Abstract
Objective:
The objective was to investigate the neuroprotective role of Beta vulgaris in Parkinson's disease (PD).
Materials and Methods:
PD was induced by administration of reserpine (5 mg/kg/day, i.p for 5 consecutive days), haloperidol (1 mg/kg, i.p.), and tacrine (2.5 mg/kg, i.p.) in experimental animals. The symptoms of PD such as tremors, akinesia, rigidity, catalepsy, and vacuous chewing movements (VCMs) were evaluated. Foot shock-induced aggression (FSIA) model was used to confirm anti-parkinsonian activity. The methanolic extract of Beta vulgaris (MEBV) was administered at doses of 100, 200, and 300 mg/kg, p.o. The combination of L-dopa and carbidopa was used as a standard drug. Behavioral studies such as locomotor activity and grip strength were determined, and oxidative stress was evaluated in FSIA model in rat brain.
Results:
Pretreatment with MEBV (200 and 300 mg/kg) significantly reduced the intensity of muscular rigidity, duration of catalepsy, akinesia, the number of tremors, VCMs, and increase fighting behavior. The locomotor activity and grip strength were significantly increased by MEBV. In FSIA, the biochemical analysis of brain revealed the increased level of lipid peroxidation (LPO) and decreased levels of superoxide dismutase (SOD) and catalase (CAT). MEBV significantly reduced LPO level and restored the defensive antioxidant enzymes SOD and CAT in rat brain.
Conclusions:
The results indicated the protective role of B. vulgaris against PD. The mechanism of protection may be due to augmentation of cellular antioxidants.
KEY WORDS: Beta vulgaris, Parkinson's disease, reserpine, tacrine
Introduction
Parkinson's disease (PD) is a chronic progressive neurodegenerative disorder of the central nervous system (CNS).[1] Reserpine induces depletion of central catecholamines stores which causes Parkinson's like symptoms in rats.[2,3] Haloperidol, an antipsychotic drug, which blocks central dopamine receptor in the striatum, produces a behavioral state (catalepsy) in experimental animals.[4] Foot-shock induced aggression (FSIA) model is used for evaluation of centrally acting drugs.[5] The anticholinesterase, tacrine is used therapeutically to improve memory function in patients with Alzheimer's disease. One of the motor effects produced by cholinomimetics is tremulous jaw movements also known as vacuous chewing moments (VCM).[6]
Traditional medicines play vital roles to cover basic health in developing countries. Beta vulgaris (Chenopodiaceae) commonly known as beetroot. In traditional medicine, it has been mentioned that B. vulgaris has the neuroprotective potential to treat several CNS related diseases. The literature survey indicates that B. vulgaris possesses pharmacological actions such as antidepressant, antioxidant, anticonvulsant, cerebroprotective, and hepatoprotective activities.[7] However, in spite of reported antioxidant and neuroprotective activities, no major investigative reports found pertaining to its neuroprotective potential. Thus, the present study was designed to check the utility of B. vulgaris in the management of PD.
Materials and Methods
Experimental Animals
Wistar rats of either sex, weighing 230–250 g were obtained from National Toxicological Centre, Pune, India were used for the experiment. Animals were housed in colony cages and maintained under the standard laboratory environmental conditions; temperature 25 ± 2°C, 12 h light: 12 h dark cycle and 50 ± 5% relative humidity with free access to food and water ad libitum. Animals were acclimatized to laboratory conditions before the test. Each group consisted of six (n = 6) animals. All the experiments were carried out during the light period (08:00–16:00 h). The studies were carried out in accordance with the guidelines given by Committee for the Purpose of Control and Supervision of Experiments on Animals, New Delhi (India). The Institutional Animal Ethical Committee approved the protocol of the study (IAEC/2013/04).
Drugs and Chemicals
Levodopa and carbidopa (GlaxoSmithKline Pharmaceuticals, Nashik, India); thiobarbituric acid (TBA) (Research Lab, Fine Chem, Mumbai); nitrobluetetrazolium chloride (NBT) (Research Lab, Fine Chem, Mumbai); 5,5’-dithiobis-2-nitro benzoic acid (Alfa Aesar, A Johnson Mathey Company). All the chemicals used were of analytical grade and purchased from standard manufacturers.
Collection and Authentication of Plant Material
The leaves of B. vulgaris were collected in the month of August from the local area of Nashik and authenticated at the Botanical Survey of India, Pune where a voucher specimen was deposited (Voucher No. ABK-1).
Extract Preparation
The leaves were shade dried and reduced to coarse powder. The powdered leaves of B. vulgaris were defatted with petroleum ether (60–80°C) under soxhlet extraction. The defatted marc was air-dried and put for exclusive extraction under soxhlet using methanol. The extract was then filtered and evaporated to dryness under reduced pressure (yield 6.4% w/w). The suspension of methanolic extract of Beta vulgaris (MEBV) was prepared in 0.5% carboxymethyl cellulose (CMC) in distilled water. The extract was administered in doses of 100, 200, and 300 mg/kg (p.o.). Vehicle group was given only vehicle (0.5% CMC in distilled water) in a volume equivalent to that of the plant extract.
Phytochemical Screening of Beta vulgaris
Phytochemical screening of the MEBV for the presence of flavonoids, glycosides, alkaloids, saponins, and tannins were carried out in accordance with procedures previously described.[8]
Acute Toxicity Study in Mice (Acute Toxicity Study Determination)
Acute toxicity study (LD50) was carried out according to the Organization for Economic Co-operation and Development (OECD) guidelines (OECD, 2001). The MEBV was administered with the minimum dose to individual animals and observed for next 24 h. If no mortality was seen then, the dose was increased with 50, 100, 200, 500, 800, 1000, 1500, and 2000 mg/kg, p.o.[9,10]
Sub Acute Toxicity Study in Rats
The animals were divided into two groups (n = 10). The first group serves as a control, received 0.5% CMC in distilled water (5 ml/kg, p.o.); the second group received MEBV 2000 mg/kg for 2 weeks. All rats were observed daily for physiological and behavioral changes. The parameters observed were hyperactivity, grooming, convulsions, and loss of righting reflex, respiration, salivation, urination, and defecation. All the survived animals under experimental condition were observed for biochemical and hematological parameters.[9,10] The blood samples were collected by retro-orbital puncture, using capillary tubes for hematological and biochemical studies. The parameters included: Red blood cell count, white blood cell count, hemoglobin. For biochemical analysis, blood was centrifuged at 2000–3000 rpm for 10 min to obtain serum, for determination of glucose, aspartate aminotransferase, alanine aminotransferase, total cholesterol, triglycerides, high-density lipoprotein, and very low-density lipoprotein.
Experimental Design
Animals were randomly assigned into six groups; each group consisted of six animals (n = 6). Group I-vehicle. Group II-reserpine (5 mg/kg, i.p., for 5 consecutive days), or haloperidol (1 mg/kg, i.p.) or tacrine (2.5 mg/kg, i.p.) or foot-shock (0.8 mA for 5 s followed by 5 s shock free interval for 3 min). PD was induced by administration of reserpine, haloperidol, and tacrine in animals. Further, FSIA model was used to support the anti-Parkinsonian activity of test drugs. Group III-MEBV (100 mg/kg, p.o.). Group IV-MEBV (200 mg/kg, p.o.). Group V-MEBV (300 mg/kg, p.o.). Group VI-L-dopa + carbidopa (30 mg/kg, p.o.) were used for the treatment of Parkinsons symptoms.
Reserpine-induced Parkinson Disease
Reserpine, the antihypertensive agent, induces depletion of central catecholamines stores. Injection of reserpine in rats causes hypokinesia, rigidity, tremors, and immobility.[3] The animals were treated with reserpine (5 mg/kg, i.p., for 5 consecutive days). After 24 h of last treatment animals were tested for induction of severity of tremors by giving the scores as follows: No tremors-0, occasional twitches-1, moderate or intermittent twitches-2, continues tremors-3. The number of tremors was counted for 5 min. If animals were not showing tremors then 0 score was given, if animals showed 1 or 2 tremors then 1 score was given, animals showed 3 or 5 tremors in 5 min then 2 score was given and for 6 or more tremors, score was given 3. Akinesia was determined by holding the tail of animal and putting the front paws on the platform and let the animal to walk while holding (number of steps taken with forelimbs of animal were counted for 3 min) and muscular rigidity was determined by suspending the animal with forelimbs on middle part of horizontal glass rod (0.5 cm diameter) at the height of 25 cm above the table top and time to fall on the bottom surface was measured. The cut-off was kept for 1 min. The animals were treated with MEBV (100, 200 and 300 mg/kg, p.o., respectively), or L-dopa-carbidopa (30 mg/kg, p.o.) 60 min before administration of reserpine for 5 consecutive days.[3]
Locomotor Activity
After evaluation of tremors, akinesia, and muscular rigidity, locomotor activity was evaluated by using actophotometer. The apparatus consist of photoelectric cells, which are connected in circuit with a counter. When the beam of light falling on the photocell is cut off by the animal, a count was recorded for 10 min.[11]
Grip Strength
Subsequently latency to grip strength was evaluated by using rotarod apparatus. The rod is 75 cm in length and 3 cm in diameter, divided into six sections by plastic discs adjusted at the 50 cm above the table top. Each animal was placed on rotating rod (20 rpm) and latency to fall down was recorded in different groups of animals.[2]
Haloperidol-induced Catalepsy
This model may provide novel approaches for the development of drugs, which can reverse cataleptic state in patients with PD and can reverse or prevent the extrapyramidal side effects associated with antipsychotic treatment.[4,12] After 30 min of administration of haloperidol (1 mg/kg, i.p.), the duration of catalepsy was measured for 5 min at an interval of 30, 60, 90, and 120 min. Duration of catalepsy was determined by placing an animal on the horizontal metal bar at a height of 6 cm in such a way that the fore-limbs of the animal should be on the horizontal bar while the hind-limb touches the surface.[13] The animals were treated with MEBV (100, 200, and 300 mg/kg, p.o. respectively), or L-dopa-carbidopa (30 mg/kg, p.o.) 60 min prior administration of haloperidol.[12]
Tacrine-induced Vacuous-chewing Movements
The VCMs are characterized by rapid, vertical deflections of the lower jaw that resemble chewing but are not directed at any stimulus.[6] The VCMs share some characteristics with human Parkinsonian symptoms. Tacrine-induced tremulous jaw movements can be suppressed by anti-Parkinsonian agents. Tacrine (2.5 mg/kg, i.p.) was administered to the animals and after 30 min animals were observed for VCMs or jaw movements for 10 min at the interval of 0, 30, 60, 90, and 120 min. The test groups were administered with MEBV (100, 200, and 300 mg/kg, p.o., respectively) or standard drug 60 min before administration of tacrine.[14]
Foot-shock Induced Aggression
In this method, fighting behavior of rats, which become extremely vicious after foot-shock was determined. The method is used for evaluation of centrally acting drugs.[5] The animals were placed (in pairs) in a box with grid floor consisting of steel rods. The total numbers of fights were recorded for each pair during a 0.8 mA current was delivered for 5 s followed by 5 s intermission for 3 min. The MEBV (100, 200, and 300 mg/kg, p.o., respectively) or standard drug were administered 60 min before delivery of foot-shock for 7 days and the number of fights were recorded on day 7th.
Dissection and Homogenization
On the 7th day, immediately after behavioral assessments the animals were sacrificed by decapitation. The brain was removed, rinsed with isotonic saline, and weighted. A 10% (W/V) tissue homogenate was prepared in 0.1 M phosphate buffer (pH 7.4). The postnuclear fraction for catalase (CAT) assay was obtained by centrifugation (Remi - C-30, Remi Industries Ltd. Mumbai, India) of the homogenate at 1000 g for 20 min at 4°C; for other enzyme assays, centrifugation was at 12000 g for 60 min at 4°C. An Elico biospectrophotometer-BL200 was used for subsequent assays.[15]
Catalase Activity
Catalase activity was assessed by the method of Luck, where the breakdown of H2O2 was measured at 240 nm. The assay mixture consisted of 3 ml of H2O2 phosphate buffer (0.0125 M H2O2), and 0.05 ml of supernatant of brain homogenate and the change in absorbance was measured at 240 nm. The enzyme activity was calculated using millimolar extinction coefficient of H2O2 (0.071). The results were expressed as micromole of H2O2 decomposed per minute per milligram of protein.[16]
Superoxide Dismutase Activity
Superoxide dismutase (SOD) activity was assayed according to the method of Kono, where the reduction of NBT was inhibited by SOD and measured at 560 nm. The reaction was initiated by addition of hydroxylamine hydrochloride to the reaction mixture containing NBT and postnuclear fraction of brain homogenate. Results were expressed as percentage inhibition of reduction of NBT.[17]
Lipid Peroxidation Assay
Quantitative estimation of lipid peroxidation (LPO) was carried out according to the method of Wills. The amount of malondialdehyde (MDA) formed was measured by reaction with TBA at 532 nm. The results were expressed as nmoles of MDA/mg protein using molar extinction coefficient of the chromophore (1.56 × 105 M-1 cm-1).[18]
Statistical Analysis
The results were expressed as mean ± standard error of the mean data were subjected to one-way analysis of variance (ANOVA) followed by Dunnett's test.
Results
Phytochemical Screening
The phytochemical screening of MEBV revealed the presence of glycosides, flavonoids, tannins, and saponins.
Acute Toxicity Study
The results of the LD50 indicated that MEBV administration by oral route with the dose of 2.0 g/kg did not produce any sign of toxicity or death, suggesting LD50 above 2.0 g/kg by oral route.
Sub Acute Toxicity Study
The results of the sub-LD50 indicated that oral administration of MEBV did not induce significant alterations in almost all biochemical and hematological parameters. MEBV was found to be safe in sub LD50 at high dose (2 g/kg) in experimental animals. Chronic toxicity, mutagenicity, and carcinogenicity studies are further necessary to support the safe and sound use of this plant.
Reserpine Antagonism
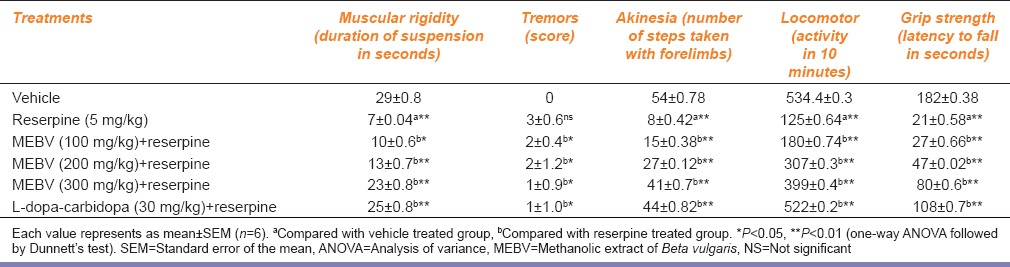
Treatment with reserpine for 5 days produces muscular rigidity, tremors, and akinesia in animals. The intensity of muscular rigidity, numbers of tremors, and akinesia were significantly decreased by MEBV at 100, 200, and 300 mg/kg (P < 0.05, P < 0.01, respectively) as compared with reserpine treated group. L-dopa-carbidopa treated group also showed a significant reversal of reserpine induced Parkinsons symptoms (P < 0.01).
The locomotor activity and grip strength were significantly decreased by reserpine as compared to the vehicle group. The locomotor activity and grip strength was significantly (P < 0.01) restored by MEBV at 100, 200, and 300 mg/kg and L-dopa-carbidopa [Table 1].
Table 1.
Effect of Beta vulgaris on muscular rigidity, tremors, akinesia, locomotor activity, and grip strength in reserpine-induced motor defects
Haloperidol-induced Catalepsy
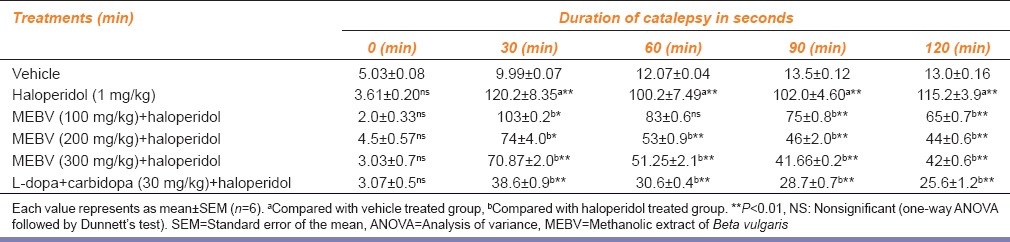
Pretreatment with MEBV 200 and 300 mg/kg significantly reduced the duration of catalepsy induced by haloperidol (P < 0.01 respectively) as compared to haloperidol treated group. Duration of catalepsy was also significantly decreased (P < 0.01) by L-dopa-carbidopa [Table 2].
Table 2.
Effect of Beta vulgaris on duration of haloperidol-induced catalepsy
Tacrine-induced Vacuous Chewing Movements
Tacrine produced VCMs in experimental animals. Pretreatment with MEBV 200 and 300 mg/kg significantly reduced the number of VCMs (P < 0.01) as compared to tacrine treated group. The number of VCMs was also significantly reduced (P < 0.01) in L-dopa-carbidopa treated group [Figure 1].
Figure 1.
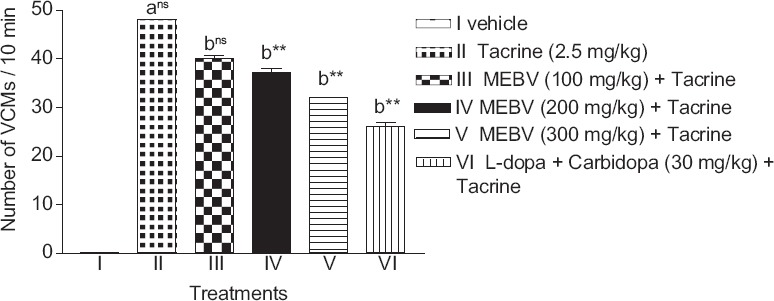
Effect of Beta vulgaris on tacrine-induced vacuous chewing movements. Each column represents as mean ± standard error of the mean (n = 6). (a) compared with vehicle treated group. (b) compared with tacrine treated group **P < 0.01, ns-nonsignificant (one-way analysis of variance followed by Dunnett's test)
Foot-shock Induced Aggression
Administration of MEBV (300 mg/kg) showed significantly (P < 0.01) increase in the number of fights in FSIA, as compared with the vehicle group. L-dopa-carbidopa treated group also showed an increase in the number of fights [Figure 2].
Figure 2.
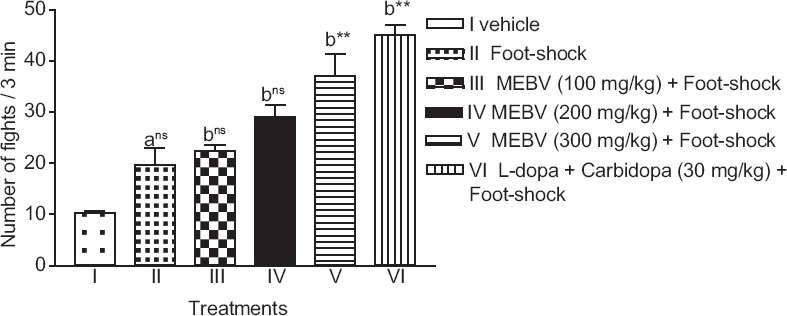
Effect of Beta vulgaris on the number of fights in foot shock-induced aggression model. Each column represents as mean ± standard error of the mean (n = 6). (a) compared with vehicle treated group. (b) compared with foot-shock group **P < 0.01, ns-nonsignificant (one-way analysis of variance followed by Dunnett's test)
Biochemical Estimation
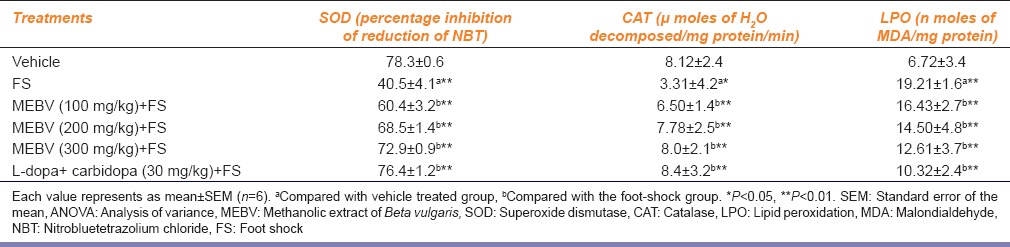
The SOD and CAT levels were significantly decreased in animals delivered foot-shock for 7 days as compared to the vehicle group. The LPO level was significantly increased after foot-shock. MEBV significantly (P < 0.01) increased SOD and CAT levels with concomitant decrease in LPO level in rat brain (P < 0.01) [Table 3].
Table 3.
Effect of Beta vulgaris on brain SOD, CAT and LPO levels
Discussion
The present study demonstrates anti-Parkinson activity of B. vulgaris in different animal models. The symptoms of Parkinsons disease were induced by administration of reserpine, haloperidol, tacrine, and foot-shock induced aggression in experimental animals.
Reserpine induces muscular rigidity, tremors, and akinesia by depletion of central catecholamine storage.[3] MEBV (200 and 300 mg/kg, p.o.) and the combination of L-dopa and carbidopa produced a significant reduction in these symptoms in rats. B. vulgaris was also able to increase the locomotor activity and grip strength in rats. Reserpine induced motor defect was significantly reversed by B. vulgaris. Amelioration of symptoms of reserpine by B. vulgaris demonstrates anti-Parkinsons activity.
In the present study, animals were treated with haloperidol (1 mg/kg) showed cataleptic behavior similar to the symptoms of PD. Haloperidol, an antipsychotic drug, which blocks central dopamine receptor in the striatum, produces a behavioral state in animals such as mice and rats in which they fail to correct externally imposed postures. This is referred to as catalepsy.[18] Haloperidol-induced catalepsy is one of the animal models to test the extrapyramidal side effects of antipsychotic drugs. The haloperidol, (a nonselective D2 dopamine antagonist) induced catalepsy is primarily due to the blockade of dopamine receptors in the striatum. The agent, increasing dopamine transmission inhibits neuroleptic induced catalepsy.[19] The MEBV (200 and 300 mg/kg, p.o.) showed a significant reduction in the duration of catalepsy demonstrating antiparkinson activity. The inhibition of catalepsy indicates the ability of the drug to potentiate dopaminergic transmission in the striatum.
The VCMs are one of the common symptoms of anticholinesterase which was increased in animals treated with tacrine. The anticholinesterase, tacrine is used therapeutically to improve memory function in patients with early and late onset of Alzheimer's disease. However, it can cause parkinsonian side-effects such as bradykinesia, rigidity, and tremors. One of the motor effects produced by cholinomimetics is tremulous jaw movements (also known as “VCM” or “purposeless” chewing).[6] It was found that the number of VCMs was significantly reduced in animals treated with MEBV (200 and 300 mg/kg, p.o.). The results indicated that the investigational plant has the ability to balance the level between dopamine and acetylcholine by providing the dopaminergic activity in vivo. The numbers of VCMs also decreased in animals treated with a combination of L-dopa and carbidopa as a standard treatment.
In the present investigation, an attempt has been made to study the effect of B. vulgaris on dopaminergic transmission. The FSIA model was used to evaluate the anti-Parkinson activity of B. vulgaris. Central monoaminergic neurons appear to play an essential role in modulation of aggressive behavior. Central D2 dopamine receptors are involved in the modulation of foot-shock aggression in mice. Brain dopamine level has been reported to increase in FSIA.[20] The 0.8 mA current was given to the animals consecutively for 7 days. Pretreatment with B. vulgaris (300 mg/kg) for 7 days significantly increased the number of fighting attacks, therefore suggesting a possible dopaminergic activity of B. vulgaris in FSIA. Fighting behavior also significantly increased in animals treated with L-dopa and carbidopa.
Oxidative stress is one of the major reasons for nerve damage in many neurodegenerative disorders.[21] In Parkinsons disease, oxidation of dopamine by monoamine oxidase-B, and aldehyde dehydrogenase generates hydroxyl free radicals in the presence of ferrous ions (basal ganglia are rich in iron).[22] Hence, to find out oxidative stress at various levels the defensive antioxidant enzymes in rat brain were measured. SOD is the most important enzymes in the antioxidant defense system of the body. The major function of SOD is to catalyze the conversion of superoxide anion radicals to H2O2 and hence reduces the toxic effects due to this radical or other free radicals derived from secondary reactions. CAT, which is present virtually in all mammalian cells, is responsible for the removal of H2O2. LPO is the measure of the excessive oxidation of the lipids in the body indicating increased superoxide production. Therefore, the oxidative stress indices were estimated in rat brain such as SOD, CAT, and LPO. There was a significant reduction in antioxidant defensive enzymes SOD and CAT and increase in LPO content observed in FSIA model. The levels of SOD, CAT, and LPO were significantly restored by treatment with MEBV. B. vulgaris reduced oxidative stress in foot-shock-induced rat brain suggesting the antioxidant activity of the plant. The neuroprotective activity of the plant may be due to its antioxidant property, which reinforces antiparkinson activity of B. vulgaris.
The above behavioral and biochemical results suggest that B. vulgaris has the ability to improve symptoms of Parkinsonism, in part, by the restoring the level of dopamine, and by the regulation of the antioxidant system. Thus, antioxidant and neuroprotective activities may be responsible for antiparkinsons effect. Hence, B. vulgaris may be useful as a neuroprotective agent in the treatment of PD. The above observed beneficial effects of B. vulgaris may be attributed to diverse chemical components namely flavonoids, glycosides, saponins, and tannins.
Conclusions
B. vulgaris significantly reduced the symptoms of PD may be due to antioxidant and neuroprotective activities or increase in the level of brain dopamine similar to L-dopa and carbidopa. Thus, B. vulgaris may have therapeutic potential in the treatment of PD.
Further, it is necessary to estimate the brain dopamine level and isolate the individual constituents responsible for neuroprotective potential.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Rooke ED, Vesterinen HM, Sena ES, Egan KJ, Macleod MR. Dopamine agonists in animal models of Parkinson's disease: A systematic review and meta-analysis. Parkinsonism Relat Disord. 2011;17:313–20. doi: 10.1016/j.parkreldis.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 2.Vogel HG. 2nd ed. New York: Springer Publication; 2002. Drug Discovery and Evaluation: Pharmacological Assays; p. 577. [Google Scholar]
- 3.Colpaert FC. Pharmacological characteristics of tremor, rigidity and hypokinesia induced by reserpine in rat. Neuropharmacology. 1987;26:1431–40. doi: 10.1016/0028-3908(87)90110-9. [DOI] [PubMed] [Google Scholar]
- 4.Ferré S, Guix T, Prat G, Jane F, Casas M. Is experimental catalepsy properly measured? Pharmacol Biochem Behav. 1990;35:753–7. doi: 10.1016/0091-3057(90)90354-k. [DOI] [PubMed] [Google Scholar]
- 5.Slattery DA, Markou A, Cryan JF. Evaluation of reward processes in an animal model of depression. Psychopharmacology (Berl) 2007;190:555–68. doi: 10.1007/s00213-006-0630-x. [DOI] [PubMed] [Google Scholar]
- 6.Mayorga AJ, Carriero DL, Cousins MS, Gianutsos G, Salamone JD. Tremulous jaw movements produced by acute tacrine administration: Possible relation to parkinsonian side effects. Pharmacol Biochem Behav. 1997;56:273–9. doi: 10.1016/s0091-3057(96)00225-0. [DOI] [PubMed] [Google Scholar]
- 7.Asolkar LV, Kakkar KK, Chakre OJ. New Delhi: BS Publisher; 1992. Second Supplement to Glossary to Indian Medicinal Plants with Active Principals, Part I (A-K) pp. 945–50. [Google Scholar]
- 8.Kokate CK, Purohit AP, Gokhale SB. 47th ed. Pune: Nirali Prakashan; 2011. Pharmacognosy; pp. 615–6.19. [Google Scholar]
- 9.France: OECD; 2001. Organization Economic for Cooperation and Development (OECD). Guidelines for testing of chemicals. Acute Oral Toxicity – Up and Down Procedure; pp. 1–26. [Google Scholar]
- 10.Curtis DK, John BW, Casarett D. 1st ed. New York: McGraw-Hills Publication; 2002. Essentials of Toxicology; p. 1103. [Google Scholar]
- 11.Kulkarni SK. 3rd ed. New Delhi: Vallabh Prakashan; 2005. Experimental pharmacology; pp. 117–8. [Google Scholar]
- 12.Gyertyán I, Sághy K. The selective dopamine D3 receptor antagonists, SB 277011 - A and S 33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol. 2007;572:171–4. doi: 10.1016/j.ejphar.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 13.Cousins MS, Carriero DL, Salamone JD. Tremulous jaw movements induced by the acetylcholinesterase inhibitor tacrine: Effects of antiparkinsonian drugs. Eur J Pharmacol. 1997;322:137–45. doi: 10.1016/s0014-2999(97)00008-3. [DOI] [PubMed] [Google Scholar]
- 14.Naidu PS, Singh A, Kulkarni SK. Effect of Withania somnifera root extract on haloperidol-induced orofacial dyskinesia: Possible mechanisms of action. J Med Food. 2003;6:107–14. doi: 10.1089/109662003322233503. [DOI] [PubMed] [Google Scholar]
- 15.Luck H. Catalase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1971. pp. 885–93. [Google Scholar]
- 16.Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–95. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- 17.Wills ED. Mechanisms of lipid peroxide formation in animal tissues. Biochem J. 1966;99:667–76. doi: 10.1042/bj0990667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott PJ, Close SP, Walsh DM, Hayes AG, Marriott AS. Neuroleptic-induced catalepsy as a model of Parkinson's disease. I. Effect of dopaminergic agents. J Neural Transm Park Dis Dement Sect. 1990;2:79–89. doi: 10.1007/BF02260896. [DOI] [PubMed] [Google Scholar]
- 19.Rang HP, Dale MM, Ritter JM, Flower RJ. 6th ed. New York: Churchill Livingstone; 2007. Pharmacology; pp. 512–517. [Google Scholar]
- 20.Yadav AV, Nade VS. Anti-dopaminergic effect of the methanolic extract of Morus alba L. leaves. Indian J Pharmacol. 2008;40:221–6. doi: 10.4103/0253-7613.44154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nade VS, Shendye NV, Kawale LA, Patil NR, Khatri ML. Protective effect of nebivolol on reserpine-induced neurobehavioral and biochemical alterations in rats. Neurochem Int. 2013;63:316–21. doi: 10.1016/j.neuint.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Tripathi KD. 6th ed. New Delhi: Jaypee Publication; 2009. Essentials of Medical Pharmacology; p. 414. [Google Scholar]


