Abstract
Aims:
The aim of this study was to evaluate the bilirubin lowering and wound healing property of aqueous extract of Calotropis procera (AECP) leaves in Wistar rats.
Materials and Methods:
Albino Wistar rats of either sex were used for the study. Bilirubin lowering property of C. procera leaves was evaluated using phenylhydrazine and paracetamol as inducing agents followed by measuring the concentration of serum total bilirubin in hyperbilirubinemic rats. Wound healing property was evaluated using incision and excision models by measuring tensile breaking strength, percentage wound contractions, and epithelization days, respectively.
Statistical Analysis:
Statistical comparison between groups in each experiment was done with one-way analysis of variance followed by Dunnett's test.
Results:
AECP showed a significant (P < 0.05) decrease in concentrations of serum total bilirubin in hyperbilirubinemic rats as well as significant (P < 0.05) increase in breaking strength and percentage wound contractions with decreased epithelization period when compared to control groups.
Conclusions:
AECP showed significant bilirubin lowering and wound healing property in Wistar rats.
KEY WORDS: Calotropis procera, excision, hyperbilirubinemia, incision, paracetamol, phenylhydrazine, wound healing
Introduction
The human race started using plants or plant products successfully as a mean of treatment of disease and injuries as effective therapeutic tools from early days of civilization to the modern age.[1] Calotropis procera Linn (Family: Asclepiadaceae) is an ayurvedic plant with important medicinal properties. It is known by various vernacular names such as Swallow-Wort (English), Madar (Hindi), Alarka (Sanskrit), and commonly referred to as Ark, Swallow wart or milkweed. It occurs frequently in Indonesia, Malaysia, China, India as wasteland weed and also found in most parts of the world with a warm climate in dry, sandy, and alkaline soils. C. procera Linn is an erect, tall large, highly branched, and perennial shrub or small tree that grows to a height of 5.4 m with milky latex throughout the plant.[2]
The phytochemistry of plant reveals presence of triterpenoids, flavonoids, cardiac glycosides, cardenolides, anthocyanins, α-amyrin, β-amyrin, lupeol, β-sitosterol, flavanols, mudarine, resins, a powerful bacteriolytic enzyme calactin, a nontoxic proteolytic enzyme calotropin, and a wax.[3] The parts of plants used in ayurvedic medicine are leaves, fresh or dried, the roots, root bark, and flowers. The powdered leaves are useful for fast healing of wounds, as purgative, to treat liver problems, to promote sexual health, to relieve stomach ache, headache, also applied in sprain to ease swelling and pain.
Traditionally, the plant has been used as anti-fungal, antipyretic, analgesic. The dried leaves are used as an expectorant, anti-inflammatory, for the treatment of paralysis and rheumatic pain. The dried latex and roots are used as an antidote for snake poisoning. It is also used as an abortifacient for the treatment of piles and intestinal worms. The tender leaves are also used to treat migraine.[4]
Therefore, by taking into limelight the traditional uses[5] of Calotropis procera, the present study was performed to provide a pharmacological base for use of plant in treatment of hyperbilirubinemia induced by phenylhydrazine (PHZ) and paracetamol[6,7] and wound healing.[8,9]
Materials and Methods
Animals
Adult Wistar rats of either sex weighing about 180–250 g were purchased from Bharat serum and vaccines, Thane. The animals were housed in standard laboratory conditions in groups of three at 25°C ± 2°C, humidity 60% ± 2%, and 12 h light: Dark cycle. Animals had a free access to standard laboratory food purchased from Amrut rat and mice feed, Nashik, India and water. The animals were acclimatized to the laboratory conditions 1 week prior to the experimentation. All experiments were performed during a light portion of 12-12 h. The protocol was approved from Institutional Animal Ethics Committee of MGV's Pharmacy College, Nashik (MGV/PC/XXVIII/01/2013-14).
Drugs and Chemicals
Phenylhydrazine (Sigma-Aldrich, USA), paracetamol (Merck), Silymarin (Silybon, Micro labs), Dexamethasone (DXM) injection (Life care pharmaceuticals), ketamine HCl (Aneket, Neon labs), Total Bilirubin kit, alanine transaminase (ALT), and aspartate transaminase (AST) (Coral Clinical Systems, Mumbai) were used in present study. Drug solutions were prepared fresh in distilled water and stored in a refrigerator at 4°C. The doses prepared were based upon the previous studies and shown to be pharmacologically active.[10,11,12]
Preparation of Plant Extract
Leaves of C. procera were collected from Tapovan garden, Panchvati, Nashik. The leaves were authenticated by Dr. (Mrs.) A. G. Bhaskarwar, Ayurvedic Seva Sangh, Panchvati, Nashik. The collected leaves were shade dried and powdered using a grinder. The aqueous extract of C. procera (AECP) was prepared by boiling the powdered leaf matter with 16 times of its weight in distilled water and reducing its volume up to 1/32 times. The extract obtained was stored in refrigerator till used.
Phenylhydrazine Induced Hyperbilirubinemia
The animals were treated with PHZ (5 mg/kg i.p.) for 5 days to develop jaundice following standard procedure[10] with slight modification. LD50 (993 mg/kg) was found to be reported in rats.[13] Hence, the doses selected for study were 25 mg/kg and 50 mg/kg.
The animals were randomly distributed into five groups (n = 5). Group I received vehicle (distilled water, 5 ml/kg, p.o.), Group II received PHZ (5 mg/kg, i.p.), Group III received PHZ (5 mg/kg, i.p.) and Silymarin suspension (100 mg/kg, p.o.), Group IV received PHZ (5 mg/kg, i.p.) and AECP (25 mg/kg, p.o.), and Group V received PHZ (5 mg/kg, i.p.) and AECP (50 mg/kg, p.o.). The concentration of serum total bilirubin was determined by Mod. Jendrassik and Grof's method[14] and hemoglobin (Hb) by Sahli-Hellige method[15] on day 1 and day 5 after 6 h of administration of PHZ to confirm jaundiced condition of animals. The treatment of jaundiced groups with Silymarin (100 mg/kg, p.o.) and AECP (25 and 50 mg/kg, p.o.) was started on day 6 and continued up to day 10. Blood was collected from tail vein of rats on day 1 (normal), day 5 (after 6 h of PHZ administration), and on day 10 to determine serum concentration of total bilirubin and Hb level.
Paracetamol Induced Hyperbilirubinemia
The animals were treated with paracetamol (2 g/kg p.o.)[11] for 5 days to develop jaundice following standard procedure[10] with slight modification. The animals were randomly distributed into five groups (n = 5). Group I received vehicle (distilled water, 5 ml/kg, p.o.), Group II received paracetamol (2 g/kg p.o.), Group III received paracetamol (2 g/kg p.o) and Silymarin suspension (100 mg/kg p.o.), Group IV received paracetamol (2 g/kg p.o) and AECP (25 mg/kg, p.o.), Group V received paracetamol (2 g/kg p.o) and AECP (50 mg/kg, p.o.). The concentration of serum total bilirubin was determined by Mod. Jendrassik and Grof's method, ALT and AST by Reitman and Frankel's method[16] on day 1 and day 5 after 6 h of administration of paracetamol to confirm jaundice in animals. The treatment for jaundice with Silymarin (100 mg/kg, p.o.) and AECP (25 and 50 mg/kg, p.o.) was started on day 6 and continued up to day 10. Blood was collected from tail vein of rats on day 1 (normal), day 5 (after 6 h of paracetamol administration), and on day 10 to determine serum concentration of total bilirubin, ALT and AST.
Incision Wound Model
Animals were distributed into four groups (n = 5) as follows: Group I received vehicle (distilled water, 5 ml/kg, p.o.), Group II received DXM (0.34 mg/kg i.m. on 1st day and 0.17 mg/kg i.m. on alternative days for 7 days), Group III received AECP (25 mg/kg, p.o.), Group IV received AECP (50 mg/kg, p.o.). All procedures were carried out under ketamine anesthesia (60 mg/kg i.p.).[17] On the depilated backs of Wistar rats, two paravertebral incisions of 2.5 cm were made cutting through the full thickness of the skin. Interrupted sutures, 1 cm apart were placed to approximate the cut edges of skin by ethilon 4-0.[18] The sutures were removed after 7 days and skin breaking tensile strength was measured on day 10 by continuous water flow technique of Lee.[19]
Excision Wound Model
Animals were randomly distributed into four groups (n = 5). Group I received vehicle (distilled water, 5 ml/kg, p.o.), Group II received DXM (0.34 mg/kg i.m. on 1st day and 0.17 mg/kg i.m. on alternative days till epithelization), Group III received AECP (25 mg/kg, p.o.), Group IV received AECP (50 mg/kg, p.o.), and received their respective treatment. An excision wound was inflicted by cutting away a circular piece of 0.5 mm to the full thickness of skin on a predetermined area on depilated back. Epithelization period was noted as the number of days required for Eschar to fall off leaving no raw wound behind. Wound contraction rate was monitored by measuring wound area on alternate days. Reduction in wound area expressed as a percentage of original wound size.[20]
Statistical Analysis
All data expressed as mean ± standard error of mean (SEM) of value for corresponding parameters. Statistical comparison between groups in each experiment was performed with One-way analysis of variance followed by Dunnett's test. Statistical analysis was performed using Primer software.
Results
Phenylhydrazine Induced Hyperbilirubinemia
Serum total bilirubin level
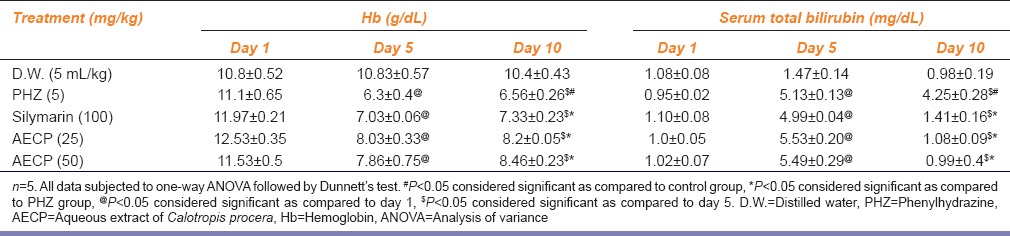
Animals treated with PHZ (5 mg/kg i.p.) showed significant (P < 0.05) increase in serum bilirubin level on day 5 compared to day 1 and significant (P < 0.05) decrease in serum bilirubin on day 10 compared to day 5. Animals showed significant (P < 0.05) increase in the level of serum total bilirubin compared to vehicle treated group. Hyperbilirubinemic rats treated with Silymarin (100 mg/kg p.o.) and AECP (25 and 50 mg/kg p.o.) showed significant (P < 0.05) decrease in levels of serum total bilirubin as compared to PHZ treated group on day 10 of study [Table 1].
Table 1.
Effect of AECP on Hb and serum total bilirubin in PHZ treated rats on day 1, 5 and 10
Hemoglobin
Animals treated with PHZ (5 mg/kg i.p) showed significant (P < 0.05) decrease in Hb level on day 5 compared to day 1 and significant (P < 0.05) increase in Hb level on day 10 compared to day 5. Animals showed significant (P < 0.05) decrease in levels of Hb compared to vehicle treated group. Hyperbilirubinemic rats treated with Silymarin (100 mg/kg p.o) and AECP (25 and 50 mg/kg, p.o.) showed significant (P < 0.05) increase in levels of Hb as compared to the PHZ treated group on day 10 of study [Table 1].
Paracetamol Induced Hyperbilirubinemia
Serum total bilirubin level
Animals treated with paracetamol (2 g/kg p.o.) showed significant (P < 0.05) increase in serum bilirubin level on day 5 compared to day 1 and significant (P < 0.05) decrease in serum bilirubin on day 10 compared to day 5. Animals showed significant (P < 0.05) increase in the level of total bilirubin compared to vehicle treated group. Hyperbilirubinemic rats treated with Silymarin (100 mg/kg p.o.) and AECP (25 and 50 mg/kg p.o.) showed significant (P < 0.05) decrease in levels of serum total bilirubin as compared to paracetamol treated group on day 10 of study [Figure 1].
Figure 1.
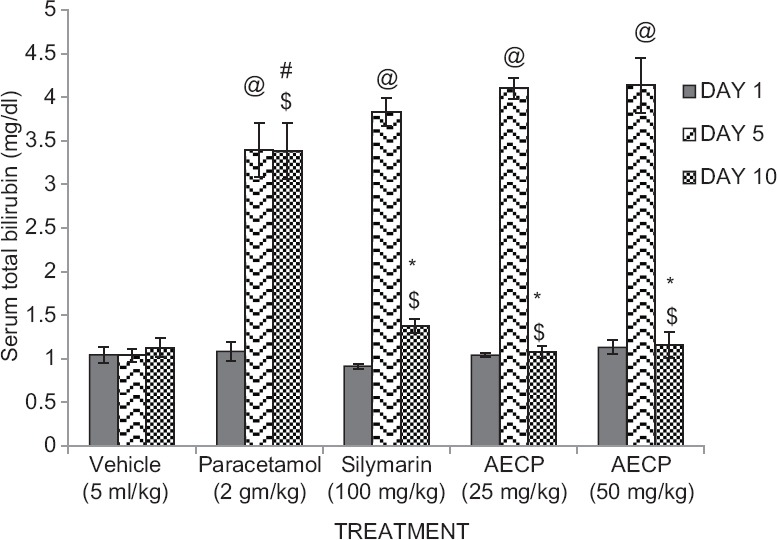
Effect of aqueous extract of Calotropis procera (AECP) on serum total bilirubin levels in paracetamol treated rats on day 1, 5, and 10 (n = 5. All data subjected to one-way analysis of variance followed by Dunnett's test. #P < 0.05 considered significant as compared to control group.*P < 0.05 considered significant as compared to phenylhydrazine group. @P < 0.05 considered significant as compared to day 1. $P < 0.05 considered significant to day 5)
Serum alanine transaminase and aspartate transaminase level
Animals treated with paracetamol (2 g/kg p.o.) showed significant (P < 0.05) increase in serum level of ALT and AST on day 5 compared to day 1 and significant (P < 0.05) decrease in serum level of ALT and AST on day 10 compared to day 5. Animals showed significant (P < 0.05) increase in serum levels of ALT and AST compared to vehicle treated group. Hyperbilirubinemic rats treated with Silymarin (100 mg/kg p.o.) and AECP (25 and 50 mg/kg p.o.) showed significant (*P < 0.05) decrease in serum levels of ALT and AST compared to the paracetamol treated group on day 10 of study [Figures 2 and 3].
Figure 2.
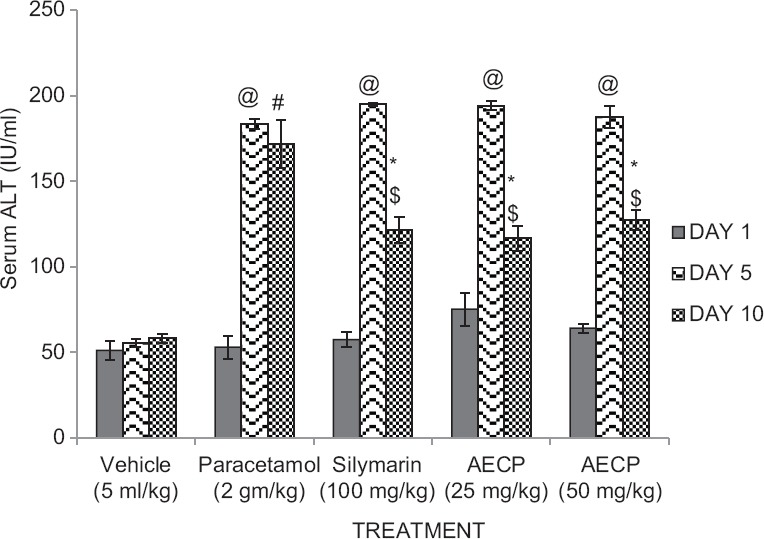
Effect of aqueous extract of Calotropis procera on (AECP) on serum alanine transaminase levels in paracetamol treated rats on day 1, 5, and 10 (n = 5. All data subjected to one-way analysis of variance followed by Dunnett's test. #P < 0.05 considered significant as compared to control group. *P < 0.05 considered significant as compared to phenylhydrazine group. @P < 0.05 considered significant as compared to day 1. $P < 0.05 considered significant to day 5)
Figure 3.
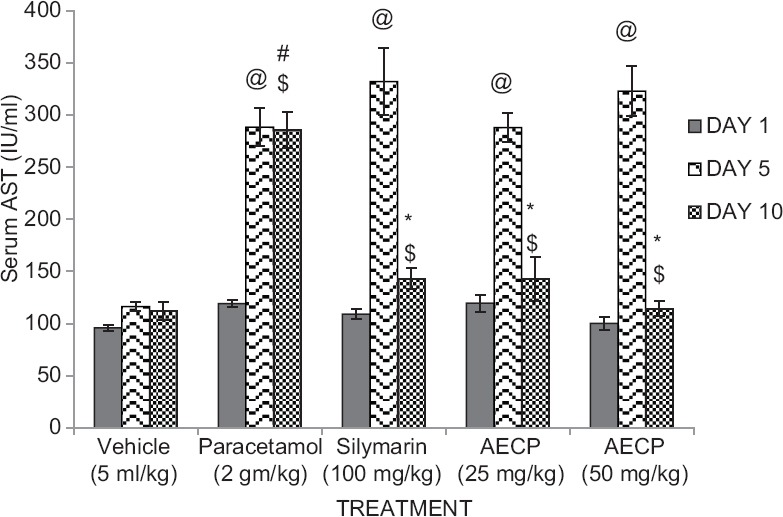
Effect of aqueous extract of Calotropis procera on serum aspartate transaminase levels in paracetamol treated rats on day 1, 5, and 10 (n = 5. All data subjected to one-way analysis of variance followed by Dunnett's test. #P < 0.05 considered significant as compared to control group. *P < 0.05 considered significant as compared to phenylhydrazine group. @P < 0.05 considered significant as compared to day 1. $P < 0.05 considered significant to day 5)
Wound Healing Property
Incision model
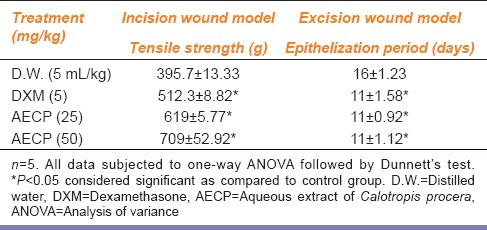
Animals treated with DXM (0.34 mg/kg i.m. on first day and 0.17 mg/kg i.m. on alternative days for 7 days) and AECP (25 and 50 mg/kg p.o.) showed significant (P < 0.05) increase in tensile breaking strength of sutured skin compared to control group [Table 2].
Table 2.
Effect of AECP on tensile strength in incision wound model and epithelization period in excision wound model
Excision model
Percentage wound healing and epithelization days: Animals treated with DXM (0.34 mg/kg i.m. on 1st day and 0.17 mg/kg i.m. on alternative days till epithelization) and AECP (25 and 50 mg/kg p.o.) showed significant (P < 0.05) increase in wound contractions [Figure 4] and decrease in epithelization period as compared to control group [Table 2].
Figure 4.
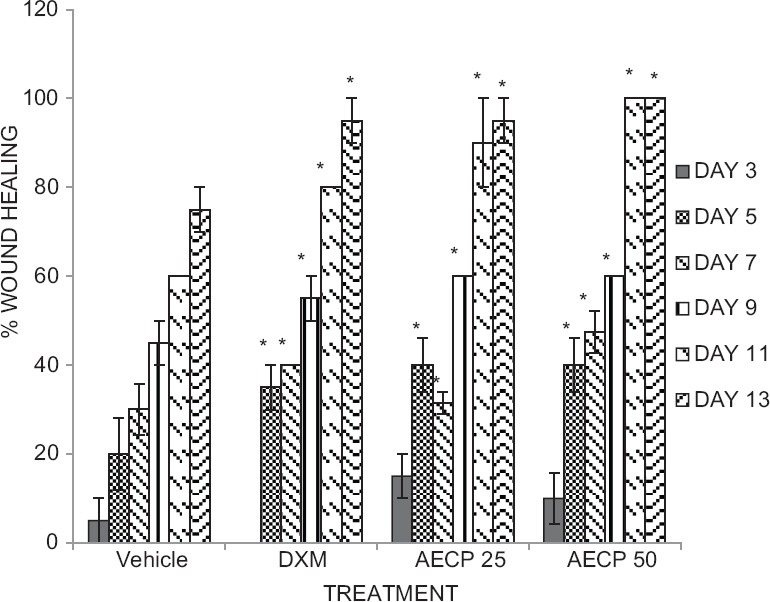
Effect of aqueous extract of Calotropis procera on percent wound healing in rats (N = 5 All data were subjected to one-way analysis of variance followed by Dunnett's test. *P < 0.05 was considered significant as compared to control group and Dexamethasone group, respectively. Vertical lines represent standard error of mean)
Discussion
In order to establish a scientific basis for utilization of C. procera in the treatment of hyperbilirubinemia, it was decided to evaluate the bilirubin lowering activity in PHZ and paracetamol-induced hyperbilirubinemic rats. Earlier reports revealed that PHZ induced hyperbilirubinemic rats showed marked increase in serum total bilirubin levels,[21] the reason for which would be excess hemolysis of the RBC's leading to over production of the bilirubin causing hyperbilirubinemia. Paracetamol treated rats showed marked increase in serum total bilirubin, ALT and AST levels,[22] the mechanism of which is acute hepatocyte necrosis due to formation of N-acetyl-p-benzoquinoneimine (NAPQI) and saturation of sulfate and glucuronide pathways of paracetamol metabolism.[23]
Silymarin a unique flavonoid complex is a substance with documented hepatoprotective property by its cell membrane stabilizing property.[24] Similarly, the number of flavonoid components, flavonolignans were found to be present in C. procera leaves.[25] Hence, the bilirubin lowering activity of AECP was studied along with Silymarin and comparing both with jaundiced groups.
In the present study, results of both hyperbilirubinemic model viz., PHZ and paracetamol, revealed a significant (P < 0.05) decrease in the serum total bilirubin levels in PHZ and paracetamol treated animals with increase in Hb in PHZ treated animals and decrease in Serum ALT and AST levels when compared with vehicle-treated group and also results were observed to be similarly effective as that of Silymarin treated groups.
Dexamethasone a glucocorticoid possess, a marked anti-inflammatory property along with its capacity to stimulate connective tissue growth factor (CTGF) expressions in normal tissues and organs causing fibroblast proliferation and extracellular matrix deposition[26] which may serve as a basis for its use as wound healing agent for a preclinical study. Wound healing property of C. procera was studied using two different models viz., incision and excision wound model. The results of incision wound showed a significant increase in breaking strength of sutured skin. In excision study, the animals treated with AECP showed a significant increase in wound contraction, increased percentage wound healing with a decrease in epithelization period. This result was in agreement with that of a previous study by Shilpa et al. who reported that treatment with Crinium defixum possess potent wound healing activity in excision and incision wound model.[27]
Results of the present study revealed that AECP possess a marked bilirubin lowering property which resulted in decrease in serum concentration of total bilirubin in both models of hyperbilirubinemia and also possess a wound healing property which resulted in an increased tensile breaking strength of sutured skin, increased percentage of wound healing with decreased epithelization period in incision and excision model, respectively.
The above obtained results may be possibly due to presence of various phytochemicals which play role as antioxidants thereby stabilizing the cellular membrane of hepatocytes exhibiting bilirubin lowering property, as well as chemical moieties which cause increase in fibrinogenesis leading to wound healing property.
Hence, the further detailed scientific study of C. procera such as screening, isolation, and characterization along with anti-oxidant and histopathological study can discover the particular chemical moiety with its possible mode of action for above-obtained results.
Acknowledgement
The authors are grateful to the management and Prin. Dr. R. S. Bhambar for support and encouragement.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Yesmin N, Uddin SN, Mubassara S, Muhammad AA. Antioxidant and antibacterial activities of Calotropis procera Linn. American-Eurasian J Agric Environ Sci. 2008;4:550–3. [Google Scholar]
- 2.Sharma AK, Kharb R, Kaur R. Pharmacognostical aspects of Calotropis procera (Ait.) R. Br. Int J Pharm Bio Sci. 2011;2:480–8. [Google Scholar]
- 3.Sharma R, Thakur GS, Sanodiya BS, Savita A, Pandey M, Sharma A, et al. Therapeutic potential of Calotropis procera: A giant milkweed. ISOR J Pharm Bio Sci. 2012;4:42–57. [Google Scholar]
- 4.Meena AK, Yadav A, Rao MM. Ayurvedic uses and pharmacological activities of Calotropis procera Linn. Asian J Tradit Med. 2011;6:45–53. [Google Scholar]
- 5.Misra MK, Mohanty MK, Das PK. Studies on the method – Ethnobotany of Calotropis gigantea and C. procera. Anc Sci Life. 1993;13:40–56. [PMC free article] [PubMed] [Google Scholar]
- 6.Rice AC, Shapiro SM. A new animal model of hemolytic hyperbilirubinemia-induced bilirubin encephalopathy (kernicterus) Pediatr Res. 2008;64:265–9. doi: 10.1203/PDR.0b013e31817d9be0. [DOI] [PubMed] [Google Scholar]
- 7.Pandit A, Sachdev T, Bafna P. Drug-induced hepatotoxicity: A Review. J Appl Pharm Sci. 2012;2:233–43. [Google Scholar]
- 8.Deshmukh PT, Fernandes J, Atul A, Toppo E. Wound healing activity of Calotropis gigantea root bark in rats. J Ethnopharmacol. 2009;125:178–81. doi: 10.1016/j.jep.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 9.Akkol EK, Süntar I, Erdogan TF, Keles H, Gonenç TM, Kivçak B. Wound healing and anti-inflammatory properties of Ranunculus pedatus and Ranunculus constantinapolitanus: A comparative study. J Ethnopharmacol. 2012;139:478–84. doi: 10.1016/j.jep.2011.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Raju S, Rao MU, Reddy SK, Ramya G, Kumar VG. Effect of Benzoin resin on the serum bilirubin levels in temporary jaundice induced by Phenylhydrazine: A preliminary study. Asian J Pharm Res Healthc. 2011;3:68–71. [Google Scholar]
- 11.Usmani S, Kushwaha P. Hepatoprotective activity of extracts of leaves of Calotropis gigantean. Asian J Pharm Clin Res. 2010;3:195–6. [Google Scholar]
- 12.Khairnare RM. Nashik: Pune University; 2011. Immunomodulatory and wound healing activity of aqueous extract of Terminalia tomentosa barks in laboratory animals [Dissertation]. MGV's Pharm Colg; p. 2. [Google Scholar]
- 13.Manivannan E, Rajaram S, Kothari R, Atul B, Jayakar B. Effect of Calotropis procera Linn against paracetamol induced hepatotoxicity in rats. Int J Res Pharm Biomed Sci. 2011;2:701–3. [Google Scholar]
- 14.Jendrassik L, Grof P. Colorimetric Method of Determination of bilirubin. Biochem Z. 1938 297-81-2. [Google Scholar]
- 15.Kale SR, Kale RR. 16th ed. Pune: Nirali Prakashan; 2006. Practical Human Anatomy and Physiology; pp. 5–16. [Google Scholar]
- 16.Reitman S, Frankel S. In vitro determination of transaminase activity in serum. Am J Clin Pathol. 1957;28:56–60. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Youth RA, Simmerman SJ, Newell R, King RA. Ketamine anesthesia for rats. Physiol Behav. 1973;10:633–6. doi: 10.1016/0031-9384(73)90236-9. [DOI] [PubMed] [Google Scholar]
- 18.Lee KH. Studies on the mechanism of action of salicylates 3. Effect of vitamin A on the wound healing retardation action of aspirin. J Pharm Sci. 1968;57:1238–40. doi: 10.1002/jps.2600570736. [DOI] [PubMed] [Google Scholar]
- 19.Lee KH, Tong TG. Mechanism of action of retinyl compounds on wound healing. II. Effect of active retinyl derivatives on granuloma formation. J Pharm Sci. 1970;59:1195–7. doi: 10.1002/jps.2600590836. [DOI] [PubMed] [Google Scholar]
- 20.Morton JJ, Malone MH. Evaluation of vulneray activity by an open wound procedure in rats. Arch Int Pharmacodyn Ther. 1972;196:117–26. [PubMed] [Google Scholar]
- 21.Fareed KN, Woode E, Ebenzer O, Christopher L. Bilirubin lowering potential of Annona muricata (Linn.) in temporary jaundiced rats. Am J Pharmacol Toxicol. 2012;7:33–40. [Google Scholar]
- 22.Hemamalini K, Krishna RV, Vasireddy U, Bhargav A. Hepatoprotective activity of Tabebuia rosea and Solanum pubescens against paracetamol induced hepatotoxicity in rats. Asian J Pharm Clin Res. 2012;5:153–6. [Google Scholar]
- 23.Heard KJ. Acetylcysteine for acetaminophen poisoning. N Engl J Med. 2008;359:285–392. doi: 10.1056/NEJMct0708278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraschini F, Demartini G, Esposti D. Pharmacology of Silymarin. Clin Drug Invest. 2002;22:51–6. [Google Scholar]
- 25.Mahajan RT, Chaudhari GM. A novel approach towards phytosomal flavonoids. Pharm Sci Monit. 2012;3:2079–105. [Google Scholar]
- 26.Dammeier J, Beer HD, Brauchle M, Werner S. Dexamethasone is a novel potent inducer of connective tissue growth factor expression. Implications for glucocorticoid therapy. J Biol Chem. 1998;273:18185–90. doi: 10.1074/jbc.273.29.18185. [DOI] [PubMed] [Google Scholar]
- 27.Shilpa K, Rajendra Y, Sanjeevkumar A, Vinaykumar D, Vinodkumar R, Gnananath K. Evaluation of wound healing potential in Crinium defixum ker gawl bulbs. Asian J Pharm Clin Res. 2013;6:61–3. [Google Scholar]


