Abstract
Nicotine has been shown to enhance the motivational properties of non-nicotine stimuli. This reinforcement-enhancing property of nicotine has the potential to promote the use of other illicit substances as well as maladaptive patterns of food intake. Therefore, the current study aimed to examine whether nicotine enhances preference for contexts paired with cocaine or sucrose utilizing a place conditioning procedure. Separate groups of adult male rats were administered sucrose or cocaine in one of two compartments of a standard CPP chamber on four consecutive days. Preference was then assessed following no injection, a single subcutaneous (s.c.) injection of nicotine, and a s.c. saline injection. Animals preferred the chamber paired with either sucrose or cocaine, as evident from an increased time spent in the paired chamber compared to baseline. Nicotine further increased the time spent in the sucrose- or cocaine-paired chamber, consistent with a reinforcement-enhancement effect. Previous results demonstrate an interaction between nicotine and intake of other drugs or food. The present findings provide an additional mechanism that may underlie these effects and which may have implications for drug dependence and obesity.
Keywords: Cocaine, Conditioned place preference, Enhancement, Nicotine, Reinforcement, Reward, Sucrose
1. Introduction
Despite widespread knowledge of its negative health consequences, tobacco use constitutes the leading cause of preventable death in the United States (Centers for Disease Control and Prevention 2011). Research into the reinforcing effects of nicotine suggests that while nicotine, the principal reinforcing agent in tobacco, is important in sustained tobacco dependence (Anthony et al., 1994; Caggiula et al., 2001; Goldberg et al., 1981; Rose and Corrigall, 1997), environmental stimuli play a critical role in nicotine reinforcement (Bevins and Caggiula, 2009; Conklin and Tiffany, 2001; Rose et al., 2000). This work has demonstrated both the ability of nicotine to transform neutral, non-drug stimuli into conditioned reinforcers (Geier et al., 2000; Palmatier et al., 2007a; Perkins et al., 1994; Rose and Behm, 1991; Rose and Levin, 1991) and the ability of nicotine to non-associatively enhance responding for other reinforcers (Barret and Bevins, 2013; Barrett and Bevins, 2012; Caggiula et al., 2009; Chaudhri et al., 2006b; Palmatier et al., 2006). Like nicotine, other drug of abuse, particularly psychostimulants, also impact the reinforcing properties of other stimuli through both associative and non-associative effects (Chaudhri et al., 2006b; Graves and Napier; Weiss et al., 2000; Zhou et al., 2005).
The reinforcement-enhancing property of nicotine may promote the use of other substances such as rewarding, palatable food or other drugs of abuse. It has previously been demonstrated in preclinical investigations that prolonged nicotine exposure enhances sensitization and conditioned place preference to cocaine, as well as multiple markers of neuronal activity following cocaine (Levine et al., 2011). Likewise, both clinical and preclinical evidence point to interactions between nicotine and other drugs of abuse (Doyon et al., 2013; Huang et al., 2013; Levine et al., 2011; Richter et al., 2002). For example, cigarette smoking may intensify the subjective effects of cocaine and cocaine craving (Brewer et al., 2013) and clinical data suggest a negative relationship between smoking and cocaine abstinence (Shoptaw et al., 1996). Nicotine also effects feeding behaviors (Jo et al., 2002). Nicotine has anorectic effects on food consumption (Wellman et al., 2005) in rats, but increases operant responding for sucrose pellets (Palmatier et al., 2012; Schassburger et al., 2013) or solution (Barret and Bevins, 2013), which may indicate enhanced motivation to respond for palatable food (Donny et al., 2011).
Although nicotine is known to enhance responding for drugs of abuse and palatable food, studies of the interaction between nicotine and reward-paired stimuli are limited. These studies are important because they can provide insight into the manner in which nicotine impacts reinforced behavior. For example, nicotine may alter the likelihood of seeking out contexts predictive of reinforcement independent of any action on the rewarding outcome associated with the drug or food. The current study tested this hypothesis by examining the ability of nicotine to enhance a context paired with either sucrose or cocaine.
We utilized a place conditioning procedure to investigate this interaction between nicotine and food or drug cues. The bulk of the work to date examining the nicotine reinforcement-enhancing effect has exclusively used behavioral models involving an operant response (but see (Thiel et al., 2009). While some of this work has made significant progress in delineating varied mechanisms by which nicotine may increase responding for non-nicotine reinforcers (Barret and Bevins, 2013; Cassidy and Dallery, 2012), the evaluation of acquisition and expression of learned associations less clear in operant behavioral procedures, and nicotine’s effects on locomotor activity may serve as a confounding variable. Therefore, the ability to examine the reinforcement-enhancing effects of nicotine in alternative models would be highly useful. The designed experiments address whether nicotine enhances the expression of a preference for sucrose or drug-paired cues, while simultaneously demonstrating whether such investigations are feasible using a place conditioning procedure. We predicted that a single s.c. injection of nicotine could enhance the preference for environments associated with palatable food or drug reward.
2. Materials and Methods
2.1 Subjects
Male, Sprague-Dawley rats (n=36, Harlan Farms, Indianapolis, IN), were ordered to weigh 200–225 g upon arrival, were singly housed in suspended, wire mesh cages in a temperature and humidity-controlled colony room on a reversed light/dark cycle (lights off 7 am) with ad lib access to food and water. They were handled and weighed daily, and weights ranged between 265–317g throughout testing. All conditioning and testing took place during the dark hours of the cycle. After one week of acclimatization, rats remained on ad lib water but were food restricted to 20 g of food per day. Rats in the cocaine CPP study remained on this diet for the duration of the experiment, whereas animals in the sucrose CPP study were further restricted to 15 g of food per day prior to initial sucrose exposure through the duration of the experiment. This measure was taken to encourage sucrose consumption during the conditioning phase of the study. Practices utilized in this study were approved by the University of Pittsburgh Institutional Animal Care and Use Committee (IACUC) and carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2 Drugs
Cocaine hydrochloride (Sigma, St. Louis, MO) was dissolved in sterile 0.9% saline solution. A 10 mg/kg/ml dose was selected based on previous studies demonstrating cocaine CPP (Harris and Aston-Jones, 2003). Nicotine hydrogen tartrate (Sigma, St. Louis, MO) was dissolved in 0.9% saline solution. The pH of the solution was adjusted to 7.0 (± 0.2) using dilute NaOH. The dose of nicotine used was 0.4 mg/kg/ml (free base concentration) based on the results of previous studies demonstrating the reinforcement-enhancing effects of subcutaneous nicotine injections (Caggiula et al., 2009; Wing and Shoaib, 2010). Both nicotine and cocaine solutions were passed through a 0.22 µm filter to ensure sterility.
2.3 Sucrose Pre-exposure
Rats designated as part of the sucrose CPP experiment received two, 2-hr exposures to 25% sucrose in their home cage to reduce novelty-induced hypophagia. The percent sucrose solution was chosen based on previous literature (White and Carr, 1985), with the goal of attaining a modest preference to allow for measurement of a possible nicotine-enhancement effect. Sucrose was made by dissolving crystalline sucrose (Fisher Scientific, Waltham, MA) in tap water. Bottles were placed opposite cage water bottles during sucrose exposure. Bottles were weighed before and after sucrose exposure to measure sucrose consumption.
2.4 Place conditioning
2.4.1 Conditioned Place Preference Chamber
Rats were conditioned and tested in CPP chambers (MED-CPP-RS, Med Associates, Inc., St. Albans, VT). Each chamber had three compartments with distinct wall patterns and floor textures. Compartments A and B were equal in size and dimension and were separated by a small middle compartment. Manually operated guillotine doors separated all compartments. Lighting in compartments A and B were set at 0.2 lux each, whereas center chamber lighting was set to 8.3 lux to reduce inherent preference for the small, center compartment. Infra-red photo beam sensors in each chamber recorded how much time the animal spent in each compartment during testing. Each chamber was encased in a sound-attenuating cabinet.
2.4.2 Initial Preference Assessment
On Day 1, all animals underwent a 20 min initial preference assessment to establish baseline preference. Manually operated guillotine doors were open during the testing to allow free access to all chamber compartments. Rats were placed into the center compartment, allowed to freely explore for 20 min, and then removed and returned to their home cage. Time spent in each compartment was recorded. Rats (n=9) that spent more than 60% of their time in any single chamber during the initial preference test were excluded from the study and not subject to further procedures to maintain minimal contribution of novelty-seeking or extreme bias to the procedures. Rats were randomly assigned to sucrose (n=14) or cocaine (n=13) conditioning groups.
2.4.3 Conditioning Sessions: sucrose
Conditioning sessions were counterbalanced by order and UCS-paired compartment (random, nonbiased design) and conducted over 4 days with one UCS and one control session per day separated by 4-hr. Rats were placed directly into the conditioning compartment. Ten min into each UCS conditioning session, bottles were inserted into CPP compartments (control session– empty, sucrose session- 25% sucrose). Rats were removed and returned to the home cage after another 10 min (20 min total session).
2.4.4 Preference Tests: sucrose
Three preference tests were conducted on consecutive days 24-hr after conditioning was complete. For each test, rats were placed into the center compartment of the apparatus and left undisturbed with free access to all compartments (no bottles present) for 20 min. Five min prior to preference test 2, each animal received a single injection of 0.4 mg/kg nicotine. Five min before preference test 3, each animal received a 0.3 ml injection of 0.9% saline. Animals spent the five min between s.c. injections and the start of preference tests in their home cages. Rats were returned to their home cage after completion of the 20 min test. Time spent in each compartment was recorded.
2.4.5 Conditioning sessions: cocaine
Conditioning sessions were counterbalanced by order and UCS-paired compartment (random, nonbiased design) and conducted over 4 days with one UCS and one control session per day separated by 4-hr. This allowed for minimal carryover of cocaine effects to the control session due to the short half-life of cocaine (Sun et al., 2002). Rats began each conditioning session with a cocaine (10 mg/kg/ml, i.p.) or saline injection, followed by immediate placement into the conditioning compartment. Animals remained in the compartment for 60 minutes until they were removed and returned to their home cages.
2.4.6 Preference test: cocaine
Three preference tests were conducted on consecutive days 24-hr after conditioning was complete. For each test, rats received a saline injection as a control for the previous cocaine injections during conditioning sessions, and were placed immediately in the center compartment of the apparatus and left undisturbed with free access to all chambers for 20 min. The effect of nicotine was examined using a crossover design. Five min prior to preference test 2, half the animals received a single injection of 0.4 mg/kg nicotine, and half received saline control injections. Five min before preference test 3, half the animals received a single injection of 0.4 mg/kg nicotine, and half received saline control injections. Animals spent the five min between s.c. nicotine or saline control injections and the start of preference tests in their home cages. After 20 min, rats were removed from the chamber and returned to their home cage. Time spent in each compartment was recorded.
2.5 Data Analysis
To verify the development of preference, total time spent in the UCS compartment was compared in the initial preference test (pre-conditioning) vs. the first preference test (post-conditioning) with paired t-tests. To examine nicotine enhancement, difference scores (UCS compartment time postconditioning – preconditioning) were calculated for each animal for each post-conditioning preference test. A repeated measures one-way ANOVA compared difference scores across the three post-conditioning tests. Significant main effects were further dissected via Tukey’s post-hoc comparisons. The relation between initial post-conditioning preference (difference score on saline test day 1) and nicotine enhancement (difference score on nicotine test day – difference score on saline test day 1) of that preference was evaluated through use of two-tailed correlational analyses. Cocaine CPP data analyses failed to reveal a significant effect of injection order. For this reason, crossover injection group data were integrated for statistical analysis and graphical representations. Data were considered statistically significant at p<0.05.
3. Results
3.1 The reinforcement-enhancing effect of nicotine on sucrose CPP
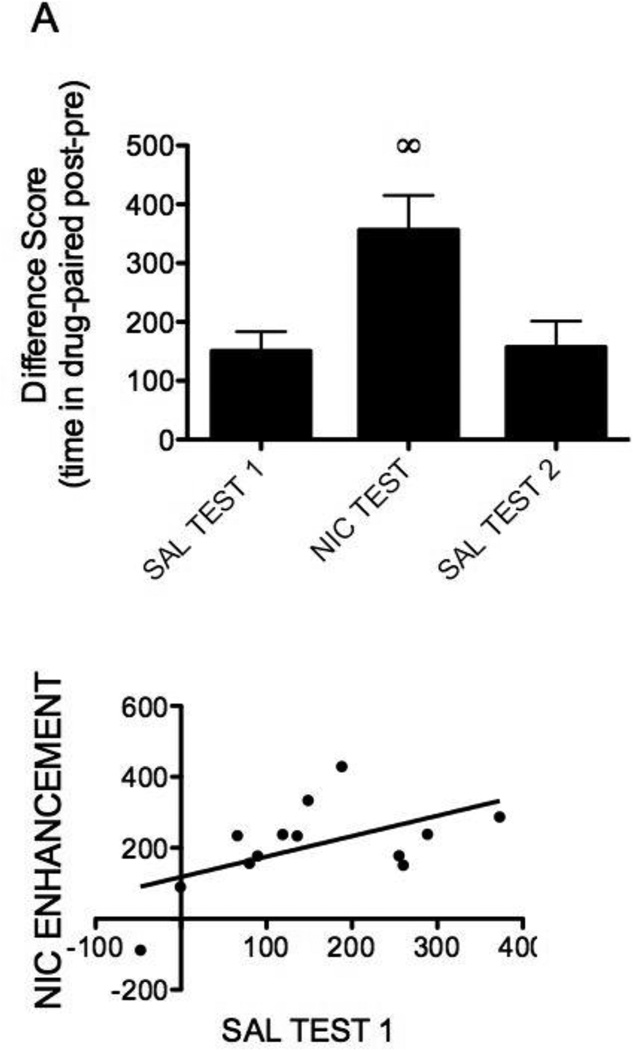
Rats displayed a significant conditioned place preference to the sucrose-paired compartment (t(13)=2.76, p<0.05). Further, nicotine significantly increased the amount of time spent in the sucrose-paired compartment (F(2,26)=8.50, p<0.05, Figure 1A), as the nicotine test difference score was significantly different from tests 1 and 3 (ps<0.05). The difference scores between tests 1 and 3 were not significantly different from one another (p>0.05, Figure 1A). Individual data demonstrate that 64% of rats showed a preference for the sucrose-paired compartment after conditioning (Figure 1B). Correlation analyses failed to reveal a significant relation between initial sucrose preference and nicotine enhancement (R2=0.001, p=0.91, Figure 1B).
Fig. 1.
A) Nicotine enhances the expression of a sucrose conditioned place preference. B) Baseline preference for sucrose had no relationship to nicotine enhancement of preference. Data are represented as the mean difference score (UCS compartment time pre – post-conditioning; ±SEM). ◦Denotes statistically different from all other tests
3.2: The reinforcement-enhancing effect of nicotine on cocaine CPP
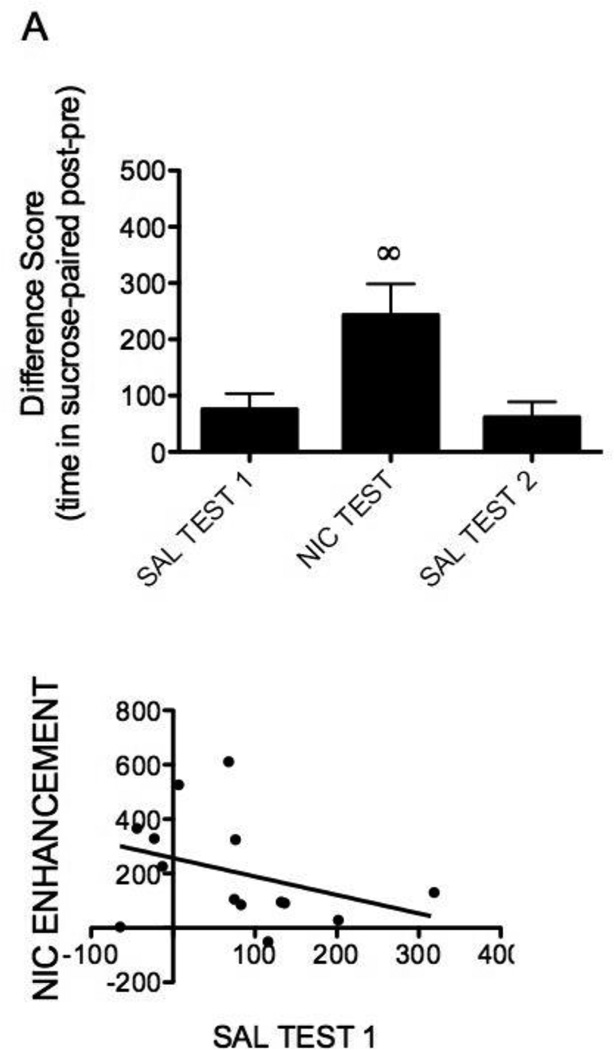
Rats displayed a significant conditioned place preference to the cocaine-paired compartment (t(12)=4.53, p<0.05). Further, nicotine significantly increased the amount of time spent in the cocaine-paired compartment (F(2,24)=10.19, p<0.05, Figure 2A), as the nicotine test difference score was significantly different from both saline injections (ps<0.05), which were not significantly different from one another (p>0.05, Figure 2A). Individual data show that 85% of rats showed a preference for the cocaine-paired compartment after conditioning. Correlation analyses revealed a significant relationship between initial sucrose preference and nicotine enhancement (R2=0.77, p<0.0001, Figure 2B).
Fig. 2.
A)Nicotine enhances the expression of a cocaine conditioned place preference. B) Significant relationship between initial preference for cocaine and nicotine enhancement of cocaine preference. Data are represented as the mean difference score (UCS compartment time pre – post-conditioning; ±SEM). ◦Denotes statistically different from all other tests
4. Discussion
The current results demonstrate that nicotine enhances the preference for contexts that have previously been paired with sucrose or cocaine. In nicotine-naïve rats, a single injection of nicotine caused an increase in the expression of a conditioned place preference for either cocaine or sucrose. In addition to demonstrating that the reinforcement-enhancing effect of nicotine previously seen in operant procedures extends to CPP, these data have clear relevance to two primary, yet less well-studied, challenges facing the field attempting to combat the negative health consequences of nicotine. First, the ability of nicotine to enhance seeking of cues or contexts previously paired with alternative drugs of abuse may increase rates of relapse, as exposure to drug-paired cues or environments may induce craving and promote relapse. Second, nicotine has a complicated interaction with feeding behavior; these data provide another mechanism by which nicotine may promote maladaptive patterns of food intake - via increased seeking of environments previously associated with palatable food.
The findings of this experiment are consistent with those of past studies investigating the reinforcement-enhancing effects of nicotine (Chaudhri et al., 2006b; Olausson et al., 2004; Palmatier et al., 2007b; Raiff and Dallery, 2006). The production of a robust effect after a single injection exclusively during the expression phase of the CPP experiment provides further evidence that the reinforcement-enhancing effect of nicotine is a non-associative process that depends on the initial rather than the long-term pharmacological effects of nicotine (Caggiula et al., 2009; Chaudhri et al., 2006b; Donny et al., 2003). The current studies also provide important novel information about the effects of nicotine. First, a pronounced effect was observed after a single injection which constitutes a notable difference between the present experiment and its operant counterparts in which repeated nicotine exposures appear to be necessary to produce a significant effect (Caggiula et al., 2009; Chaudhri et al., 2006b; Palmatier et al., 2012; Raiff and Dallery, 2006). This distinction suggests that CPP may be a more sensitive measure of the reinforcement-enhancing effects of nicotine. Future research could investigate the effect of single vs. repeated exposure to nicotine to determine whether repeated nicotine injections also potentiate the effects of nicotine on the expression of CPP. Second, nicotine in the current study led to a decreased exploration of the entire place conditioning apparatus concomitant with the increased time spent in the paired chamber. This should reduce concerns that the nicotine enhancement effect is driven by increased locomotor activity (which has been a concern in operant models (Frenk and Dar, 2004). Third, the use of the CPP procedure for the measurement of nicotine enhancement may provide a novel means by which to examine potential nicotine enhancement effects on aversive stimuli; the operant conditioning procedure is not well-suited to examine such questions. Future experiments could provide important information regarding how the reinforcement enhancing effects of nicotine may impact the persistence of smoking behavior. For example, it has been well documented that many stimuli, presented in isolation in, lead to reinstatement (Shaham et al., 2003) of drug- or sucrose-seeking (Nair et al., 2006; Rauhut et al.; Simms et al.) in animal models of relapse. These stimuli can interact to promote increased levels of reinstatement as well. Exposure to acute nicotine can prompt reinstatement of nicotine-seeking behavior (Budzynska and Biala; Chiamulera et al., 1996); however, it is not known if nicotine could enhance the ability of other stimuli to promote reinstatement as well.
The reinforcement enhancing effect of nicotine positively correlated to the initial preference of rats for the cocaine context. That is, rats that had a higher baseline preference for the cocaine compartment also showed higher levels of nicotine enhancement of this preference. These data are consistent with previous results demonstrating that the enhancing properties of nicotine are related to the value of the primary non-nicotine reinforcer. Behavior supported by stimuli that are weakly reinforcing is not readily enhanced by nicotine, whereas modestly reinforcing stimuli are enhanced by nicotine (Chaudhri et al., 2006a; Palmatier et al., 2007c). However, this effect did not extend to the sucrose-paired context. However, with the exception of a single outlier, the range of preference scores in the sucrose data set was considerably less (−80 – 200) than that for the cocaine group (−50 – 400). Further, only 65% of rats showed a preference for the sucrose-paired compartment, while 85% of rats showed preference for the cocaine-paired compartment. Either of these factors, along with the complexity of the relationship between nicotine and food (see below), may explain a difference in the correlation of baseline preference and nicotine enhancement of the preference between cocaine and sucrose-conditioned rats.
Many previous studies have documented an interaction between tobacco and other drugs of abuse (Sullivan and Covey, 2002), including cocaine (Epstein et al., 2010). High levels of drug dependence and increased rates of psychostimulant use are associated with increased tobacco use (Henningfield et al., 1990). Cocaine-dependent smokers begin cocaine use at an earlier age and use cocaine more often (Budney et al., 1993). Previous research indicates that nicotine increases cue-induced cocaine craving and the desire to use cocaine in users (Reid et al., 1998), and smoking and tobacco craving are often related to cocaine craving as well (Epstein et al., 2010). Reductions in use of tobacco may improve abstinence from cocaine as well (Shoptaw et al., 1996). The current results demonstrating an interaction of nicotine with environments previously associated with cocaine provides an additional mechanism by which nicotine may accelerate relapse during abstinence.
The effect of nicotine on cocaine CPP could involve mechanisms related to their shared discriminative and/or common neuropharmacological effects. Previous research has demonstrated that in rats trained to discriminate cocaine from saline, nicotine will fully substitute for cocaine (Desai and Terry, 2003). Hence, animals in the present study may have spent more time in the cocaine-associated chamber after nicotine because nicotine produced cocaine-like effects. However, this is unlikely, as cocaine injections in rats with a history of cocaine CPP do not show enhanced preference beyond that measured initially, only reinstatement of the existing preference (Aguilar et al., 2009; Cordery et al., 2012; Mueller and Stewart, 2000). Further, more recent studies have shed doubt on the ability of nicotine to fully substitute for cocaine (Gould et al., 2011; Mello and Newman, 2011). The fact that the nicotine effect was similar in direction and magnitude across experiments (cocaine and sucrose) further decrease the likelihood that these results are driven by a substitution effect. Finally, the data showing nicotine enhancement of a visual stimulus make it unlikely nicotine’s effects are due to a lack of discrimination between nicotine and the enhanced stimuli.
Alternatively, the effect of nicotine could have been attributed to a disruption of extinction processes. To examine this more fully, we performed a second set of t-tests between the initial preference test (preconditioning) and the final conditioning test (after the nicotine enhancement) for both groups. This analysis showed a significant difference, indicating the preference for the cocaine or sucrose-paired chamber was still intact after 3 post-conditioning tests. This is consistent with previous data that show the extinction of a cocaine preference takes at least 7 days or more (Aguilar et al., 2009; Cordery et al., 2012; Mueller and Stewart, 2000). Therefore, the actions of nicotine on cocaine CPP likely represent a true reinforcement-enhancement effect.
The enhancement of a preference for sucrose-paired environments adds to a complex literature on the relationship between nicotine and feeding. Most research on the effects of nicotine on feeding behavior emphasizes the anorectic effects of nicotine and the weight loss associated with nicotine delivery (Bellinger et al., 2003; Bellinger et al., 2005; Blaha et al., 1998; Grunberg et al., 1987; Miyata et al., 2001). These effects can be modulated by the fat content of the diet and the feeding status of the rats (Wellman et al., 2005)). Such research might suggest that nicotine should reduce the motivational effects of sucrose and attenuate a sucrose-induced CPP. However, as we have previously discussed, nicotine may enhance the reinforcing efficacy of food like it does other reinforcers (Donny et al., 2011). Indeed, nicotine has previously been shown to enhance responding for sucrose-paired stimuli (Chaudhri et al., 2006a), and recent data indicate that nicotine can affect responding for sucrose pellets (Schassburger et al., 2013) or solution (Barret and Bevins, 2013). However, other reports suggest that nicotine does not enhance the palatability (Parker and Doucet, 1995) or intake (Palmatier et al., 2012) of sweet tastes such as sucrose. One important difference between studies examining nicotine and feeding and those looking at operant responding for food or sucrose is that in the latter, rats are generally food restricted, which should alter reinforcer value. The reinforcement-enhancing effects of nicotine are clearly complex and modified by conditioning and dietary factors. Our results suggest that nicotine may promote maladaptive patterns of feeding-related behavior by enhancing the motivational properties of environments associated with palatable food reward, which could contribute to overeating or eating despite satiety.
The neural mechanisms by which nicotine enhances the ability of stimuli to motivate behavior has received much speculation. While other studies have reported on the ability of nicotine to enhance long-term potentiation, neurotransmitter levels, and immediate early gene expression caused by other stimuli (Levine et al., 2011; Li et al.), how these effects relate to reinforcement-enhancement, specifically, is unclear. It has been suggested that potentiation of dopaminergic signaling is a likely candidate for the site of interaction (Rice and Cragg, 2004). This is consistent with the current data set, as dopamine is involved in conditioned place preference to cocaine (Adams et al., 2001) and palatable foods (Agmo et al., 1995). This is a goal of future studies, however, and remains to be directly tested.
In summary, nicotine enhances the preference for environments previously paired with either sucrose or cocaine reward. These observations are consistent with earlier studies that demonstrated similar effects using operant procedures, and should facilitate expanded research on the reinforcement-enhancing effects of nicotine with novel behavioral approaches. Furthermore, these data suggest that nicotine may potentiate the seeking of environments that have previously been associated with other rewards. This provides an interesting behavioral mechanism by which nicotine could support an increased likelihood of engagement in those other behaviors (e.g., drug use, feeding) and drive the cycle of addiction or maladaptive consumption of food.
Highlights.
Rats displayed a place preference to contexts paired with cocaine or sucrose
Nicotine increased expression of a place preference for sucrose or cocaine
Magnitude of enhancement was significantly correlated to initial cocaine preference
Acknowledgements
Research was supported by NIH grant R01 DA010464 (ECD). The authors would also like to thank Maysa Gharib, Colleen Silky, Melinda Moran, Angela Lutheran, and Rick Jacobson for their technical assistance. All aspects of these studies were in compliance with the current laws of the United States.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors have no conflicts of interest. Authors have full control of all primary data and will allow representatives of Pharmacology, Biochemistry and Behavior to review the data if they request to see it.
References
- Adams JU, Careri JM, Efferen TR, Rotrosen J. Differential effects of dopamine antagonists on locomotor activity, conditioned activity and conditioned place preference induced by cocaine in rats. Behav. Pharmacol. 2001;12:603–611. doi: 10.1097/00008877-200112000-00004. [DOI] [PubMed] [Google Scholar]
- Agmo A, Galvan A, Talamantes B. Reward and reinforcement produced by drinking sucrose: two processes that may depend on different neurotransmitters. Pharmacol. Biochem. Behav. 1995;52:403–414. doi: 10.1016/0091-3057(95)00128-j. [DOI] [PubMed] [Google Scholar]
- Aguilar MA, Rodriguez-Arias M, Minarro J. Neurobiological mechanisms of the reinstatement of drug-conditioned place preference. Brain Res Rev. 2009;59:253–277. doi: 10.1016/j.brainresrev.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Anthony JC, Warner AL, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the national comorbidity survey. Exp Clin Psychopharmacol. 1994;2:224–268. [Google Scholar]
- Barret ST, Bevins RA. Nicotine enhances operant responding for qualitatively distinct reinforcers under maintenance and extinction conditions. Pharmacol. Biochem. Behav. 2013:114–115. 9–15. [PMC free article] [PubMed] [Google Scholar]
- Barrett ST, Bevins RA. A quantitative analysis of the reward-enhancing effects of nicotine using reinforcer demand. Behav. Pharmacol. 2012;23:781–789. doi: 10.1097/FBP.0b013e32835a38d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger L, Cepeda-Benito A, Wellman PJ. Meal patterns in male rats during and after intermittent nicotine administration. Pharmacol. Biochem. Behav. 2003;74:495–504. doi: 10.1016/s0091-3057(02)01033-x. [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Wellman PJ, Cepeda-Benito A, Kramer PR, Guan G, Tillberg CM, Gillaspie PR, Hill EG. Meal patterns in female rats during and after intermittent nicotine administration. Pharmacol. Biochem. Behav. 2005;80:437–444. doi: 10.1016/j.pbb.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Caggiula AR. Nicotine, tobacco use, the 55th Nebraska Symposium on Motivation. Nebr. Symp. Motiv. 2009;55:1–3. doi: 10.1007/978-0-387-78748-0_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaha V, Yang ZJ, Meguid M, Chai JK, Zadak Z. Systemic nicotine administration suppresses food intake via reduced meal sizes in both male and female rats. Acta Medica (Hradec Kralove) 1998;41:167–173. [PubMed] [Google Scholar]
- Brewer AJ, 3rd, Mahoney JJ, 3rd, Nerumalla CS, Newton TF, De la Garza R., 2nd The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacol. Biochem. Behav. 2013;106:132–136. doi: 10.1016/j.pbb.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J. Subst. Abuse. 1993;5:117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- Budzynska B, Biala G. Effects of bupropion on the reinstatement of nicotine-induced conditioned place preference by drug priming in rats. Pharmacol Rep. 63:362–371. doi: 10.1016/s1734-1140(11)70502-3. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, Palmatier MI, Liu X, Chaudhri N, Sved AF. The role of nicotine in smoking: a dual-reinforcement model. Nebr. Symp. Motiv. 2009;55:91–109. doi: 10.1007/978-0-387-78748-0_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol. Biochem. Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Cassidy RN, Dallery J. Effects of economy type and nicotine on the essential value of food in rats. J. Exp. Anal. Behav. 2012;97:183–202. doi: 10.1901/jeab.2012.97-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Operant responding for conditioned and unconditioned reinforcers in rats is differentially enhanced by the primary reinforcing and reinforcement-enhancing effects of nicotine. Psychopharmacology (Berl) 2006a;189:27–36. doi: 10.1007/s00213-006-0522-0. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006b;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 1996;127:102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- Conklin CA, Tiffany ST. The impact of imagining personalized versus standardized urge scenarios on cigarette craving and autonomic reactivity. Exp Clin Psychopharmacol. 2001;9:399–408. doi: 10.1037//1064-1297.9.4.399. [DOI] [PubMed] [Google Scholar]
- Cordery SF, Taverner A, Ridzwan IE, Guy RH, Delgado-Charro MB, Husbands SM, Bailey CP. A non-rewarding, non-aversive buprenorphine/naltrexone combination attenuates drug-primed reinstatement to cocaine and morphine in rats in a conditioned place preference paradigm. Addict Biol. 2012 doi: 10.1111/adb.12020. [DOI] [PubMed] [Google Scholar]
- Desai RI, Terry P. Evidence of cross-tolerance between behavioural effects of nicotine and cocaine in mice. Psychopharmacology (Berl) 2003;166:111–119. doi: 10.1007/s00213-002-1319-4. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Weaver MT, Levin ME, Sved AF. The reinforcement-enhancing effects of nicotine: implications for the relationship between smoking, eating and weight. Physiol. Behav. 2011;104:143–148. doi: 10.1016/j.physbeh.2011.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem. Pharmacol. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Marrone GF, Heishman SJ, Schmittner J, Preston KL. Tobacco, cocaine, and heroin: Craving and use during daily life. Addict. Behav. 2010;35:318–324. doi: 10.1016/j.addbeh.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenk H, Dar R. Reward potentiation or behavioral activation? A comment on Donny et al. Psychopharmacology (Berl) 2004;171:472–473. doi: 10.1007/s00213-003-1622-8. author reply 474–476. [DOI] [PubMed] [Google Scholar]
- Geier A, Mucha RF, Pauli P. Appetitive nature of drug cues confirmed with physiological measures in a model using pictures of smoking. Psychopharmacology (Berl) 2000;150:283–291. doi: 10.1007/s002130000404. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gould RW, Czoty PW, Nader SH, Nader MA. Effects of varenicline on the reinforcing and discriminative stimulus effects of cocaine in rhesus monkeys. J. Pharmacol. Exp. Ther. 2011;339:678–686. doi: 10.1124/jpet.111.185538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graves SM, Napier TC. Mirtazapine alters cue-associated methamphetamine seeking in rats. Biol. Psychiatry. 69:275–281. doi: 10.1016/j.biopsych.2010.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg NE, Winders SE, Popp KA. Sex differences in nicotine's effects on consummatory behavior and body weight in rats. Psychopharmacology (Berl) 1987;91:221–225. doi: 10.1007/BF00217067. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Critical role for ventral tegmental glutamate in preference for a cocaine-conditioned environment. Neuropsychopharmacology. 2003;28:73–76. doi: 10.1038/sj.npp.1300011. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Clayton R, Pollin W. Involvement of tobacco in alcoholism and illicit drug use. Br. J. Addict. 1990;85:279–291. doi: 10.1111/j.1360-0443.1990.tb03084.x. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel DB, Kandel ER, Levine A. Nicotine primes the effect of cocaine on the induction of LTP in the amygdala. Neuropharmacology. 2013;74:126–134. doi: 10.1016/j.neuropharm.2013.03.031. [DOI] [PubMed] [Google Scholar]
- Jo YH, Talmage DA, Role LW. Nicotinic receptor-mediated effects on appetite and food intake. J. Neurobiol. 2002;53:618–632. doi: 10.1002/neu.10147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A, Huang Y, Drisaldi B, Griffin EA, Jr, Pollak DD, Xu S, Yin D, Schaffran C, Kandel DB, Kandel ER. Molecular mechanism for a gateway drug: epigenetic changes initiated by nicotine prime gene expression by cocaine. Sci Transl Med. 2011;3:107ra109. doi: 10.1126/scitranslmed.3003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Bu Q, Chen B, Shao X, Hu Z, Deng P, Lv L, Deng Y, Zhu R, Li Y, Zhang B, Hou J, Du C, Zhao Q, Fu D, Zhao Y, Cen X. Mechanisms of metabonomic for a gateway drug: nicotine priming enhances behavioral response to cocaine with modification in energy metabolism and neurotransmitter level. PLoS ONE. 9:e87040. doi: 10.1371/journal.pone.0087040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Newman JL. Discriminative and reinforcing stimulus effects of nicotine, cocaine, and cocaine + nicotine combinations in rhesus monkeys. Exp Clin Psychopharmacol. 2011;19:203–214. doi: 10.1037/a0023373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata G, Meguid MM, Varma M, Fetissov SO, Kim HJ. Nicotine alters the usual reciprocity between meal size and meal number in female rat. Physiol. Behav. 2001;74:169–176. doi: 10.1016/s0031-9384(01)00540-6. [DOI] [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav. Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Nair SG, Gray SM, Ghitza UE. Role of food type in yohimbine- and pellet-priming-induced reinstatement of food seeking. Physiol. Behav. 2006;88:559–566. doi: 10.1016/j.physbeh.2006.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Caggiula AR, Donny EC, Sved AF. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology. 2007a;32:1098–1108. doi: 10.1038/sj.npp.1301228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Liu X, Matteson GL, Donny EC, Caggiula AR, Sved AF. Conditioned reinforcement in rats established with self-administered nicotine and enhanced by noncontingent nicotine. Psychopharmacology (Berl) 2007b;195:235–243. doi: 10.1007/s00213-007-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Matteson GL, Black JJ, Liu X, Caggiula AR, Craven L, Donny EC, Sved AF. The reinforcement enhancing effects of nicotine depend on the incentive value of non-drug reinforcers and increase with repeated drug injections. Drug Alcohol Depend. 2007c;89:52–59. doi: 10.1016/j.drugalcdep.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, O'Brien LC, Hall MJ. The role of conditioning history and reinforcer strength in the reinforcement enhancing effects of nicotine in rats. Psychopharmacology (Berl) 2012;219:1119–1131. doi: 10.1007/s00213-011-2439-5. [DOI] [PubMed] [Google Scholar]
- Parker LA, Doucet K. The effects of nicotine and nicotine withdrawal on taste reactivity. Pharmacol. Biochem. Behav. 1995;52:125–129. doi: 10.1016/0091-3057(95)00060-a. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Epstein LH, Grobe J, Fonte C. Tobacco abstinence, smoking cues, and the reinforcing value of smoking. Pharmacol. Biochem. Behav. 1994;47:107–112. doi: 10.1016/0091-3057(94)90118-x. [DOI] [PubMed] [Google Scholar]
- Raiff BR, Dallery J. Effects of acute and chronic nicotine on responses maintained by primary and conditioned reinforcers in rats. Exp Clin Psychopharmacol. 2006;14:296–305. doi: 10.1037/1064-1297.14.3.296. [DOI] [PubMed] [Google Scholar]
- Rauhut AS, Fenton L, Bardo MT. Renewal of sucrose-seeking behavior in rats: Role of D(2) dopamine receptors. Pharmacol. Biochem. Behav. 96:354–362. doi: 10.1016/j.pbb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP. An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Depend. 1998;49:95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat. Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97:861–869. doi: 10.1046/j.1360-0443.2002.00162.x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM. Psychophysiological interactions between caffeine and nicotine. Pharmacol. Biochem. Behav. 1991;38:333–337. doi: 10.1016/0091-3057(91)90287-c. [DOI] [PubMed] [Google Scholar]
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol. Biochem. Behav. 2000;67:71–81. doi: 10.1016/s0091-3057(00)00301-4. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology (Berl) 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Rose JE, Levin ED. Inter-relationships between conditioned and primary reinforcement in the maintenance of cigarette smoking. Br. J. Addict. 1991;86:605–609. doi: 10.1111/j.1360-0443.1991.tb01816.x. [DOI] [PubMed] [Google Scholar]
- Schassburger RL, Rupprecht LE, Smith TT, Buffalari DM, Thiels E, Donny EC, Sved AF. Neuroscience. San Diego, California: 2013. Nicotine Enhances the Rewarding Properties of Sucrose, Program No. 545.30. Abstracts 2013; Online. [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shoptaw S, Jarvik ME, Ling W, Rawson RA. Contingency management for tobacco smoking in methadone-maintained opiate addicts. Addict. Behav. 1996;21:409–412. doi: 10.1016/0306-4603(95)00066-6. [DOI] [PubMed] [Google Scholar]
- Simms JA, Richards JK, Mill D, Kanholm I, Holgate JY, Bartlett SE. Induction of multiple reinstatements of ethanol- and sucrose-seeking behavior in Long-Evans rats by the alpha-2 adrenoreceptor antagonist yohimbine. Psychopharmacology (Berl) 218:101–110. doi: 10.1007/s00213-011-2451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MA, Covey LS. Current perspectives on smoking cessation among substance abusers. Curr Psychiatry Rep. 2002;4:388–396. doi: 10.1007/s11920-002-0087-5. [DOI] [PubMed] [Google Scholar]
- Sun H, Shen ML, Pang YP, Lockridge O, Brimijoin S. Cocaine metabolism accelerated by a re-engineered human butyrylcholinesterase. J. Pharmacol. Exp. Ther. 2002;302:710–716. doi: 10.1124/jpet.302.2.710. [DOI] [PubMed] [Google Scholar]
- Thiel KJ, Sanabria F, Neisewander JL. Synergistic interaction between nicotine and social rewards in adolescent male rats. Psychopharmacology (Berl) 2009;204:391–402. doi: 10.1007/s00213-009-1470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Maldonado-Vlaar CS, Parsons LH, Kerr TM, Smith DL, Ben-Shahar O. Control of cocaine-seeking behavior by drug-associated stimuli in rats: effects on recovery of extinguished operant-responding and extracellular dopamine levels in amygdala and nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 2000;97:4321–4326. doi: 10.1073/pnas.97.8.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman PJ, Bellinger LL, Cepeda-Benito A, Susabda A, Ho DH, Davis KW. Meal patterns and body weight after nicotine in male rats as a function of chow or high-fat diet. Pharmacol. Biochem. Behav. 2005;82:627–634. doi: 10.1016/j.pbb.2005.11.002. [DOI] [PubMed] [Google Scholar]
- White NM, Carr GD. The conditioned place preference is affected by two independent reinforcement processes. Pharmacol. Biochem. Behav. 1985;23:37–42. doi: 10.1016/0091-3057(85)90127-3. [DOI] [PubMed] [Google Scholar]
- Wing VC, Shoaib M. A second-order schedule of food reinforcement in rats to examine the role of CB1 receptors in the reinforcement-enhancing effects of nicotine. Addict Biol. 2010;15:380–392. doi: 10.1111/j.1369-1600.2009.00203.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, Zhang F, Tang S, Liu H, Gu J, Yang G. The dissociation of heroin-seeking patterns induced by contextual, discriminative, or discrete conditioned cues in a model of relapse to heroin in rats. Psychopharmacology (Berl) 2005;181:197–206. doi: 10.1007/s00213-005-2262-y. [DOI] [PubMed] [Google Scholar]