | 13.0 | 7.8 | 0.30 |
| Acidemia | 16.1 | 28.3 | 11.3 | <0.01 |
| Uremic manifestations | 12.4 | 4.3 | 15.7 | 0.06 |
| Oliguria | 39.8 | 34.8 | 41.7 | 0.42 |
| Fluid overload | 32.9 | 28.3 | 34.8 | 0.43 |
ICU, intensive care unit.
P values for comparison of developed versus emerging countries; P≤0.006 represents a significant difference, with the Bonferroni correction.
Figure 2.
Causes of AKI. The causes of AKI are nonexclusive. Intravascular fluid losses included hemorrhage, burns, and hypovolemia due to other causes. Increased vascular capacity included sepsis and other causes. Extracorporeal fluid losses included vomiting, diarrhea, and diabetes insipidus, while vascular causes included artery/vein thrombosis, emboli, or trauma. P values for the difference between developed and emerging countries for each cause of AKI are, respectively, as follows: prerenal, P<0.001; intravascular fluid losses, P=0.004; sepsis, P<0.001; cardiac failure, P<0.001; liver diseases, P=0.06; extracorporeal fluid losses, P=0.02; acute tubular necrosis, P<0.001; multisystemic, P<0.01; contrast-induced, P=0.03; interstitial nephritis, P<0.001; GN, P<0.001; obstruction, P=0.003; vascular, P=0.31. With the Bonferroni correction, P≤0.004 represents a significant difference. Other causes were unknown or undetermined.
At AKI diagnosis, most patients met AKIN stage 1 criteria (developed countries versus emerging countries: initial AKIN stage 1, 98.1% versus 95.4%; stage 2, 1.3% versus 1.8%; and stage 3, 0.6% versus 2.8%, respectively; P=0.09). The maximal AKI severity is illustrated in Table 2. Compared with patients from emerging countries, patients from developed countries had less severe AKI (P<0.001). After exclusion of patients with CKD, patients from developed and emerging countries had similar AKI severity (P=0.12).
AKI Outcomes
RRT.
Overall, 23.7% of patients required RRT, 1.0 (IQR, 0–5.0) day after AKI diagnosis. Patients from developed countries required RRT less often (15.5% versus 30.2%; P<0.001) and later after AKI diagnosis (2.0 [IQR, 0.75–5.0] days versus 0 [IQR, 0–5.0] days; P=0.02). Both groups had a similar duration of RRT (5.5 [IQR, 1.0–12.75] days versus 6.0 [IQR, 2.0–14.0] days; P=0.76). Multiple modalities were used more often in emerging countries (24.4% in developed countries versus 40.0% in emerging countries; P=0.06). Continuous RRT, intermittent hemodialysis, sustained low-efficiency dialysis (SLED), and peritoneal dialysis were used in 72.7%, 30.4%, 33.5%, and 1.9% of patients, respectively. SLED was most often used in emerging countries (45.2% versus 4.3%; P<0.001). Other modalities were used similarly (developed versus emerging countries: continuous RRT, 78.3% versus 70.4% [P=0.31]; intermittent hemodialysis, 39.1% versus 27.9% [P=0.13]; peritoneal dialysis, 0% versus 2.6% [P=0.27]). Reasons for initiating RRT were similar among countries; the most common were oliguria and fluid overload (Table 2).
Mortality, Length of Stay, and Renal Functional Recovery.
Crude hospital mortality was 22.0% and was higher in developed countries (27.6% versus 17.6%; P=0.002). Crude hospital mortality was 32.3% in patients requiring RRT. The lengths of stay in the ICU (5.0 [IQR, 2.0–9.0] days in developed countries versus 6.0 [IQR, 2.5–10.0] days in emerging countries; P=0.27) and hospital (11.0 [IQR, 6.0–22.0] days versus 10.0 [IQR, 4.5–23.0] days, respectively; P=0.42) were similar between groups. Survivors from emerging countries were less likely to recover kidney function (52.2% in emerging countries versus 71.7% in developed countries; P<0.001) and more frequently dialysis-dependent at hospital discharge (18.5% versus 5.7%; P<0.001).
Risk Factors for Mortality and Absence of Renal Recovery.
In the entire cohort, independent risk factors for mortality were older age, residence in an emerging country, use of mechanical ventilation, higher APACHE score, and AKI stage 3 with RRT (Table 3). Vasopressors and CFB presented significant interactions with the developed/emerging status. Vasopressors were associated with mortality in emerging countries only and with higher cumulative fluid balance in developed countries only.
Table 3.
Risk factors for hospital mortality in patients with AKI from developed and emerging countries (n=745)
| Risk Factor | OR (95% CI) |
|---|---|
| Age | 1.03 (1.01 to 1.05) |
| Emerging country | 2.32 (1.14 to 4.74) |
| On vasopressorsa | 1.84 (1.05 to 3.25) |
| In developed countries | 1.07 (0.42 to 2.71) |
| In emerging countries | 3.51 (1.54 to 7.99) |
| On mechanical ventilation | 2.41 (1.23 to 4.73) |
| APACHE III score | 1.03 (1.02 to 1.04) |
| Cumulative fluid balancea | 1.07 (1.03 to 1.12) |
| In developed countries | 1.12 (1.05 to 1.20) |
| In emerging countries | 0.98 (0.92 to 1.05) |
| AKI stage 3 with RRT | 2.03 (1.07 to 3.83) |
Variables included in the multivariate model were age, race, developing or emerging country, surgery status, Acute Physiology and Chronic Health Evaluation (APACHE) III score, sepsis status, use of vasopressors, mechanical ventilation, starches, RRT, cumulative fluid balance, and significant interaction terms (between country type and vasopressors, between country type and cumulative fluid balance). Area under the receiver-operating characteristic curve (c statistic): 0.87 (95% confidence interval, 0.83 to 0.90); Hosmer–Lemeshow: 0.60. OR, odds ratio; 95% CI, 95% confidence interval.
Vasopressors and cumulative fluid balance presented significant interactions with the developed/emerging status.
We did not include AKI duration in the models on mortality and absence of renal recovery due to collinearity with AKI severity and renal recovery. In the entire cohort, unadjusted mortality associated with an AKI duration ≤2 days was 13.3%; 3–4 days, 12.0%; 5–7 days, 25.0%; and >7 days, 29.5% (P<0.001). Unadjusted mortality was similar for AKI duration <7 days between developed and emerging countries (15.2% versus 12.3%; P=0.46) and higher in patients from developed countries not recovering within 7 days (45.1% versus 21.1%; P<0.001).
The median time for follow-up of kidney function between ICU admission and hospital discharge was 11.0 (IQR, 5.0–24.0) days. Risk factors for the absence of renal recovery in survivors at hospital discharge included living in an emerging country and higher severity of AKI with the use of RRT (Table 4). Mechanical ventilation and APACHE score had significant interactions with developed/emerging status (Table 4). A higher APACHE score was associated with a decreased probability of renal recovery in emerging countries only. The use of mechanical ventilation was associated with a higher probability of renal recovery in developed countries only. We also included results with a less stringent definition of renal recovery (Supplemental Table 2).
Table 4.
Risk factors for the absence of renal recovery in survivors from developed and emerging countries at hospital discharge (n=581)
| Renal recovery criteria | OR (95% CI) |
|---|---|
| Emerging country | 2.91 (1.76 to 4.80) |
| APACHE III scorea | 1.01 (1.00 to 1.02) |
| In developed countries | 1.01 (0.99 to 1.03) |
| In emerging countries | 1.02 (1.00 to 1.04)b |
| AKI stage 3 with RRT | 2.69 (1.53 to 4.72) |
| Mechanical ventilationa | 0.56 (0.33 to 0.97) |
| In developed countries | 0.42 (0.18 to 0.98) |
| In emerging countries | 0.64 (0.31 to 1.35) |
Variables included in the multivariate model were developing or emerging country, coronary artery disease, surgery status, Acute Physiology and Chronic Health Evaluation (APACHE) III score, use of vasopressors, mechanical ventilation, starches, RRT, and significant interaction terms (between country type and mechanical ventilation, between country type and APACHE III). Area under the receiver-operating characteristic curve (c statistic): 0.75 (95% confidence interval, 0.71 to 0.80). Hosmer–Lemeshow: 0.98. OR, odds ratio; 95% CI, 95% confidence interval.
APACHE III score and mechanical ventilation presented significant interactions with the developed/emerging status.
P=0.01.
Discussion
Although 85% of the world’s population resides in emerging countries, systematic prospective studies in this population have been limited (33). While formal comparisons between emerging and developed countries are sparse, there are differences in the cause, natural history, and management of AKI (14,23). We describe the findings of an ongoing multinational study of AKI in ICU patients in 14 large urban centers. Our findings provide a novel assessment of commonalities and differences in the natural history and management of mild to severe AKI.
In our study, patients with AKI from emerging countries had more CKD, less sepsis, and lower severity-of-illness scores. Causes of AKI differed, with more GN and AIN in emerging countries and more prerenal AKI, septic AKI, and ATN in developed countries. However, it is unclear whether these differences are real because kidney biopsies were performed exclusively in emerging countries. Most patients were diagnosed with stage 1 AKI, but more patients in emerging countries developed stage 3 AKI and required RRT. This result can be partly related to the lower baseline kidney function in patients from emerging countries. Resource utilization also differed, with a lower use of vasopressors and mechanical ventilation in emerging countries. Patients from emerging countries also used multiple RRT modalities more frequently and were treated with SLED more often.
The reasons underlying these differences cannot be fully understood with the data available. Patients from developed countries are usually considered to have easier access to specialized medical equipment, while the sickest patients from emerging countries may not have had access to optimal treatment because of perceived limited prognosis, restricted resources, or late transfer to large medical centers (23). In our cohort, some patients from India and China had to pay for their dialysis therapy.
During the last few years, several studies have focused on the relationships between the severity and duration of AKI and outcomes (8,34). More severe AKI has been associated with long-term CKD (8). Brown et al. (35) reported that long-term mortality was proportional to AKI duration independent of the severity (35). Renal recovery was recently associated with lower mortality or CKD after discharge (34). Our data further confirm the association between AKI severity, as reflected by the use of RRT, and outcomes.
While there are no standard definitions of renal recovery, most publications have focused on dialysis independence. We have used a conservative definition of renal recovery. In our cohort, patients from emerging countries were less likely to achieve renal recovery. Among survivors, 19% of patients from emerging countries were dialysis dependent at hospital discharge. This result is of particular concern as access to outpatient RRT is limited and long-term RRT is frequently unaffordable (23,36).
Following interaction tests, risk factors for the absence of renal recovery at discharge were slightly different in developed and emerging countries. Some studies have identified similar predictors, such as the severity of illness and AKI (37–40). Mechanical ventilation was associated with better renal recovery with our definition. This result was still present after adjustment for ICU or hospital stays, which might reflect the decrease in creatinine with loss of muscle mass following prolonged hospitalization (data not shown) (41). These results were not reproduced in patients from emerging countries, with a less strict definition of renal recovery (creatinine at discharge within 50% of reference creatinine) or with the duration of mechanical ventilation (data not shown). Living in an emerging country was a risk factor for nonrenal recovery. The underlying reasons cannot be easily determined because we adjusted for baseline kidney function. Observational studies have shown that continuous RRT may facilitate renal recovery (42,43). In our cohort, the proportion of patients treated with continuous RRT and intermittent hemodialysis was similar between emerging and developed countries; we did not include dialysis modality in our model because of collinearity. The limited results regarding the risk factors for the absence of renal recovery in developed and emerging countries highlights the need for future research on this subject.
In BEST (2) and PICARD (16), >60% of patients required RRT compared with 24% in our study, and their overall mortality was much higher. In BEST (2) and PICARD (44), 13.8% and 20.2% of survivors were dialysis dependent at hospital discharge compared with 13.3% in this study. The incidence of RRT dependence in our cohort was even lower in developed countries (6%). These findings reflect the differences in AKI severity: The PICARD and BEST cohorts largely included patients with stage 2 and 3 disease, while our population included more than 60% with stage 1 disease (23,36).
The overall mortality rate (22%) was lower than previously reported in developed and emerging countries (2,16,24), and the mortality rate in patients receiving RRT (32.3%) was much lower than in BEST (60.3%) and PICARD (45.3%) (2,44). These results are probably related to higher mean APACHE scores in PICARD (86 versus 56 in our study) (16). Indeed, our crude mortality rate was higher in developed countries (56.5% versus 22.6%), which was partly related to a higher APACHE score (2,45).
As in our study, a higher severity of illness and severity of AKI with the use of RRT have been reported as independent risk factors for mortality in a few large AKI studies (15,16). Both use of vasopressors and mechanical ventilation were independent risk factors for mortality in BEST (2). The association between CFB and mortality has been shown in several AKI studies in developed countries (46–49), including PICARD (50). However, we did not observe this result in patients from emerging countries. In addition, the association between country status and mortality has not been reported in BEST and needs to be evaluated further. Some patients from India and China may not have been able to afford dialysis, which could have affected their mortality rates.
Our study has several limitations. First, this is a convenience sample of patients from large urban centers, excluding European centers, which could introduce sample bias. Second, some centers required informed consent. However, the characteristics of enrolled and nonenrolled patients with AKI were similar except for race, diabetes, and main diagnosis. Third, there may be some selection bias from participating centers with an interest in AKI. Our data reflect AKI diagnosis based on serum creatinine only, which may affect AKI incidence. We reported pertinent information for 7 days after AKI diagnosis, which may not represent all factors affecting outcomes. Residual confounding may still be present, given variations in access to care, timing of presentation, availability of resources (e.g., frequency of laboratory testing), and local practices, which were not captured in our study.
In summary, this multinational study provides for the first time a contemporary overview of clinical factors, management, and outcomes of mild to severe AKI in the ICU in emerging and developed countries. Our data suggest that emerging countries have worse AKI outcomes that may be influenced by variations in care. These factors highlight the need to consider regional and country-specific differences in the prevalence of comorbidities, contributing factors for AKI, and management strategies for multicenter studies.
Disclosures
None.
Supplementary Material
Acknowledgments
We would like to thank the study personnel at all the hospitals involved in this trial for their help in conducting this study.
This research was supported through the UAB-UCSD O’Brien Center for Acute Kidney Injury (National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease grant DK079337). J.B. is a scholar of the Fonds de la Recherche du Québec-Santé.
None of these funding sources had a role in the design of the study, collection, analysis, and interpretation of the data; drafting or revising the manuscript; or the decision to submit the manuscript for publication.
Some of the results of this study have been presented as poster presentations at the 2011 and 2012 American Society of Nephrology meetings and at the 2013 Conference on Continuous Renal Replacement Therapies.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.04360514/-/DCSupplemental.
References
- 1.Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ, French Study Group on Acute Renal Failure : Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. Crit Care Med 24: 192–198, 1996 [DOI] [PubMed] [Google Scholar]
- 2.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Ronco C, Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: a multinational, multicenter study. JAMA 294: 813–818, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Liu KD, Chertow GM: Diagnosis, epidemiology and outcomes of acute kidney injury. Clin J Am Soc Nephrol 3: 844–861, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW: Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 16: 3365–3370, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA: Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 23: 1970–1974, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Brandt MM, Falvo AJ, Rubinfeld IS, Blyden D, Durrani NK, Horst HM: Renal dysfunction in trauma: Even a little costs a lot. J Trauma 62: 1362–1364, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoste EA, Schurgers M: Epidemiology of acute kidney injury: How big is the problem? Crit Care Med 36[Suppl]: S146–S151, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: A systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network : Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lafrance JP, Miller DR: Acute kidney injury associates with increased long-term mortality. J Am Soc Nephrol 21: 345–352, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewington AJ, Cerdá J, Mehta RL: Raising awareness of acute kidney injury: A global perspective of a silent killer. Kidney Int 84: 457–467, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Mendonça A, Vincent JL, Suter PM, Moreno R, Dearden NM, Antonelli M, Takala J, Sprung C, Cantraine F: Acute renal failure in the ICU: Risk factors and outcome evaluated by the SOFA score. Intensive Care Med 26: 915–921, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Mehta RL, Pascual MT, Soroko S, Savage BR, Himmelfarb J, Ikizler TA, Paganini EP, Chertow GM, Program to Improve Care in Acute Renal Disease : Spectrum of acute renal failure in the intensive care unit: The PICARD experience. Kidney Int 66: 1613–1621, 2004 [DOI] [PubMed] [Google Scholar]
- 17.Metnitz PG, Krenn CG, Steltzer H, Lang T, Ploder J, Lenz K, Le Gall JR, Druml W: Effect of acute renal failure requiring renal replacement therapy on outcome in critically ill patients. Crit Care Med 30: 2051–2058, 2002 [DOI] [PubMed] [Google Scholar]
- 18.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, Rocco M, Alessandri E, Giunta F, Michetti V, Iannuzzi M, Belluomo Anello C, Brienza N, Carlini M, Pelaia P, Gabbanelli V, Ronco C, NEFROINT Investigators : Prospective multicenter study on epidemiology of acute kidney injury in the ICU: A critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol 77: 1072–1083, 2011 [PubMed] [Google Scholar]
- 19.Al-Homrany M: Epidemiology of acute renal failure in hospitalized patients: Experience from southern Saudi Arabia. East Mediterr Health J 9: 1061–1067, 2003 [PubMed] [Google Scholar]
- 20.Wang Y, Cui Z, Fan M: Retrospective analysis on Chinese patients diagnosed with acute renal failure hospitalized during the last decade (1994-2003). Am J Nephrol 25: 514–519, 2005 [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Cui Z, Fan M: Hospital-acquired and community-acquired acute renal failure in hospitalized Chinese: A ten-year review. Ren Fail 29: 163–168, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Obialo CI, Okonofua EC, Tayade AS, Riley LJ: Epidemiology of de novo acute renal failure in hospitalized African Americans: Comparing community-acquired vs hospital-acquired disease. Arch Intern Med 160: 1309–1313, 2000 [DOI] [PubMed] [Google Scholar]
- 23.Cerdá J, Bagga A, Kher V, Chakravarthi RM: The contrasting characteristics of acute kidney injury in developed and developing countries. Nat Clin Pract Nephrol 4: 138–153, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Anandh U, Renuka S, Somiah S, Vincent L: Acute renal failure in the tropics: Emerging trends from a tertiary care hospital in South India. Clin Nephrol 59: 341–344, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Lombardi R, Yu L, Younes-Ibrahim M, Schor N, Burdmann EA: Epidemiology of acute kidney injury in Latin America. Semin Nephrol 28: 320–329, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Jha V, Parameswaran S: Community-acquired acute kidney injury in tropical countries. Nat Rev Nephrol 9: 278–290, 2013 [DOI] [PubMed] [Google Scholar]
- 27.Kohli HS, Bhat A, Jairam A, Aravindan AN, Sud K, Jha V, Gupta KL, Sakhuja V: Predictors of mortality in acute renal failure in a developing country: A prospective study. Ren Fail 29: 463–469, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Cerdá J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, Bagga A, Levin A: Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 3: 881–886, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 30.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 20: 864–874, 1992 [PubMed] [Google Scholar]
- 31.Knaus WA, Wagner DP, Draper EA, Zimmerman JE, Bergner M, Bastos PG, Sirio CA, Murphy DJ, Lotring T, Damiano A: The APACHE III prognostic system. Risk prediction of hospital mortality for critically ill hospitalized adults. Chest 100: 1619–1636, 1991 [DOI] [PubMed] [Google Scholar]
- 32.Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PM, Thijs LG: The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22: 707–710, 1996 [DOI] [PubMed] [Google Scholar]
- 33.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL, Acute Kidney Injury Advisory Group of the American Society of Nephrology : World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pannu N, James M, Hemmelgarn B, Klarenbach S, Alberta Kidney Disease Network : Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 8: 194–202, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown JR, Kramer RS, Coca SG, Parikh CR: Duration of acute kidney injury impacts long-term survival after cardiac surgery. Ann Thorac Surg 90: 1142–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okunola O, Akinsola A, Ayodele O: Kidney diseases in Africa: Aetiological considerations, peculiarities and burden. Afr J Med Med Sci 41: 119–133, 2012 [PubMed] [Google Scholar]
- 37.Srisawat N, Murugan R, Lee M, Kong L, Carter M, Angus DC, Kellum JA, Genetic and Inflammatory Markers of Sepsis (GenIMS) Study Investigators : Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int 80: 545–552, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Macedo E, Zanetta DM, Abdulkader RC: Long-term follow-up of patients after acute kidney injury: Patterns of renal functional recovery. PLoS ONE 7: e36388, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lin YF, Ko WJ, Chu TS, Chen YS, Wu VC, Chen YM, Wu MS, Chen YW, Tsai CW, Shiao CC, Li WY, Hu FC, Tsai PR, Tsai TJ, Wu KD, NSARF Study Group : The 90-day mortality and the subsequent renal recovery in critically ill surgical patients requiring acute renal replacement therapy. Am J Surg 198: 325–332, 2009 [DOI] [PubMed] [Google Scholar]
- 40.Bagshaw SM, Mortis G, Godinez-Luna T, Doig CJ, Laupland KB: Renal recovery after severe acute renal failure. Int J Artif Organs 29: 1023–1030, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Schetz M, Gunst J, Van den Berghe G: The impact of using estimated GFR versus creatinine clearance on the evaluation of recovery from acute kidney injury in the ICU. Intensive Care Med 40: 1709–1717, 2014 [DOI] [PubMed] [Google Scholar]
- 42.Schneider AG, Bellomo R, Bagshaw SM, Glassford NJ, Lo S, Jun M, Cass A, Gallagher M: Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: A systematic review and meta-analysis. Intensive Care Med 39: 987–997, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Wald R, Shariff SZ, Adhikari NK, Bagshaw SM, Burns KE, Friedrich JO, Garg AX, Harel Z, Kitchlu A, Ray JG: The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: A retrospective cohort study. Crit Care Med 42: 868–877, 2014 [DOI] [PubMed] [Google Scholar]
- 44.Khosla N, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini E, Mehta RL, Program to Improve Care in Acute Renal Disease (PICARD) : Preexisting chronic kidney disease: A potential for improved outcomes from acute kidney injury. Clin J Am Soc Nephrol 4: 1914–1919, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang IK, Wang ST, Chang HY, Lin CL, Kuo HL, Chen TC, Lee CH, Chuang FR: Prognostic value of acute physiology and chronic health evaluation II and organ system failure in patients with acute renal failure requiring dialysis. Ren Fail 27: 663–669, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Payen D, de Pont AC, Sakr Y, Spies C, Reinhart K, Vincent JL, Sepsis Occurrence in Acutely Ill Patients (SOAP) Investigators : A positive fluid balance is associated with a worse outcome in patients with acute renal failure. Crit Care 12: R74, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heung M, Wolfgram DF, Kommareddi M, Hu Y, Song PX, Ojo AO: Fluid overload at initiation of renal replacement therapy is associated with lack of renal recovery in patients with acute kidney injury. Nephrol Dial Transplant 27: 956–961, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF, Jr, Hite RD, Harabin AL, National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network : Comparison of two fluid-management strategies in acute lung injury. N Engl J Med 354: 2564–2575, 2006 [DOI] [PubMed] [Google Scholar]
- 49.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lee J, Lo S, McArthur C, McGuiness S, Norton R, Myburgh J, Scheinkestel C, Su S, RENAL Replacement Therapy Study Investigators : An observational study fluid balance and patient outcomes in the Randomized Evaluation of Normal vs. Augmented Level of Replacement Therapy trial. Crit Care Med 40: 1753–1760, 2012 [DOI] [PubMed] [Google Scholar]
- 50.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, Program to Improve Care in Acute Renal Disease (PICARD) Study Group : Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.