Abstract
Background and objectives
Recent studies highlighting a role of C4d− antibody-mediated rejection (ABMR) have debated whether C4d staining has independent value as a rejection marker. Considering the presumed role of complement as an important effector of graft injury, this study hypothesized that capillary C4d, a footprint of antibody-triggered complement activation, indicates a particularly severe manifestation of ABMR.
Design, setting, participants, & measurements
This large retrospective clinicopathologic study sought to assess the clinical predictive value of C4d staining in relation to ABMR morphology. Overall, 885 renal allograft recipients who underwent transplantation between 1999 and 2006 (median duration of follow-up, 63.3 [interquartile range, 40.6–93.5] months; 206 graft losses) were included if they had had one or more indication biopsies. A total of 1976 biopsy specimens were reevaluated for capillary C4d staining (C4d data were available for 825 patients) and distinct morphologic lesions suggestive of ABMR, including glomerulitis, peritubular capillaritis, capillary microthrombi, transplant glomerulopathy, and severe intimal arteritis.
Results
C4d+ patients, with or without ABMR features, had worse death-censored 8-year graft survival (53% or 67%) than C4d− patients (66% or 81%; P<0.001). In Cox regression analysis, C4d was associated with a risk of graft loss independently of baseline confounders and ABMR morphology (hazard ratio, 1.85 [95% confidence interval, 1.34 to 2.57]; P<0.001). The risk was higher than that observed for C4d− patients, a finding that reached statistical significance in patients showing fewer than two different ABMR lesions. Moreover, in a mixed model, C4d was independently associated with a steeper decline of eGFR (slope per year, −8.23±3.97 ml/min per 1.73 m2; P<0.001).
Conclusions
These results suggest that detection of intragraft complement activation has strong independent value as an additional indicator of ABMR associated with adverse kidney transplant outcomes.
Keywords: antibody-mediated rejection, C4d, complement, kidney transplantation, transplant outcomes
Introduction
Linear deposition of C4d along peritubular capillaries (PTCs), a footprint of alloantibody-triggered classic complement activation, is widely accepted as a marker of antibody-mediated rejection (ABMR) (1,2). Initial studies have shown that C4d predicts adverse graft outcome and is associated with circulating donor-specific antibodies (DSAs) and characteristic morphologic lesions in the microcirculation (3–7). More recently, however, its diagnostic value has been challenged by the finding that distinct morphologic and molecular ABMR features may also frequently occur in the absence of detectable C4d deposits (8–11). Studies have suggested complement-independent mechanisms of antibody-mediated graft injury (12–14). Nevertheless, several lines of evidence indicate a dominant effector role of complement (15–17). One study recently showed that the formation of complement-fixing DSA may entail a particular risk of graft failure (16). Moreover, blocking antibody-triggered complement activation was suggested to be highly effective in preventing ABMR in recipients at high immunologic risk (15). Considering the presumed role of complement in rejection, it is tempting to speculate that even in the context of a refined repertoire of sensitive diagnostic strategies C4d staining can help uncover a particular risk of graft injury and predict responsiveness to treatment targeting complement.
To clarify the role of C4d as an independent and additive rejection marker, we studied a large cohort of 885 kidney transplant recipients who had undergone biopsy to evaluate long-term transplant function and survival in relation to the results of a comprehensive morphologic and immunohistochemical biopsy workup.
Materials and Methods
This retrospective study was approved by the institutional ethics committee and conducted according to the Declaration of Helsinki. The clinical and research activities reported are consistent with the principles outlined in the Declaration of Istanbul on Organ Trafficking and Transplant Tourism.
Patients and Immunosuppression
Overall, 885 of 1248 consecutive adult recipients of an ABO-compatible kidney allograft transplanted at the Medical University Vienna, Austria, between January 1999 and April 2006 were included. Patients included had undergone indication biopsies for unexplained graft dysfunction and/or proteinuria (for baseline characteristics, see Table 1). Three hundred fifty-four patients had one biopsy, 231 had two, and 300 three or more.
Table 1.
Patient baseline characteristics in relation to the results of indication biopsies
| Characteristic | All Patientsv (n=885) | C4d in PTCsa | ABMR Featuresb | ||||
|---|---|---|---|---|---|---|---|
| Yes (n=154) | No (n=671) | P Value | Yes (n=380) | No (n=494) | P Value | ||
| Recipient age (yr) | 52 (41–62) | 50 (40–60) | 52 (41–62) | 0.08 | 48 (38–58) | 55 (44–64) | <0.001 |
| Donor age (yr) | 50 (39–60) | 46 (36–59) | 51 (40–60) | 0.003 | 50 (39–59) | 51 (40–60) | 0.10 |
| Deceased donor transplants | 783 (89) | 140 (91) | 587 (88) | 0.25 | 335 (88) | 438 (88) | 0.82 |
| Prior allograft | 161(18) | 53 (34) | 100 (15) | <0.001 | 105 (27) | 55 (11) | <0.001 |
| ≥10% current CDC-PRA | 191 (22) | 65 (42) | 120 (18) | <0.001 | 113 (30) | 78 (16) | <0.001 |
| HLA mismatch (A, B, DR) | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.30 | 3 (2–4) | 3 (2–4) | 0.001 |
| Cold ischemia time (h) | 13 (8–19) | 13 (9–19) | 13 (8–19) | 0.12 | 13 (8–19) | 12 (8–18) | 0.21 |
| Initial immunosuppressive therapy | |||||||
| Cyclosporine | 656 (74) | 127 (83) | 484 (72) | 0.008 | 297 (78) | 351 (71) | 0.02 |
| Tacrolimus | 148 (17) | 20 (13) | 118 (18) | 0.17 | 58 (15) | 89 (18) | 0.27 |
| IL-2 receptor antibody | 161 (18) | 19 (12) | 130 (19) | 0.05 | 51 (13) | 108 (22) | 0.001 |
| Depleting antilymphocyte antibody | 122 (14) | 46 (30) | 68 (10) | <0.001 | 67 (18) | 53 (11) | 0.004 |
| Peritransplant IA | 66 (8) | 30 (20) | 33 (5) | <0.001 | 47 (12) | 19 (4) | <0.001 |
| mTOR inhibitor with or without CNI | 99 (11) | 8 (5) | 82 (12) | 0.01 | 33 (9) | 58 (12) | 0.15 |
Values are median (interquartile range) or number (percentage). For comparative analysis, Fisher exact and Mann–Whitney U tests were applied. CDC-PRA, complement-dependent cytotoxicity panel-reactive antibody; mTOR, mammalian target of rapamycin; IA, immunoadsorption; CNI, calcineurin inhibitor; PTCs, peritubular capillaries; ABMR, antibody-mediated rejection.
C4d staining results were available for 825 of the 885 included study patients. Patients with C4d scores > 0 were categorized as C4d+.
Biopsy material for a histomorphologic evaluation of ABMR-typical lesions (g, ptc, cg, v3 lesions, capillary microthrombi) was available for 874 of the 885 included study patients.
On the basis of prospective C4d staining, done as part of our routine biopsy workup since 1999, most patients showing focal or diffuse C4d in PTCs had undergone changes in maintenance immunosuppression and/or rejection treatment with or without apheresis for alloantibody depletion. Following our local standard at that time, such treatment included tacrolimus rescue therapy or high-dose steroids (n=25) (18), a depleting antilymphocyte antibody (n=33), or immunoadsorption with or without a depleting antilymphocyte antibody (n=56), applied as a desensitization strategy in patients with broad panel reactivity (19) or as a treatment of established C4d+ rejection (20).
The outcomes studied were graft loss, patient death, and eGFR calculated according to the Mayo Clinic equation (21). Patients receiving dialysis were considered to have an eGFR of 5 ml/min per 1.73 m2.
Biopsies
Overall, 1976 indication biopsies, which were performed after a median of 0.8 months after transplantation (interquartile range, 0.3–6.7 months), were reevaluated for C4d staining and ABMR histomorphology. Most biopsies (n=1460) were done within the first 6 months. C4d staining was performed by immunohistochemistry on paraffin sections (22). Specimens were evaluated by two observers (Z.K. and N.K.) according to the Banff 2009 scheme (23). C4d was scored as 0 (negative), 1 (minimal), 2 (focal), and 3 (diffuse). In addition, the presence or absence of five different lesions suggestive of ABMR was documented (peritubular capillaritis [ptc score >0], glomerulitis [g score >0], capillary microthrombi, transplant glomerulopathy [cg score >0], and severe intimal arteritis [v score of 3]). Because thrombotic microangiopathy (TMA) may be triggered by a variety of nonalloimmune factors, we included a separate analysis in which cases of C4d− TMA without any other features of ABMR were classified as ABMR−. For biopsies showing GN, we did not document g and cg scores. Patient categories were defined according to C4d scores and the finding of ABMR features. In the case of two or more biopsies, for each of the documented features, maximum scores obtained during follow-up were recorded.
Statistical Analyses
Variables were compared by using chi-square, Fisher exact, Mann–Whitney U, or Kruskal–Wallis tests as appropriate. For correlation analysis, the Spearman test was applied. Kaplan–Meier analysis was used to calculate patient and graft survival, and the Mantel–Cox log-rank test was performed to compare survival between groups. For Cox regression analysis, the included variables were determined by the purposeful selection algorithm (24). This algorithm selects variables significant in a univariate model as well as variables that change the parameter estimate of others by >30%. We included C4d, number of ABMR features, recipient age, donor age, number of HLA mismatches (A, B, DR), and cold ischemia time; these confounders were selected from a pool of variables that also consisted of female sex, first or repeated transplant, living or deceased donor, presensitization (complement-dependent cytotoxicity panel reactive antibody ≥10%), and type of initial immunosuppression (cyclosporine-, tacrolimus-, or sirolimus/everolimus-based treatment; induction therapy with a depleting antilymphocyte antibody or an IL-2 receptor antibody, with or without preemptive immunoadsorption). To assess associations of biopsy results with eGFR course, we performed a mixed-model analysis with longitudinal data. The variables included were the same as in the Cox model, with eGFR as dependent variable and an additional interaction term of C4d and time. The nonlinearity in this model was accounted for by an additional parameter for measurements after 7 years. A two-sided P value <0.05 was considered to represent a statistically significant difference. Statistical calculations were performed by using PASW for Windows, version 18.0 (SPSS Inc., Hong Kong), and SAS 9.3 for Windows (SAS Institute, Cary, NC).
Results
This study included 885 kidney transplant recipients who underwent indication biopsies (median duration of follow-up, 63.3 [interquartile range, 40.6–93.5] months). Baseline characteristics are summarized in Table 1.
C4d Staining in PTCs and Kidney Allograft Survival
For 825 recipients adequate material was available for retrospective evaluation of C4d staining. Hundred fifty-four (19%) patients showed capillary C4d in at least one of the biopsy specimens. Thirty-nine patients were categorized as having minimal (C4d1), 54 as having focal (C4d2), and 61 as having diffuse (C4d3) C4d deposits (Table 2). Compared with C4d− (C4d score of 0), C4d+ recipients (C4d score ≥1) were younger; were more frequently presensitized and retransplant recipients; and had more frequently been subjected to more intense initial immunosuppression, including antilymphocyte antibody induction or immunoadsorption for desensitization (Table 1). As illustrated in Figure 1, the worst 8-year death-censored graft survival was observed in patients scored as C4d3 (49%), followed by C4d2 (56%), C4d1 (66%), and C4d0 (77%) (P<0.001). Cox regression analysis revealed independent effects of both C4d2 and C4d3 (hazard ratios, 2.29 and 2.72, respectively; P<0.001) on death-censored graft survival (Table 3). For C4d1 we found a trend toward a higher risk of graft loss (hazard ratio, 1.73; P=0.07).
Table 2.
Relationship between C4d scores and morphologic features suggestive of antibody-mediated rejection
| Morphologic resultsa | All Patients (n=874) | C4d Score: 0 (n=671) | C4d Score: 1 (n=39) | C4d Score: 2 (n=54) | C4d Score: 3 (n=61) | P Value |
|---|---|---|---|---|---|---|
| Individual ABMR lesionsb,c(%) | ||||||
| Glomerulitis (g) | ||||||
| g score >0 | 19 | 12 | 19 | 50 | 58 | <0.001 |
| g score: 1 | 5 | 4 | 5 | 9 | 17 | |
| g score: 2 | 7 | 4 | 6 | 20 | 18 | |
| g score: 3 | 7 | 4 | 8 | 21 | 23 | |
| Peritubular capillaritis (ptc) | ||||||
| ptc score >0 | 31 | 26 | 50 | 51 | 61 | <0.001 |
| ptc score: 1 | 13 | 11 | 22 | 19 | 23 | |
| ptc score: 2 | 15 | 12 | 28 | 30 | 25 | |
| ptc score: 3 | 3 | 3 | 0 | 2 | 14 | |
| Transplant glomerulopathy (cg) | ||||||
| cg score >0 | 12 | 9 | 19 | 26 | 36 | <0.001 |
| cg score: 1 | 5 | 4 | 8 | 5 | 14 | |
| cg score: 2 | 3 | 2 | 0 | 11 | 3 | |
| cg score: 3 | 4 | 3 | 11 | 9 | 19 | |
| Capillary microthrombi | 9 | 7 | 16 | 9 | 30 | <0.001 |
| Severe intimal arteritis (v3) | 1 | 0.6 | 0 | 2 | 3 | 0.10 |
| Number of different ABMR features (%) | ||||||
| 0 lesions | 56 | 63 | 33 | 22 | 12 | <0.001 |
| 1 lesion | 31 | 29 | 59 | 43 | 38 | <0.001 |
| 2 lesions | 10 | 6 | 5 | 32 | 34 | <0.001 |
| ≥3 lesions | 3 | 2 | 3 | 4 | 16 | <0.001 |
ABMR, antibody-mediated rejection.
For patients with two or more biopsies, for each individual lesion the maximum score documented during follow-up was recorded.
Adequate material for re-evaluation of single criteria suggestive of ABMR was available for 817 (g), 754 (ptc), 846 (capillary microthrombi), 821 (v3), and 801 (cg) patients.
Lesions were scored according to the Banff 2009 scheme (23).
Figure 1.
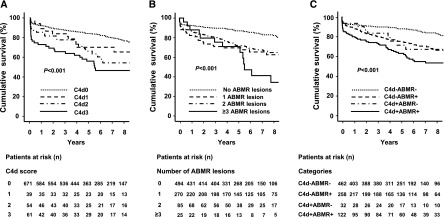
Association of biopsy results with death-censored graft survival. Kaplan–Meier analysis of death-censored kidney allograft survival to evaluate the effect of (A) C4d Banff scores, (B) the number of individual histomorphologic antibody-mediated rejection (ABMR) features, and (C) C4d staining (C4d+, C4d scores of 1–3; C4d−, C4d score of 0) in relation to the presence (ABMR+) or absence (ABMR−) of one or more ABMR features. For univariate comparison the log-rank test was used.
Table 3.
Multivariable models of biopsy results and graft loss
| Variable | Patients (n) | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|---|
| Independent effect of C4d scoring resulta,b | |||
| 0 | 671 | Reference | |
| 1 | 39 | 1.73 (0.95 to 3.14) | 0.07 |
| 2 | 54 | 2.29 (1.44 to 3.66) | <0.001 |
| 3 | 61 | 2.72 (1.79 to 4.12) | <0.001 |
| Independent effect of number of ABMR lesionsa,c | |||
| 0 lesions | 494 | Reference | |
| 1 lesion | 275 | 2.02 (1.45 to 2.79) | <0.001 |
| 2 lesions | 85 | 2.63 (1.69 to 4.08) | <0.001 |
| 3 or more lesions | 25 | 4.15 (2.32 to 7.44) | <0.001 |
| Independent effect of C4d versus ABMR morphologya | |||
| Overall cohort | 806e | ||
| C4d in PTCsd | 1.85 (1.34 to 2.57) | <0.001 | |
| ABMR morphology | 1.91 (1.38 to 2.66) | <0.001 | |
ABMR, antibody-mediated rejection; PTCs, peritubular capillaries.
Confounding variables were selected according to the purposeful selection algorithm as described in the Materials and Methods section (recipient age, donor age, HLA-mismatch, cold ischemia time).
C4d staining in PTCs was scored according to the Banff scheme.
Documented ABMR lesions were glomerulitis (g score >0), peritubular capillaritis (ptc score >0), severe intimal arteritis (v score of 3), thrombotic microangiopathy, and glomerulopathy (cg score >0).
C4d positivity was defined as a C4d score ≥1.
Number of patients for whom all confounding variables included in the model were available.
Histomorphologic Lesions Suggestive of ABMR and Graft Survival
For 380 recipients (44%), at least one of five characteristic histomorphologic ABMR lesions was recorded. The most frequent lesion was peritubular capillaritis (Table 2). Patients with ABMR morphology were younger, were more frequently presensitized and recipients of prior transplants, and more often received antibody induction therapy and immunoadsorption (Table 1). Evaluating the total number of different ABMR features, we identified 270 patients showing one, 85 showing two, and 25 showing three or more different lesions. The finding of individual ABMR features was associated with adverse 8-year death-censored graft survival (g, 58% versus 77% [no lesion], P=0.001; cg, 58% versus 76%, P<0.01; ptc, 63% versus 80%, P<0.001; capillary microthrombi, 43% versus 76%, P<0.001; v3, 14% versus 79%, P<0.001). We found a strong relationship between the number of different ABMR features and graft loss rates, being highest when three or more ABMR features were present (Figure 1). This effect was also observed in a multivariate model showing a stepwise increase in hazard ratio in relation to the number of ABMR features (Table 3).
Independent Effect of C4d Staining and ABMR Morphology on Clinical Outcomes
For each of the recorded individual ABMR features, we found associations with C4d scores (P<0.0001); the exception was grade 3 intimal arteritis, for which only a trend was observed (Table 2). Moreover, there was a highly significant correlation between C4d scoring and the number of ABMR lesions (r=0.41; P<0.001). Overall, ABMR morphology was more frequent in C4d+ (79%) than in C4d− patients (36%).
Thirty-two patients were C4d+ (C4d score ≥1) without ABMR-typical features (C4d+ABMR−); only four of them showed acute tubular injury with minimal inflammation. The time to the first C4d+ biopsy specimen in these patients did not differ from that observed for C4d+ABMR+ patients (median, 0.75 versus 0.65 months; P=0.53). Of the 109 C4d+ABMR+ patients with two or more biopsies, 12 recipients showed the sequence of a C4d+ABMR− biopsy specimen preceding the occurrence of ABMR features in subsequent specimens. However, for 20 patients the opposite was the case.
As illustrated in Figure 1, C4d+ABMR+ patients exhibited the worst 8-year graft survival (53%), followed by C4d+ABMR− (67%), C4d−ABMR+ (66%) and C4d−ABMR− patients (81%). Comparing patient groups individually, C4d+ABMR+, C4d+ABMR−, and C4d−ABMR+ patients showed higher graft loss rates than C4d−ABMR− patients (P≤0.001). While survival differed significantly between C4d+ABMR+ and C4d−ABMR+ patients (P=0.01), this was not the case for a comparison of C4d+ABMR+ with C4d+ABMR− recipients (P=0.25).
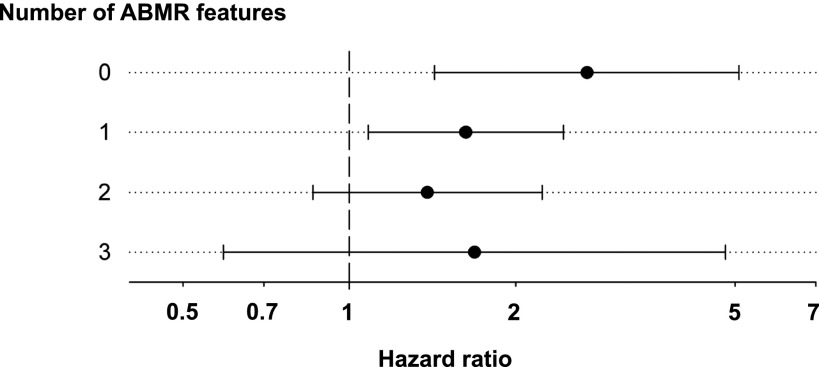
In a Cox model, both C4d positivity and ABMR morphology were independent risk factors of graft loss associated with an approximately 2-fold higher risk (Table 3). Figure 2 illustrates the association between C4d and graft loss in relation to the discrete number of ABMR features. In this analysis, C4d positivity was associated with graft loss independently of the morphologic presentation, with hazard ratios that were consistently higher than those computed for C4d− patients with or without ABMR morphology; this difference achieved statistical significance in patients showing fewer than two different ABMR lesions (Figure 2).
Figure 2.
Forest plot of the risk of graft failure in relation to biopsy findings. Hazard ratios (95% confidence intervals) are shown for C4d (score ≥1) in relation to the discrete number of antibody-mediated rejection features. Confounding variables included in the model (recipient age, donor age, HLA-mismatch, cold ischemia time) were selected according to the purposeful selection algorithm as described in the Materials and Methods section.
Associations of C4d with graft loss were not related to an imbalance regarding the frequency of type 1 or 2 T cell–mediated rejection (C4d+: 56%; patients with ABMR morphology: 60%) or mild or moderate intimal arteritis (v1 and/or 2 score: 32% versus 36%). Moreover, in a separate analysis, 25 patients showing C4d− TMA with no other ABMR-typical lesions, a finding that may not be related to alloimmunity, were reclassified as ABMR−. Redefinition of patient groups did not considerably change associations of C4d (hazard ratio, 1.87; 95% confidence interval, 1.35 to 2.61) or ABMR morphology (hazard ratio, 1.71; 95% confidence interval, 1.24 to 2.36) with graft loss.
C4d, ABMR Morphology and eGFR Slopes
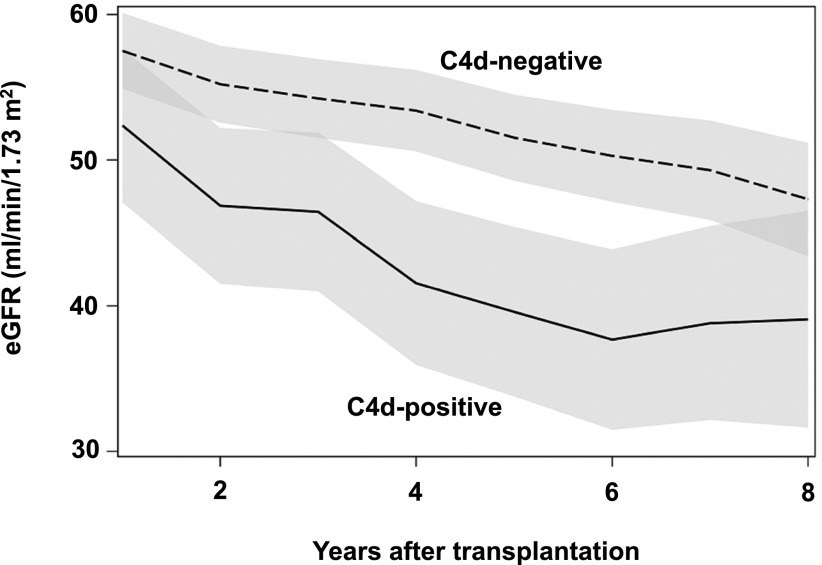
As demonstrated by multivariable mixed model analysis, C4d+ recipients showed a significantly greater eGFR slope throughout the study period (−8.23±3.97 ml/min per 1.73 m2; P<0.001), even when the presence of ABMR features were considered; these were also independently associated with a greater eGFR slope (−8.55±1.94 ml/min per 1.73 m2; P<0.001) (Table 4). The adverse adjusted course of eGFR in C4d+ patients compared with C4d− patients is illustrated in Figure 3.
Table 4.
Estimates of eGFR changes for the main effects in a mixed model
| Effect | eGFR Change per Year (ml/min per 1.73 m2) | P Value |
|---|---|---|
| C4d in PTCsa | −8.23±3.97 | <0.001 |
| ABMR morphologyb | −8.55±1.94 | <0.001 |
| Recipient age, per yr | −0.13±0.07 | 0.05 |
| Donor age, per yr | −0.59±0.06 | <0.001 |
| Cold ischemia time, per hr | −0.46±0.06 | <0.001 |
| HLA-mismatch, per number | 0.04±0.67 | 0.96 |
Changes in eGFR are expressed as mean±SD. PTCs, peritubular capillaries; ABMR, antibody-mediated rejection.
Positive C4d staining was defined as a Banff C4d score >0.
One or more histologic features suggestive of ABMR.
Figure 3.
Mixed model to analyze the effect of C4d staining on the post-transplant course of eGFR. The eGFR calculated according to the Mayo Clinic equation was evaluated for C4d+ versus C4d− recipients with yearly measurements for a standardized recipient, adjusted for median values of selected baseline confounders: recipient age (52 years), donor age (49 years), cold ischemia time (12 hours), and HLA mismatch (n=3). The shaded areas represent the 95% confidence intervals.
Discussion
In this large retrospective study we sought to define the clinical significance of C4d as a marker of humoral alloimmunity. Our results demonstrate that a positive C4d stain reflects a more severe form of ABMR, independent of and in addition to its histomorphologic presentation.
In line with previous reports (3,5,6,25), C4d was associated with inferior allograft survival. Patients with diffuse or focal C4d had a graft failure risk two to three times higher than did C4d− patients. Even for minimal C4d a trend toward adverse graft survival was observed, a result that supports the recent recommendation of considering a C4d1 score (immunohistochemistry) positive (2). A remarkable finding was that the associations of C4d with survival were independent of ABMR morphology. C4d was associated with a significant increment in the risk of graft failure, especially in transplants with fewer than two different morphologic ABMR features. Finally, in support of an outstanding prognostic value of C4d, we also found a strong independent effect in an analysis of eGFR slopes.
Our results are in line with those of an earlier study of indication biopsies showing a strong association of C4d with transplant outcome, with or without DSA, even after adjustment for glomerulitis and peritubular capillaritis (26). The previously described relationship among DSA levels, C4d staining, and the severity of ABMR (26,27) may support a cardinal role of complement as a trigger of graft injury. Nevertheless, one may also argue that C4d could be an indirect indicator that strong endothelial alloantibody binding promotes complement-independent injury, leading to a more active ABMR process.
Several previous reports have highlighted an important role of C4d− ABMR and have challenged the leading diagnostic role of C4d staining as a biomarker of ABMR (2,8–11,28–30). For example, in a study of 173 indication biopsies, the frequent finding of molecular features of endothelial injury uncovered by transcriptional profiling also predicted adverse graft survival in the absence of C4d staining (8). In a protocol biopsy study of 54 presensitized patients, many patients developed subclinical C4d− ABMR, which was associated with the development of subsequent graft injury (31). Notably, in these initial studies, the worst outcome results were reported for C4d+ allografts, suggesting that C4d could indicate a more severe form of ABMR (8,31). However, this concept was questioned by subsequent reports suggesting a strong independent clinical effect of microcirculation injury (11,32,33). Nevertheless, comparably small sample sizes in many of the cited studies may have impeded the strength of multivariable statistical models.
Approximately 40% of our C4d− patients showed morphologic features suggestive of ABMR. In support of a role of complement-independent humoral injury, ABMR morphology was independently associated with impaired graft survival. Indeed, recent studies have proposed a variety of candidate mechanisms contributing to graft injury, including natural killer cell activation, endothelial cell activation, and modulation of angiotensin receptor function (12–14). However, in interpreting our data, it is important to note that we applied immunohistochemical C4d staining, which may be less sensitive than immunofluorescence (34,35). Negative C4d staining does not necessarily exclude a certain level of intragraft complement activation that might have escaped immunohistologic detection.
Notably, 21% of the C4d+ patients presented without ABMR morphology. Analysis of the timing and sequence of biopsy results suggested that this specific phenotype may not necessarily reflect an early stage of rejection. In interpreting our data in the context of earlier studies (5,36), it is important to note that in accordance with recent Banff updates (37) minimal C4d, a common finding in C4d+ABMR− patients (41%), was included to define a positive stain. We are aware that in the absence of DSA data, a role of false-positive C4d staining cannot be excluded. Several earlier studies have shown that C4d may occur in the absence of microcirculation injury, especially in ABO-incompatible transplantation (38). The role of C4d without ABMR morphology, however, is less clear for ABO-compatible grafts. In our cohort, this finding was associated with adverse graft outcomes, whereby survival was intermediate between that of C4d−ABMR− and C4d+ABMR+ patients. In multivariate analysis, C4d staining was associated with an additional risk of graft loss, particularly in patients showed no morphologic ABMR lesions. Our findings may be consistent with a previous study of protocol biopsies demonstrating an adverse effect of C4d also in the absence of histologic signs of rejection (39).
Earlier studies have suggested considerable differences in the phenotype and clinical presentation of ABMR between presensitized recipients and patients who develop DSA later after transplantation (37,40). In our model, however, there was no significant interaction between recipient presensitization (≥10% complement-dependent cytotoxicity panel reactive antibodies) and C4d or ABMR morphology, which precluded a valid subgroup analysis in relation to presensitization status (data not shown).
A major limitation of our study is the lack of systematic protocol biopsies. Accordingly, we were unable to detect the continuous (subclinical) evolution of features of acute or chronic graft injury. For individual patients, especially for those with only one documented biopsy, we cannot exclude relevant fluctuations of levels of intragraft complement activation, as reported earlier (9,22,41).
In our cohort of consecutive transplant recipients, prospective DSA monitoring was not part of our clinical routine. Considering the limited specificity of distinct lesions (e.g., glomerulitis or peritubular capillaritis), which may also be found in DSA− diagnostic entities (28), one may argue that the lack of DSA data may have led to an overestimation of the number of C4d− ABMR cases. For this reason we did not include v1 or v2 lesions, which were recently suggested to reflect ABMR in some patients (42). Similarly, by including TMA as a possible ABMR feature (43), we cannot exclude other potential causes, especially in patients showing C4d− TMA with no other ABMR-typical lesions. In a separate analysis reclassifying these patients as ABMR−, however, the independent association between C4d or ABMR morphology with graft survival did not significantly change. On the other hand, by scoring transplant glomerulopathy according to the Banff 2009 scheme, it is possible that discrete lesions, defined as positive in a recently published update, have been missed (2,23).
Finally, many of our C4d+ patients had been subjected to intensified immunosuppression. At that time the concept of C4d− negative ABMR was not yet established, and antihumoral treatment was not considered in cases of C4d− graft dysfunction. One may speculate that antihumoral treatment, especially in C4d+ patients, may have led to a considerable bias, counteracting the independent influence of C4d in our cohort.
In conclusion, this study supports a prominent prognostic value of C4d staining as a rejection marker in ABO-compatible kidney transplantation. Our results suggest that C4d is associated with adverse kidney transplant performance independent of and in addition to histomorphologic features suggestive of ABMR.
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria—an addition to the Banff 97 classification of renal allograft rejection. Am J Transplant 3: 708–714, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, Castro MC, David DS, David-Neto E, Bagnasco SM, Cendales LC, Cornell LD, Demetris AJ, Drachenberg CB, Farver CF, Farris AB, 3rd, Gibson IW, Kraus E, Liapis H, Loupy A, Nickeleit V, Randhawa P, Rodriguez ER, Rush D, Smith RN, Tan CD, Wallace WD, Mengel M, Banff meeting report writing committee : Banff 2013 meeting report: Inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant 14: 272–283, 2014 [DOI] [PubMed] [Google Scholar]
- 3.Feucht HE, Schneeberger H, Hillebrand G, Burkhardt K, Weiss M, Riethmüller G, Land W, Albert E: Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int 43: 1333–1338, 1993 [DOI] [PubMed] [Google Scholar]
- 4.Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB: Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol 12: 574–582, 2001 [DOI] [PubMed] [Google Scholar]
- 5.Mauiyyedi S, Crespo M, Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Tolkoff-Rubin NE, Williams WW, Delmonico FL, Cosimi AB, Colvin RB: Acute humoral rejection in kidney transplantation: II. Morphology, immunopathology, and pathologic classification. J Am Soc Nephrol 13: 779–787, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Böhmig GA, Exner M, Habicht A, Schillinger M, Lang U, Kletzmayr J, Säemann MD, Hörl WH, Watschinger B, Regele H: Capillary C4d deposition in kidney allografts: A specific marker of alloantibody-dependent graft injury. J Am Soc Nephrol 13: 1091–1099, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Regele H, Böhmig GA, Habicht A, Gollowitzer D, Schillinger M, Rockenschaub S, Watschinger B, Kerjaschki D, Exner M: Capillary deposition of complement split product C4d in renal allografts is associated with basement membrane injury in peritubular and glomerular capillaries: A contribution of humoral immunity to chronic allograft rejection. J Am Soc Nephrol 13: 2371–2380, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Sis B, Halloran PF: Endothelial transcripts uncover a previously unknown phenotype: C4d-negative antibody-mediated rejection. Curr Opin Organ Transplant 15: 42–48, 2010 [DOI] [PubMed] [Google Scholar]
- 9.Loupy A, Hill GS, Suberbielle C, Charron D, Anglicheau D, Zuber J, Timsit MO, Duong JP, Bruneval P, Vernerey D, Empana JP, Jouven X, Nochy D, Legendre CH: Significance of C4d Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 11: 56–65, 2011 [DOI] [PubMed] [Google Scholar]
- 10.Haas M: Pathology of C4d-negative antibody-mediated rejection in renal allografts. Curr Opin Organ Transplant 18: 319–326, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Loupy A, Lefaucheur C, Vernerey D, Chang J, Hidalgo LG, Beuscart T, Verine J, Aubert O, Dubleumortier S, Duong van Huyen JP, Jouven X, Glotz D, Legendre C, Halloran PF: Molecular microscope strategy to improve risk stratification in early antibody-mediated kidney allograft rejection. J Am Soc Nephrol 25: 2267–2277, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor activating antibodies in renal-allograft rejection. N Engl J Med 352: 558–569, 2005 [DOI] [PubMed] [Google Scholar]
- 13.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, Russell PS, Colvin RB: A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant 12: 313–321, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Valenzuela NM, McNamara JT, Reed EF: Antibody-mediated graft injury: Complement-dependent and complement-independent mechanisms. Curr Opin Organ Transplant 19: 33–40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM: Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 11: 2405–2413, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Farrar CA, Sacks SH: Mechanisms of rejection: Role of complement. Curr Opin Organ Transplant 19: 8–13, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Schwarz C, Regele H, Huttary N, Wahrmann M, Exner M, Nagy-Bojarsky K, Kletzmayr J, Hörl WH, Böhmig GA: Rescue therapy with tacrolimus and mycophenolate mofetil does not prevent deterioration of graft function in C4d-positive chronic allograft nephropathy. Wien Klin Wochenschr 118: 397–404, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Bartel G, Wahrmann M, Regele H, Kikić Z, Fischer G, Druml W, Mühlbacher F, Böhmig GA: Peritransplant immunoadsorption for positive crossmatch deceased donor kidney transplantation. Am J Transplant 10: 2033–2042, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Böhmig GA, Wahrmann M, Regele H, Exner M, Robl B, Derfler K, Soliman T, Bauer P, Müllner M, Druml W: Immunoadsorption in severe C4d-positive acute kidney allograft rejection: A randomized controlled trial. Am J Transplant 7: 117–121, 2007 [DOI] [PubMed] [Google Scholar]
- 21.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Regele H, Exner M, Watschinger B, Wenter C, Wahrmann M, Österreicher C, Säemann MD, Mersich N, Hörl WH, Zlabinger GJ, Böhmig GA: Endothelial C4d deposition is associated with inferior kidney allograft outcome independently of cellular rejection. Nephrol Dial Transplant 16: 2058–2066, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM, 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff ’09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Bursac Z, Gauss CH, Williams DK, Hosmer DW: Purposeful selection of variables in logistic regression. Source Code Biol Med 3: 17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worthington JE, McEwen A, McWilliam LJ, Picton ML, Martin S: Association between C4d staining in renal transplant biopsies, production of donor-specific HLA antibodies, and graft outcome. Transplantation 83: 398–403, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Gaston RS, Cecka JM, Kasiske BL, Fieberg AM, Leduc R, Cosio FC, Gourishankar S, Grande J, Halloran P, Hunsicker L, Mannon R, Rush D, Matas AJ: Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 90: 68–74, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, Gandhi MJ, Dean PG, Stegall MD: Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant 8: 2684–2694, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Sis B, Jhangri GS, Riopel J, Chang J, de Freitas DG, Hidalgo L, Mengel M, Matas A, Halloran PF: A new diagnostic algorithm for antibody-mediated microcirculation inflammation in kidney transplants. Am J Transplant 12: 1168–1179, 2012 [DOI] [PubMed] [Google Scholar]
- 29.Cohen D, Colvin RB, Daha MR, Drachenberg CB, Haas M, Nickeleit V, Salmon JE, Sis B, Zhao MH, Bruijn JA, Bajema IM: Pros and cons for C4d as a biomarker. Kidney Int 81: 628–639, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M: Diagnosis and management of antibody-mediated rejection: Current status and novel approaches. Am J Transplant 14: 255–271, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loupy A, Suberbielle-Boissel C, Hill GS, Lefaucheur C, Anglicheau D, Zuber J, Martinez F, Thervet E, Méjean A, Charron D, Duong van Huyen JP, Bruneval P, Legendre C, Nochy D: Outcome of subclinical antibody-mediated rejection in kidney transplant recipients with preformed donor-specific antibodies. Am J Transplant 9: 2561–2570, 2009 [DOI] [PubMed] [Google Scholar]
- 32.de Kort H, Willicombe M, Brookes P, Dominy KM, Santos-Nunez E, Galliford JW, Chan K, Taube D, McLean AG, Cook HT, Roufosse C: Microcirculation inflammation associates with outcome in renal transplant patients with de novo donor-specific antibodies. Am J Transplant 13: 485–492, 2013 [DOI] [PubMed] [Google Scholar]
- 33.Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF: Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant 9: 2520–2531, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Troxell ML, Weintraub LA, Higgins JP, Kambham N: Comparison of C4d immunostaining methods in renal allograft biopsies. Clin J Am Soc Nephrol 1: 583–591, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Seemayer CA, Gaspert A, Nickeleit V, Mihatsch MJ: C4d staining of renal allograft biopsies: A comparative analysis of different staining techniques. Nephrol Dial Transplant 22: 568–576, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Collins AB, Schneeberger EE, Pascual MA, Saidman SL, Williams WW, Tolkoff-Rubin N, Cosimi AB, Colvin RB: Complement activation in acute humoral renal allograft rejection: Diagnostic significance of C4d deposits in peritubular capillaries. J Am Soc Nephrol 10: 2208–2214, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Mengel M, Sis B, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Cendales L, Demetris AJ, Drachenberg CB, Farver CF, Rodriguez ER, Wallace WD, Glotz D, Banff meeting report writing committee : Banff 2011 Meeting report: New concepts in antibody-mediated rejection. Am J Transplant 12: 563–570, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas M: The significance of C4d staining with minimal histologic abnormalities. Curr Opin Organ Transplant 15: 21–27, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Dickenmann M, Steiger J, Descoeudres B, Mihatsch M, Nickeleit V: The fate of C4d positive kidney allografts lacking histological signs of acute rejection. Clin Nephrol 65: 173–179, 2006 [DOI] [PubMed] [Google Scholar]
- 40.Halloran PF, de Freitas DG, Einecke G, Famulski KS, Hidalgo LG, MengeL M, Reeve J, Sellares J, Sis B: An integrated view of molecular changes, histopathology and outcomes in kidney transplants. Am J Transplant 10: 2223–2230, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Nickeleit V, Zeiler M, Gudat F, Thiel G, Mihatsch MJ: Detection of the complement degradation product C4d in renal allografts: Diagnostic and therapeutic implications. J Am Soc Nephrol 13: 242–251, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Lefaucheur C, Loupy A, Vernerey D, Duong-Van-Huyen JP, Suberbielle C, Anglicheau D, Vérine J, Beuscart T, Nochy D, Bruneval P, Charron D, Delahousse M, Empana JP, Hill GS, Glotz D, Legendre C, Jouven X: Antibody-mediated vascular rejection of kidney allografts: A population-based study. Lancet 381: 313–319, 2013 [DOI] [PubMed] [Google Scholar]
- 43.Meehan SM, Kremer J, Ali FN, Curley J, Marino S, Chang A, Kadambi PV: Thrombotic microangiopathy and peritubular capillary C4d expression in renal allograft biopsies. Clin J Am Soc Nephrol 6: 395–403, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]