Abstract
To describe recent trends in prevalence of pre-existing diabetes mellitus (PDM) (i.e., type 1 or type 2 diabetes) and gestational diabetes mellitus (GDM) among delivery hospitalizations in the United States. Data on delivery hospitalizations from 1993 through 2009 were obtained from the Health Care Cost and Utilization Project (HCUP) Nationwide Inpatient Sample. Diagnosis-Related Group codes were used to identify deliveries and diagnosis codes on presence of diabetes. Rates of hospitalizations with diabetes were calculated per 100 deliveries by type of diabetes, hospital geographic region, patient’s age, degree of urbanicity of patient’s residence, categorized median household income for patient’s ZIP Code, expected primary payer, and type of delivery. From 1993 to 2009, age-standardized prevalence of diabetes per 100 deliveries increased from 0.62 to 0.90 for PDM (trend p < 0.001) and from 3.09 to 5.57 for GDM (trend p < 0.001). In 2009, correlates of PDM at delivery included older age [40–44 vs. 15–24: odds ratio 6.45 (95 % CI 5.27–7.88)], Medicaid/Medicare versus private payment sources [1.77 (95 % CI 1.59–1.98)], patient’s ZIP Code with a median household income in bottom quartile versus other quartiles [1.54 (95 % CI 1.41, 1.69)], and C-section versus vaginal delivery [3.36 (95 % CI 3.10–3.64)]. Correlates of GDM at delivery were similar. Among U.S. delivery hospitalizations, the prevalence of diabetes is increasing. In 2009, the prevalence of diabetes was higher among women in older age groups, living in ZIP codes with lower household incomes, or with public insurance.
Keywords: Diabetes, Pregnancy, Prevalence, Surveillance
Introduction
According to data from the U.S. National Health Interview Survey, the annual incidence of diagnosed diabetes among women of childbearing potential (i.e., 18–44 years) increased from 2.2 to 3.9 per 1,000 between 1997 and 2009 [1]. Increase in incidence is of public health concern because women with diabetes who become pregnant are at increased risk for a spectrum of serious complications for themselves and their offspring. Pregnancies among women with preexisting diabetes mellitus (PDM) (i.e., either type 1 or type 2 diabetes) are at increased risk for pregnancy-related hypertension, stillbirth, birth defects and preterm birth. Pregnancies complicated by gestational diabetes mellitus (GDM) are at increased risk for pre-eclampsia, macrosomia, birth trauma, hypoglycemia and jaundice [2–6]. Given the potential significant morbidity from diabetes in pregnancy, it becomes important to assess the extent to which the recent increase in incidence of diabetes among women of childbearing potential is manifesting as an increase in prevalence of diabetes in pregnancy in the United States.
Prior to 2000, published reports on trends in prevalence of diabetes in pregnancy were few and limited by the quality of information on diabetes available from existing data sources (i.e., birth certificates and surveys of maternal reports) and by the lack of representativeness of the populations surveyed [7, 8]. A recent report based on a national administrative database, namely, Research and Quality’s Healthcare Cost and Utilization Project [AHRQ HCUP] Nationwide Inpatient Sample [NIS], showing increases in the rates of hospital stays for GDM and PDM complicating pregnancy [9], suggest that this national database maybe useful for assessing population-based trends in diabetes in pregnancy in the United States.
In this study, we use data from NIS to describe trends in national prevalence of PDM and GDM in delivery hospitalizations for women 15–44 years of age, from 1993 through 2009. We also examine possible associations of national prevalence of PDM and GDM in delivery hospitalizations in 2009 with maternal age, urbanicity of county of residence, primary payer, and median household income of ZIP Code of residence.
Methods
Data Source
We used NIS data to identify hospital discharges, and procedures for deliveries involving diabetes diagnosed prior to and during pregnancy. The NIS contains information on hospital inpatient stays from states participating in HCUP, a federal-state data collection partnership [10]. Annual data collection by HCUP includes all community hospitals from participating states stratified by rural/urban location, number of beds, geographic region, teaching status, and hospital ownership. Within each stratum, a systematic random 20 % sample of approximately 1,000 hospitals is drawn each year. The NIS includes all hospital discharges in sampled hospitals and is designed to produce national estimates of inpatient care. Our analysis included annual NIS data from 1993 through 2009, years for which the HCUP NIS data are available, reliable, and recommended for trend analysis [11]. This research was conducted according to prevailing ethical principles. This study was reviewed by the Human Subjects Coordinator at the Centers for Disease Control and Prevention and, as an analysis of secondary data without identifiers, was determined not to require Institutional Review Board review.
Definition and Identification of Delivery Discharges (Denominator)
Hospital delivery discharges were identified using Diagnosis-Related Group (DRG) codes. DRG codes comprise a patient classification system that categorizes hospital stays into groups that are clinically homogeneous with respect to resource use, including diagnosis and type of treatment/procedure. Each hospital stay has one DRG assigned to it. Delivery stays were identified by discharges having a DRG code of 767–768 and 774–775 (vaginal delivery) or 765–766 (C-section) during 2008–2009 or a DRG code 372–375 (vaginal delivery) or 370–371 (C-section) [12] for the period 1993–2007.
Definition and Identification of Diabetes in Pregnancy Discharges (Numerator)
We identified deliveries with a PDM diagnosis by the presence of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis codes 648.0× listed anywhere on the discharge record and a GDM diagnosis by the presence of ICD-9-CM codes 648.8× listed anywhere on the discharge record. For the few cases that listed both codes, cases were counted as PDM because 648.0× specifically excludes gestational diabetes.
Definition of Covariates
Variables examined include delivery year, geographic location of the hospital, patient’s age, patient’s county of residence classified into rural/urban categories, median household income of residential ZIP Code for the patient, expected primary payer (i.e., insurance status), and type of delivery, U.S. Census region where the delivery hospital was located was categorized as: Northeast, Midwest, South, and West. Age was categorized as 15–19, 20–24, 25–29, 30–34, 35–39, and 40–44 years. The degree of urbanicity of the patient’s county of residence was categorized as: [1] large metropolitan area with at least one million residents (Central); [2] small metropolitan areas with 50,000 to less than one million residents (Small metro); [3] micropolitan areas with 10,000 to less than 50,000 (Fringe); and [4] not metropolitan or micropolitan (that is, less than 10,000 residents (Rural)). Health insurance status at the time of the hospital discharge was classified according to the type of expected primary payer: private, Medicare/Medicaid, uninsured (i.e., no charge and self-pay), and public insurance other than Medicare/Medicaid (i.e., Worker’s Compensation, Civilian Health and Medical Program of the Uniformed Services, Civilian Health and Medical Program of the Department of Veterans Affairs, Title V, and other government programs). Categories of median household income for ZIP Codes of patient’s residence were created by classifying ZIP Codes based on 2008 median annual household income quartiles (1 = $1 to $38,999; 2 = $39,000–$48,999; 3 = $49,000–63,999; and 4 ≥ $64,000) for the purpose of comparing ZIP codes with median household income of < $39,000 versus everyone else. Type of delivery was categorized as C-section and vaginal based on the DRGs [12]. Because information on race/ethnicity was not collected systematically among all states for the study period, we did not examine prevalence variation by race/ethnicity.
Statistical Methods
We estimated age-specific and age-standardized prevalence of PDM and GDM. The population from the Census 2000 Summary File was used for age standardization to examine the trends in prevalence. We used Joinpoint software [13] to evaluate trends in prevalence for PDM and GDM. Details for Joinpoint regression analysis have been previously published [14]. We used SUDAAN 11.0.0 (Research Triangle Institute, Research Triangle Park, NC) to account for the sampling design in our estimation of prevalence of each type of diabetes [11]. We used Chi square statistics to test for trends in prevalence and compare frequency distributions of categorical variables between groups, and logistic regression to examine possible associations of diabetes with age group, type of delivery, ZIP Code median household income, location of hospital, and expected primary payer.
Results
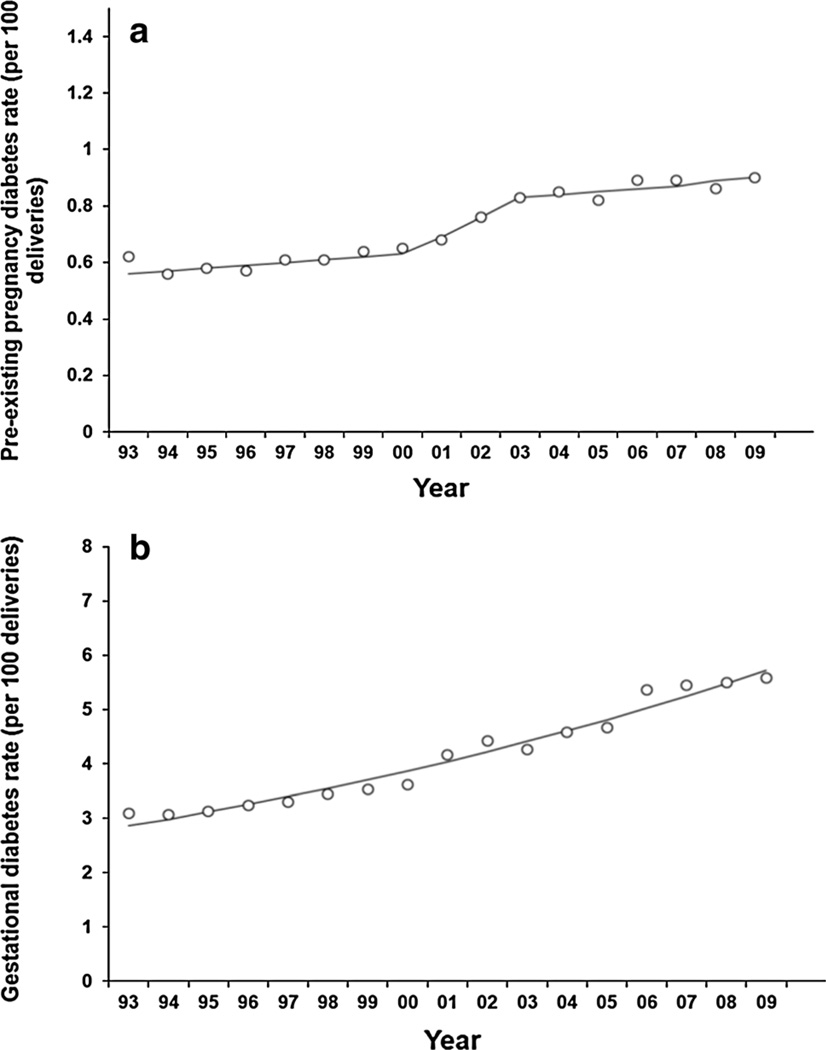
From 1993 through 2009, the age-standardized prevalence of diabetes per 100 deliveries increased from 0.62 to 0.90 for PDM (trend test, i.e., average annual percent change is different from zero, p < 0.001) (Fig. 1a) and from 3.09 to 5.57 for GDM (trend test, i.e., average annual percent change is different from zero, p < 0.001) (Fig. 1b). The average annual percent change in age-standardized prevalence per 100 deliveries was 3.6 % (95 % CI 3.0–4.2 %) for PDM and 4.4 % (95 % CI 3.9–4.8 %) for GDM.
Fig. 1.
a Annual age-standardized Prevalence of PDM per 100 delivery hospitalizations, women 15–44 years, HCUP NIS. b Annual age-standardized Prevalence of GDM per 100 Delivery Hospitalizations, Women 15–44 years, HCUP NIS
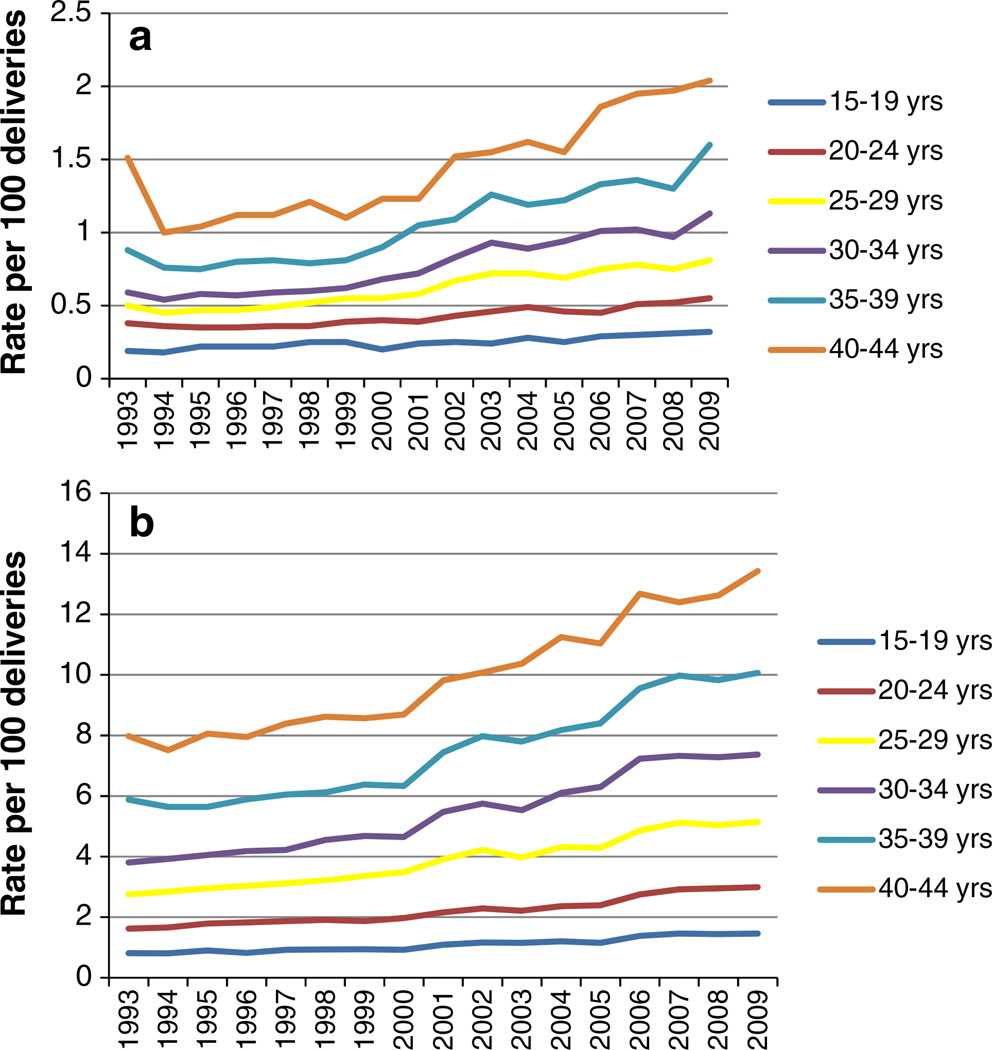
Between 1993 and 2009, the increase in prevalence of PDM and GDM over time was evident in all age groups. The increase in prevalence of PDM was greatest for the 40–44 years of age group (from 1.60 to 2.04 %) and lowest for the 15–19 years of age group (from 0.25 to 0.32 %) (Fig. 2a). Similarly, the increase in prevalence of GDM by age group was greatest for the 35–39 years of age group (from 5.91 to 10.07 %) and lowest for the 15–19 years of age group (from 0.83 to 1.46 %) (Fig. 2b).
Fig. 2.
a Annual prevalence of PDM per 100 delivery hospitalizations, women 15–44 years, HCUP NIS. b Annual prevalence of GDM per 100 delivery hospitalizations, women 15–44 years, HCUP NIS
In 2009, the NIS data included 4.1 million hospital deliveries among women 15–44 years of age, of which 264,939 were complicated by any diabetes, 36,851 (13.9 %) by PDM, and 228,088 (86.1 %) by GDM (Table 1). Deliveries complicated by PDM and GDM were more likely than deliveries not complicated by diabetes to be among older age women and to be delivered by C-section than by vaginal delivery. The overall prevalence of diabetes per 100 deliveries in 2009 was 6.47 % for any type of diabetes, 0.90 % for PDM, and 5.57 % for GDM.
Table 1.
Characteristics of hospital deliveries with diabetes and without diabetes United States, Healthcare Cost and Utilization Project Nationwide Inpatient Sample 2009
| Total hospital deliveries n (wtd %) |
Deliveries without diabetes n (wtd %) |
Deliveries with any diabetes n (wtd %) |
Deliveries with pre-existing diabetes n (wtd %) |
Deliveries with gestational diabetes n (wtd %) |
|
|---|---|---|---|---|---|
| Total | 4,097,012 | 3,832,073 (100.0) | 264,939 (100.0) | 36,851 (100.0) | 228,088 (100.0) |
| Percent of all deliveries | 100.0 | 93.53 | 6.47 | 0.90 | 5.57 |
| Percent of deliveries with diabetes | – | 100.0 | 13.9 | 86.1 | |
| U.S. Census region in which hospital is located | |||||
| Northeast | 624,842 (15.3) | 583,925 (15.2) | 40,918 (15.4) | 4,828 (13.1) | 36,090 (15.8) |
| Midwest | 874,067 (21.3) | 819,667 (21.4) | 54,400 (20.5) | 6,841 (18.6) | 47,559 (20.9) |
| South | 1,648,778 (40.2) | 1,544,407 (40.3) | 104,371 (39.4) | 16,584 (45.0) | 87,786 (38.5) |
| West | 949,325 (23.2) | 884,074 (23.1) | 65,251 (24.6) | 8,598 (23.3) | 56,653 (24.8) |
| Age group | |||||
| 15–19 years | 411,342 (10.0) | 404,005 (10.5) | 7,337 (2.8) | 1,327 (3.6) | 6,010 (2.6) |
| 20–24 | 993,554 (24.3) | 958,369 (25.0) | 35,185 (13.3) | 5,469 (14.8) | 29,716 (13.0) |
| 25–29 | 1,149,066 (28.1) | 1,080,626 (28.2) | 68,440 (25.8) | 9,352 (25.4) | 59,088 (25.9) |
| 30–34 | 953,452 (23.3) | 872,382 (22.8) | 81,069 (30.6) | 10,811 (29.3) | 70,259 (30.8) |
| 35–39 | 481,795 (11.8) | 425,567 (11.1) | 56,228 (21.2) | 7,690 (20.9) | 48,538 (21.3) |
| 40–44 | 107,804 (2.6) | 91,123 (2.4) | 16,681 (6.3) | 2,203 (6.0) | 14,478 (6.4) |
| Patient’s county of residence | |||||
| Central | 1,313,140 (32.5) | 1,221,743 (32.3) | 91,397 (34.9) | 12,548 (34.3) | 78,849 (34.9) |
| Fringe | 1,071,170 (26.5) | 1,000,329 (26.4) | 70,841 (27.0) | 8,461 (23.2) | 62,380 (27.7) |
| Small Metro | 1,051,386 (26.0) | 985,296 (26.0) | 66,090 (25.2) | 9,764 (26.7) | 56,326 (25.0) |
| Rural | 609,504 (15.1) | 575,741 (15.2) | 33,763 (12.9) | 5,766 (15.8) | 27,997 (12.4) |
| Primary payer | |||||
| Private | 1,988,648 (48.6) | 1,856,105 (48.4) | 132,543 (50.0) | 16,121 (43.8) | 116,422 (51.0) |
| Medicaid/Medicare | 1,830,398 (44.8) | 1,716,172 (44.8) | 114,227 (43.1) | 18,505 (50.2) | 95,721 (42.0) |
| Uninsured | 165,220 (4.0) | 153,319 (4.0) | 11,901 (4.5) | 1,278 (3.5) | 10,624 (4.7) |
| Other public insurance | 105,187 (2.6) | 99,294 (2.6) | 5,893 (2.2) | 897 (2.5) | 4,996 (2.2) |
| Median household income of patient’s ZIP Code | |||||
| ≥ $39,000 | 2,941,334 (73.8) | 2,749,297 (73.8) | 192,031 (75.0) | 24,558 (68.6) | 167,479 (76.0) |
| < $39,000 lowest quartile | 1,042,242 (26.2) | 978,220 (26.2) | 64,022 (25.0) | 11,257 (31.4) | 52,764 (24.0) |
| Type of delivery | |||||
| Vaginal | 2,722,661 (66.5) | 2,586,425 (67.5) | 136,235 (51.4) | 13,058 (35.4) | 123,177 (54.0) |
| C-section | 1,374,351 (33.5) | 1,245,647 (32.5) | 128,704 (48.6) | 23,793 (64.6) | 104,911 (46.0) |
Percents are rounded and may therefore not add to 100 %
‘Wtd’ indicates weighted to the U.S. population
The prevalence of diabetes increased with maternal age (Table 2). The age-standardized prevalence per 100 delivery hospitalizations was greater for GDM than for PDM. The prevalence of PDM was higher among patients located in rural areas than among other areas, whereas GDM prevalence was higher among patients living in central counties of metro areas with populations of 1 million or greater. The prevalence of PDM was higher among deliveries where the expected primary payer was Medicaid/Medicare than among deliveries where the expected primary payer was private insurance, while the opposite was true for the prevalence of GDM. For both PDM and GDM, prevalence was higher among patients residing in ZIP Codes where the median household income was in the bottom quartile and among C-section than among vaginal deliveries.
Table 2.
Prevalence for hospital deliveries with diabetes (per 100 deliveries), by maternal age, urban/rural location of hospital, expected primary payer, median household income of patient’s ZIP Code, and type of delivery, United States, Healthcare Cost and Utilization Project Nationwide Inpatient Sample, 2009
| Any diabetes Prevalencea (95 % CIb) |
Pre-existing diabetes Prevalence (95 % CI) |
Gestational diabetes Prevalence (95 % CI) |
|
|---|---|---|---|
| Overall | 6.47 (6.17, 6.77) | 0.90 (0.83, 0.98) | 5.57 (5.31, 5.84) |
| U.S. Census region in which hospital is located | |||
| Northeast | 6.13 (5.55, 6.76) | 0.73 (0.62, 0.86) | 5.40 (4.85, 6.00) |
| Midwest | 6.26 (5.53, 7.09) | 0.79 (0.66, 0.95) | 5.47 (4.81, 6.23) |
| South | 6.58 (6.10, 7.10) | 1.04 (0.92, 1.19) | 5.53 (5.13, 5.97) |
| West | 6.71 (6.23, 7.22) | 0.88 (0.75, 1.04) | 5.82 (5.40, 6.28) |
| Age group | |||
| 15–19 years | 1.78 (1.65, 1.93) | 0.32 (0.28, 0.38) | 1.46 (1.35, 1.58) |
| 20–24 | 3.54 (3.38, 3.71) | 0.55 (0.49, 0.61) | 2.99 (2.85, 3.14) |
| 25–29 | 5.96 (5.69, 6.23) | 0.81 (0.74, 0.89) | 5.14 (4.91, 5.38) |
| 30–34 | 8.50 (8.03, 9.00) | 1.13 (1.03, 1.25) | 7.37 (6.96, 7.80) |
| 35–39 | 11.67 (10.90, 12.49) | 1.60 (1.42, 1.80) | 10.07 (9.43, 10.76) |
| 40–44 | 15.47 (14.43, 16.58) | 2.04 (1.76, 2.38) | 13.43 (12.53, 14.39) |
| Patient’s county of residence | |||
| Central | 6.72 (6.05, 7.46) | 0.93 (0.79, 1.08) | 5.80 (5.21, 6.44) |
| Fringe | 6.20 (5.86, 6.55) | 0.75 (0.67, 0.83) | 5.45 (5.14, 5.79) |
| Small metro | 6.66 (6.26, 7.09) | 0.99 (0.88, 1.10) | 5.68 (5.33, 6.04) |
| Rural | 6.24 (5.93, 6.57) | 1.07 (0.96, 1.20) | 5.17 (4.91, 5.45) |
| Primary payer | |||
| Private | 5.84 (5.56, 6.13) | 0.73 (0.68, 0.79) | 5.11 (4.85, 5.38) |
| Medicaid/Medicare | 7.78 (7.37, 8.22) | 1.26 (1.14, 1.40) | 6.52 (6.17, 6.89) |
| Uninsured | 7.31 (6.09, 8.77) | 0.79 (0.61, 1.01) | 6.53 (5.40, 7.88) |
| Other public insurance | 5.90 (5.24, 6.63) | 0.89 (0.70, 1.13) | 5.01 (4.47, 5.61) |
| Median household income of patient’s ZIP Code | |||
| ≥ $39,000 | 6.25 (5.96, 6.55) | 0.80 (0.74, 0.88) | 5.45 (5.19, 5.72) |
| < $39,000 (lowest quartile) | 7.18 (6.71, 7.69) | 1.26 (1.15, 1.37) | 5.92 (5.51, 6.36) |
| Type of delivery | |||
| Vaginal | 5.21 (4.92, 5.52) | 0.50 (0.44, 0.56) | 4.71 (4.46, 4.99) |
| C-section | 8.81 (8.46, 9.17) | 1.65 (1.52, 1.80) | 7.16 (6.87, 7.46) |
Prevalence data are age-standardized to the 2009 population of deliveries
CI Confidence Interval
In 2009, the odds of PDM at delivery increased monotonically with maternal age (Table 3). The odds of PDM deliveries were higher in the South (odds ratio (OR) 1.45; 95 % CI 1.14, 1.84) than in the Northeast; among deliveries where an expected primary payer was Medicaid/Medicare (OR 1.77; 95 % CI 1.59, 1.98) or Other public insurance (OR 1.29; 95 % CI 1.01, 1.65) versus private insurance; and in patients who resided in ZIP Codes where the median household income was in the lowest quartile (OR 1.54; 95 % CI 1.41, 1.69) versus higher income areas. The odds of a GDM delivery showed a steeper increase with maternal age and were higher among deliveries to women whose primary payer was Medicaid/Medicare (OR 1.30; 95 % CI 1.21, 1.41) or Uninsured (OR 1.34; 95 % CI 1.08, 1.66) versus private insurance, and in patients residing in ZIP Codes where the median household income was less than $39,000 (OR 1.08; 95 % CI 1.00, 1.16) versus at least $39,000. The odds of a C-section delivery versus a vaginal delivery were greater for all deliveries complicated by diabetes, but were greater for PDM (OR 3.36; 95 % CI 3.10, 3.64) than for GDM (OR 1.56; 95 % CI 1.51, 1.61).
Table 3.
Adjusted odds ratios and 95 % confidence intervals (CI) for hospital deliveries complicated by diabetes and maternal age, hospital location, primary expected payer, median household income of patient’s ZIP Code, and type of delivery, United States, Healthcare Cost and Utilization Project Nationwide Inpatient Sample, 2009
| Pre-existing diabetes Adjusted odds ratio (95 % CI) |
Gestational diabetes Adjusted odds ratio (95 % CI) |
|
|---|---|---|
| U.S. Census region in which hospital is located | ||
| Northeast | 1.00 (reference) | 1.00 (reference) |
| Midwest | 1.09 (0.83, 1.44) | 1.02 (0.85, 1.22) |
| South | 1.45 (1.14, 1.84)1 | 1.03 (0.89, 1.19) |
| West | 1.23 (0.96, 1.58) | 1.09 (0.95, 1.26) |
| Age group | ||
| 15–19 years | 1.00 (reference) | 1.00 (reference) |
| 20–24 | 1.71 (1.50, 1.95)1 | 2.08 (1.95, 2.22)1 |
| 25–29 | 2.54 (2.20, 2.92)1 | 3.66 (3.40, 3.93)1 |
| 30–34 | 3.54 (3.05, 4.12)1 | 5.37 (4.94, 5.83)1 |
| 35–39 | 5.01 (4.22, 5.95)1 | 7.56 (6.90, 8.28)1 |
| 40–44 | 6.45 (5.27, 7.88)1 | 10.46 (9.48, 11.55)1 |
| Patient’s county of residence | ||
| Central | 1.00 (reference) | 1.00 (reference) |
| Fringe | 0.80 (0.68, 0.94) | 0.93 (0.83, 1.05) |
| Small metro | 1.06 (0.87, 1.29) | 0.98 (0.86, 1.11) |
| Rural | 1.14 (0.95, 1.36) | 0.88 (0.78, 1.00) |
| Primary payer | ||
| Private | 1.00 (reference) | 1.00 (reference) |
| Medicaid/Medicare | 1.77 (1.59, 1.98)1 | 1.30 (1.21,1.41)1 |
| Uninsured | 1.13 (0.87, 1.47) | 1.34 (1.08, 1.66)1 |
| Other public insurance | 1.29 (1.01, 1.65)1 | 1.00 (0.88,1.14) |
| Median household income of patient’s ZIP Code | ||
| ≥ $39,000 | 1.00 (reference) | 1.00 (reference) |
| < $39,000 | 1.54 (1.41, 1.69)1 | 1.08 (1.00, 1.16)2 |
| Type of delivery | ||
| Vaginal | 1.00 (reference) | 1.00 (reference) |
| C-section | 3.36 (3.10, 3.64)1 | 1.56 (1.51, 1.61)1 |
Odds ratios for hospital region, patient’s county of residence, primary payer, and median household income of patient’s ZIP Code are age-adjusted
p < 0.05
p = 0.05
Conclusions
Our findings of increasing trends in prevalence rates of PDM and GDM among delivery hospitalizations based on HCUP NIS data are consistent with and expand on two previous reports based on similar national data through 2004 and 2008 [9, 15], respectively; our findings indicate that such trends continued through 2009. Because the information provided by ICD-9-CM codes for classifying pregnancies complicated by PDM is not adequate for a reliable differentiation of PDM types (i.e., type 1 vs. type 2 diabetes), we were not able to derive and evaluate trends in prevalence and correlates of PDM delivery hospitalizations by type of PDM.
Our observations on the correlations of prevalence of diabetes in pregnancy with maternal age, type of expected payer and type of delivery are also consistent with previous observations [9, 16]. The observed positive correlation between older maternal age and prevalence of diabetes in pregnancy may explain in part the increasing temporal trends in pregnancies complicated by diabetes as there has been an increasing proportion of pregnancies among women in older age groups in the U.S. in recent decades. However, given the increases in prevalence of diabetes in pregnancy evident for all maternal age groups, other factors may have contributed to such trends, including increased levels of screening and diagnosis of diabetes in pregnancy and increased prevalence of vulnerable populations and/or risk factors for type 2 diabetes and GDM (e.g., obesity and physical inactivity). As the available NIS data did not include such information, we were not able to examine the contributions of these factors to the variation in prevalence of diabetes in pregnancy.
Our findings of correlations of prevalence of diabetes complicating pregnancy among deliveries to women with residence in ZIP codes with a low median household income (i.e., < $39,000) are of interest. We are not aware of previous reports of such associations, but our findings are not unexpected. A higher prevalence of diabetes has been noted among women of some minority groups or whose expected primary payer is Medicaid/Medicare [15, 17], either of whom may be disproportionately represented in ZIP codes with a low median household income. Our findings, however, do identify a subgroup of women who would benefit from targeted screening and prevention interventions for type 2 diabetes.
The higher rates of C-section versus vaginal deliveries associations complicated by PDM or GDM are not unexpected as diabetes complicating a pregnancy is an indication for C-section delivery. However, such increasing trends in delivery hospitalizations complicated by diabetes are of concern as they may have contributed to an increasing trend in the number of C-section deliveries [18], and concomitant morbidity [19].
Our findings also suggest that HCUP NIS data on discharge deliveries offer several advantages for surveillance of diabetes in pregnancy compared to data from other sources such as records of birth certificates or surveys of women of childbearing potential. Birth certificate records are available for public use; however, such records exclude delivery hospitalizations that result in stillbirths and are limited by the quality of information on history of maternal diabetes. Surveys of women such as CDC’s Pregnancy Risk Assessment Monitoring System (PRAMS) [20] are also available for public use but are limited as a data source for surveillance of diabetes in pregnancy because of the year-to-year variation in participation rates among women surveyed in previous years and limited quality of information on diabetes status in pregnancy derived from self-reports [21]. In contrast, NIS data on discharge deliveries come from large national probability samples of all delivery hospitalizations regardless of outcomes, are collected on an ongoing basis using standard procedures over time, with extensive documentation, and are available for public use. Inclusion of DRG and ICD9-CM diagnosis codes in the NIS database allows for identification of delivery hospitalizations and determination of which deliveries are complicated by PDM or GDM more accurately and consistently over time and across regions of the US than birth certificate records and surveys of women of childbearing age.
One potential limitation of NIS data for monitoring of prevalence of diabetes in pregnancy over time is the absence of data concerning deliveries that occur outside hospitals. However, because such deliveries are estimated to account for < 1 % of all deliveries [22], their exclusion from the NIS data is not likely to affect materially inferences of trends in prevalence of diabetes in pregnancy over time or of possible of variations in such prevalence with maternal characteristics. The NIS data do not include information on the extent to which deliveries captured in the NIS undergo screening for diabetes before or early in pregnancy. Therefore, we could not account for under-ascertainment of cases of diabetes in pregnancy so our estimates of prevalence of diabetes in pregnancy probably represent underestimates and our estimates of odds ratios with possible correlates of diabetes in pregnancy may be attenuated.
Surveillance of diabetes in the general population has been an important tool for assessing the burden of diabetes in the general population over time, identifying high risk or vulnerable groups in the population, and evaluating the impact of community interventions. Because women with diabetes who become pregnant and their offspring are at risk for a spectrum of serious but preventable complications, implementing a population-based surveillance system for monitoring diabetes in pregnancy can be useful in evaluating progress in prevention efforts at the population level. The representativeness and standard methodology of NIS data would seem to make such data the most practical and reliable data source for monitoring temporal trends in diabetes in pregnancy in the United States today.
Our findings contribute to the limited body of literature on the burden of diabetes in pregnancy in several respects. First, our findings corroborate the increasing trends in the population-based prevalence of both PDM and GDM noted previously [9, 15, 17], and indicate that these trends have continued through 2009, the most recent year for which NIS data were available. These trends highlight that diabetes in pregnancy remains a public health issue in need of ongoing monitoring, attention and more concerted prevention efforts. Second, our findings identify a subgroup of delivery hospitalizations among women who are older or with public insurance or no insurance as having a higher prevalence of diabetes in pregnancy and for whom screening and targeted prevention efforts need to be developed, implemented and monitored for effectiveness. Third, our findings illustrate that NIS data represent a reliable and practical data source for ongoing monitoring of pregnancies complicated by diabetes.
Our findings have implications for the prevention of type 2 diabetes among women of childbearing age and diabetes-related pregnancy complications. First, because women with GDM are at high risk for developing type 2 diabetes and effective lifestyle interventions are available to reduce their risk [23, 24], it becomes important to ensure that women with a history of GDM have follow-up screening for type 2 diabetes and are referred to a network of sites that can provide intervention services to such women. Second, for women of childbearing age with a history of type 2 diabetes, optimal management of their diabetes will require that they be empowered to receive preconception counseling and care [25]. Third, for women of childbearing age considered at risk of type 2 diabetes for other reasons (i.e., family history of diabetes, impaired fasting glucose, older age, or overweight status) and who are planning to become pregnant, it becomes important to provide access to preconception care capable of screening for and managing diabetes.
In summary, our findings show that among U.S. delivery hospitalizations, the prevalence of diabetes increased between 1993 and 2009, an observation that is consistent with previous reports of increases in prevalence of diabetes among women of childbearing age in general and of diabetes in pregnancy in particular. In addition, our findings on the prevalence of diabetes among delivery hospitalizations in 2009 identified subgroups of women for whom delivery hospitalization had a higher prevalence of diabetes and who would benefit from targeted prevention interventions, namely: women of childbearing age in older age groups, whose expected primary payer was public, or whose residence was in ZIP codes with low median household income. Lastly, our findings indicate that NIS data are a useful and a practical data source for population-based monitoring of trends in prevalence of diabetes in pregnancy and as a means for identifying subgroups of women of childbearing age at risk for diabetes for targeted screening and prevention interventions.
Acknowledgments
A.C., B.H.B. and L.S.G. researched data, contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. A.E. researched data, contributed to discussion, and reviewed and edited the manuscript. E.G contributed to discussion, wrote the manuscript, and reviewed and edited the manuscript. This study was possible because of the statewide data collection efforts of the organizations that contributed to the Healthcare Cost and Utilization Project: www.hcup-us.ahrq.gov/hcupdatapartners.jsp.
Footnotes
Disclaimer: The findings and conclusions in this report do not necessarily represent the official position of the Centers for Disease Control and Prevention or of the Agency for Healthcare Research and Quality.
Conflict of interest No potential conflicts of interest relevant to this article were reported
Contributor Information
Adolfo Correa, Email: acorrea@umc.edu, Departments of Medicine and Pediatrics, University of Mississippi Medical Center, 350 Woodrow Wilson Drive, Suite 701, Jackson, MS 39213, USA.
Barbara Bardenheier, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Anne Elixhauser, Agency for Healthcare Research and Quality, Rockville, MD, USA.
Linda S. Geiss, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA
Edward Gregg, Division of Diabetes Translation, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention, Atlanta, GA, USA.
References
- 1.CDC. [cited 2011 August 17];Incidence of diagnosed diabetes per 1,000 population aged 18–79 years, by sex and age, United States, 1997–2009. 2009 http://www.cdc.gov/diabetes/statistics/incidence/fig5.htm.
- 2.Balsells M, Garcia-Patterson A, Gich I, Corcoy R. Maternal and fetal outcome in women with type 2 versus type 1 diabetes mellitus: a systematic review and metaanalysis. Journal of Clinical Endocrinology and Metabolism. 2009;94(11):4284–4291. doi: 10.1210/jc.2009-1231. [DOI] [PubMed] [Google Scholar]
- 3.Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113(10):1126–1133. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- 4.Yogev Y, Visser GH. Obesity, gestational diabetes and pregnancy outcome. Seminars in Fetal and Neonatal Medicine. 2009;14(2):77–84. doi: 10.1016/j.siny.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Cedergren MI. Maternal morbid obesity and the risk of adverse pregnancy outcome. Obstetrics and Gynecology. 2004;103(2):219–224. doi: 10.1097/01.AOG.0000107291.46159.00. [DOI] [PubMed] [Google Scholar]
- 6.Dudley DJ. Diabetic-associated stillbirth: incidence, pathophysiology, and prevention. Clinics in Perinatology. 2007;34(4):611–626. vii. doi: 10.1016/j.clp.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 7.CDC. Prenatal care and pregnancies complicated by diabetes–U.S. reporting areas, 1989. Morbidity and Mortality Weekly Report. 1993;42(6):119–122. [PubMed] [Google Scholar]
- 8.Devlin HM, Desai J, Walaszek A. Reviewing performance of birth certificate and hospital discharge data to identify births complicated by maternal diabetes. Maternal and Child Health Journal. 2009;13(5):660–666. doi: 10.1007/s10995-008-0390-9. [DOI] [PubMed] [Google Scholar]
- 9.Wier LM, Witt E, Burgess J, Elixhauser A. Hospitalizations Related to Diabetes in Pregnancy, 2008. Rockville, MD: Agency for Healthcare Research and Quality; 2010. HCUP Statistical Brief #102. [PubMed] [Google Scholar]
- 10.Agency for Healthcare Research and Quality. Overview of the Nationwide Inpatient Sample (NIS) Agency for Healthcare Research and Quality; 2013. [Google Scholar]
- 11.Houchens RL, Elixhauser A. Using the HCUP nationwide inpatient sample to estimate trends. (Updated for 1988–2004) Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2006. HCUP Methods Series #2006-05. Online. [Google Scholar]
- 12.Kuklina EV, Whiteman MK, Hillis SD, Jamieson DJ, Meikle SF, Posner SF, et al. An enhanced method for identifying obstetric deliveries: Implications for estimating maternal morbidity. Maternal and Child Health Journal. 2008;12(4):469–477. doi: 10.1007/s10995-007-0256-6. [DOI] [PubMed] [Google Scholar]
- 13.Institute NC. Surveillance Research Program of the US National Cancer Institute. Joinpoint Regression Program version 3.5.4. Surveillance Research Program of the US National Cancer Institute 2012. http://srab.cancer.gov/joinpoint/.
- 14.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Statistics in Medicine. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Getahun D, Nath C, Ananth CV, Chavez MR, Smulian JC. Gestational diabetes in the United States: temporal trends 1989 through 2004. American Journal of Obstetrics and Gynecology. 2008;198(5):525e1–525e5. doi: 10.1016/j.ajog.2007.11.017. [DOI] [PubMed] [Google Scholar]
- 16.Bardenheier BH, Elixhauser A, Imperatore G, Devlin HM, Kuklina EV, Geiss LS, et al. Variation in Prevalence of Gestational Diabetes Among Hospital Discharges for Obstetric Delivery Across 23 States in the United States. Diabetes Care. 2013;36(5):1209–1214. doi: 10.2337/dc12-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albrecht SS, Kuklina EV, Bansil P, Jamieson DJ, Whiteman MK, Kourtis AP, et al. Diabetes trends among delivery hospitalizations in the U.S. 1994–2004. Diabetes Care. 2010;33(4):768–773. doi: 10.2337/dc09-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menacker F, Declercq E, Macdorman MF, editors. Seminars in perinatology. Amsterdam: Elsevier; 2006. Cesarean delivery: Background, trends, and epidemiology. [DOI] [PubMed] [Google Scholar]
- 19.Belizán JM, Althabe F, Cafferata ML. Health consequences of the increasing caesarean section rates. Epidemiology. 2007;18(4):485–486. doi: 10.1097/EDE.0b013e318068646a. [DOI] [PubMed] [Google Scholar]
- 20.CDC. The Pregnancy Risk Assessment Monitoring System (PRAMS) 2013 http://www.cdc.gov/prams/methodology.htm.
- 21.Goldman N, Lin IF, Weinstein M, Lin YH. Evaluating the quality of self-reports of hypertension and diabetes. Journal of Clinical Epidemiology. 2003;56(2):148–154. doi: 10.1016/s0895-4356(02)00580-2. [DOI] [PubMed] [Google Scholar]
- 22.MacDorman MF, Menacker F, Declercq E. Trends and characteristics of home and other out-of-hospital births in the United States, 1990–2006. National Vital Statistics Reports. 2010;58(11):1–14. [PubMed] [Google Scholar]
- 23.Metzger BE, Buchanan TA, Coustan DR, De Leiva A, Dunger DB, Hadden DR, et al. Summary and recommendations of the fifth international workshop-conference on gestational diabetes mellitus. Diabetes Care. 2007;30(Supplement 2):S251–S260. doi: 10.2337/dc07-s225. [DOI] [PubMed] [Google Scholar]
- 24.Liberopoulos EN, Tsouli S, Mikhailidis DP, Elisaf MS. Preventing type 2 diabetes in high risk patients: An overview of lifestyle and pharmacological measures. Current Drug Targets. 2006;7(2):211–228. doi: 10.2174/138945006775515419. [DOI] [PubMed] [Google Scholar]
- 25.Kitzmiller JL, Block JM, Brown FM, Catalano PM, Conway DL, Coustan DR, et al. Managing preexisting diabetes for pregnancy: Summary of evidence and consensus recommendations for care. Diabetes Care. 2008;31(5):1060–1079. doi: 10.2337/dc08-9020. [DOI] [PMC free article] [PubMed] [Google Scholar]