Abstract
Objectives:
Nerium oleander is traditionally used in various diseases because of its medicinal properties. One of its uses is in musculoskeletal disorder. The aim of the study was to evaluate the skeletal muscle relaxant activity of the aqueous extract of Nerium oleander flowers (AENOF) in albino rats in comparison with diazepam.
Materials and Methods:
A total of 20 Swiss albino rats aged 6–7 weeks, of either sex, weighing about 100–150 g, were taken, and after acute toxicity studies two different doses were selected. The animals were divided into four different groups. The first group was kept as the control (normal saline), second as the standard (diazepam) and the remaining two groups as Test I and Test II, and given different doses of the AENOF. Skeletal muscle relaxant activity (motor coordination) on Rotarod and locomotor activity on photoactometer was performed. Statistical analysis was carried out by using analysis of variance, followed by Dunnett's multiple comparison tests.
Results:
The result from the Actophotometer test and Rotarod test showed that the extract of AENOF significantly reduced (P < 0.05) the motor coordination of the tested animals.
Conclusions:
Our data indicates that AENOF possesses skeletal muscle relaxant activities.
KEY WORDS: Actophotometer, muscle relaxant, Nerium oleander, rotarod, soxhlet apparatus
Introduction
Nerium oleander, ganneru in Telugu is an evergreen shrub or small tree belonging to Apocynaceae family potentially toxic in all its parts. It is so widely cultivated that no precise region of origin has been identified though Southwest Asia has been suggested. According to the literature survey, it is a valuable medicinal plant used in all systems of medicine. It is a herb with different colored flowers and is suited for the dry locality.[1] Oleander is one of the most poisonous of commonly grown garden plants. Oleander grows to 2–6 m (6.6–19.7 ft) tall, with erect stems that splay outward as they mature. The leaves are in pairs or whorls of three, thick and leathery, dark-green, narrow lanceolate, 5–21 cm (2.0–8.3 inch) long and 1–3.5 cm (0.39–1.38 inch) broad, and with an entire margin. The flowers grow in clusters at the end of each branch; they are white, pink to red,[2] 2.5–5 cm (0.98–1.97 inch) diameter with a deeply 5-lobed fringed corolla round the central corolla tube. Oleander is the official flower of the city of Hiroshima, having been the first to bloom following the atomic bombing of the city in 1945.[3] The flowers are salver-shaped pink or white without any fragrance.[4] Parts of this plant are used for the treatment of various human ailments in the traditional medicine system as a folk remedy for a wide variety of conditions, including dermatitis, abscesses, eczema, psoriasis, sores, warts, corns, ringworm, scabies, herpes, skin cancer, asthma, dysmenorrheal, epilepsy, malaria, abortifacient, heart tonics, leprosy, and tumors.[5]
The leaf is used as a cardiotonic, diuretic, antibacterial in cutaneous eruptions and is also effective against snake-bites; the root is used for curing different types of cancers, ulcers, and leprosy.[6] The root-bark is used specifically against skin infection especially ringworm and the aqueous extracts of the leaves, branches, roots, and flowers are toxic to certain insects.[6] The phytochemicals identified in various parts of the plant include mainly cardiotonic glycosides, terpenoids, and steroids.[5] One study revealed that the hot aqueous extract of N. oleander leaves (anvirzel) inhibits fibroblast growth factor-2 and also has anti-tumor activity.[7] The leaf extract has antibacterial and sedative-hypnotic activities and also affects the behavioral pattern in mice.[8] The leaves and the flowers are cardiotonic, diaphoretic, diuretic and emetic, and expectorant. It has also being reported to have antibacterial and anti-diabetic activity.[9,10] The dried and fresh flowers of the plant have been reported to exhibit potent anti-inflammatory activity.[11] In our previous study, we found out that the aqueous extract of the leaves of N. oleander had reduced locomotor activity in experimental animals like albino rats.[12] In this study, we would like to elucidate whether the flowers of N. oleander plant had reduced locomotor activity as that of aqueous extract of the leaves of N. oleander.
Materials and Methods
Collection of the Nerium Oleander Flowers
The study was conducted during the period January 2014–February 2014. The flowers of N. oleander were obtained from a local garden in Khammam. The identification and authentication of the leaves were done at the Department of Botany, Government Degree College. The flowers were shade dried and powdered. The aqueous extract of flowers was prepared using the soxhlet apparatus in the Department of Pharmacology. The extract was dried under vacuum, stored at room temperature, and protected from direct sunlight. The extractive value of Nerium oleander flower (AENOF) was 15.8 GW/W.
Animals
A total of 20 Swiss albino rats aged 8–10 weeks of either sex weighing about 100–150 g were obtained from the Central Animal House. The animals were fed standard pellet diet and with water ad libitum, and were maintained under standard conditions of temperature, humidity, and light (12 h light/12 h dark cycle). The experiment complied with the guidelines for animal experimentation of our laboratory and was approved by the Institutional Animal Ethics Committee; registration number 285/CPCSEA. The guidelines for the investigation of experiments in conscious animals were followed in all tests.
Drugs and Chemicals
Diazepam (Lupin Laboratories Ltd., India), 10 mg/kg and Normal saline (0.9% NaCl solution) were administered in a volume of 10 mL/kg. The extracts were suspended in distilled water and subjected to muscle relaxant activity using the Rotarod apparatus and Actophotometer. The extracts were administered orally (p.o.,) in a volume of 10 mL/kg of body weight, in doses of 100 mg and 200 mg/kg.
Photochemical Characterization
The different extracts were subjected to general phytochemical analysis for the presence of carbohydrates, proteins, amino acids, tannins, phenolics, flavonoids, alkaloids, anthraquinones, glycosides, saponins, and steroidal nucleus using the standard methods.[13,14]
Acute Toxicity
A total of 35 mice were randomly allotted to one control and six treatment groups. The animals were fasted overnight, prior to the experimental procedure. The method of up-and-down staircase was used to determine the dose.[15] The procedure was followed as per the Organization for Economic Co-operation and Development 423 guidelines. The extract in each case was administered orally in three doses: 0.5 g/kg, 1.0 g/kg, and 2 g/kg. The animals were observed for 24 h for signs of toxicity and mortality. In the acute toxicity tests, AENOF extract treated animals exhibited no alarming signs of toxicity.[16]
Selection of Dose for Pharmacological Screening
The aqueous extract of AENOF was found to be nontoxic up to a dose of 2000 mg/kg and did not cause death, therefore, it was considered to be safe. Hence, one-tenth of this dose, that is, 200 mg/kg body weight and half of the one-tenth dose, that is, 100 mg/kg, were used for the elucidation of muscle relaxant activity.
Experimental Design
The animals were divided into four groups of five rats each. The drugs were administered as shown below:
Group I - Control rats (normal saline 10 mL/kg)
Group II - standard (diazepam 10 mg/kg)
Group III - Nerium oleander flowers 100 mg/kg
Group IV - Nerium oleander flowers 200 mg/kg.
The evaluation of skeletal muscle relaxant activity (motor coordination).
The rats were divided into four groups consisting of five animals each. Group I served as the control, which received normal saline 10 mL/kg, Group II received the standard drug diazepam, at a dose of 10 mg/kg, p.o., Group III and IV received the aqueous extract of AENOF orally at a dose of 100 and 200 mg/kg. The animals remained on Rotarod (25 rpm) for 5 min or more after successive trials were included in the study. After the administration of control, standard, and test material, the fall off time from the rotating rod was noted after 30 min. The difference in the fall off time from the rotating rod between the control and the treated rats was taken as an index of muscle relaxation.[17]
Locomotor Activity
The spontaneous locomotor activity was assessed with the help of a photoactometer.[18] Each animal was observed for a period of 5 min in a square closed field arena (30 cm × 30 cm × 30 cm) equipped with six photocells in the outer wall. Interruptions of photocell beams (locomotor activity) were recorded by means of a six digits counter. To see the locomotor activity, the Actophotometer was turned on and each mouse was placed individually in the activity cage for 5 min. The basal activity score for all the animals was noted. Control normal saline, standard diazepam, and two different doses of aqueous extract of AENOF were given orally and after 1-h of re-testing, the activity score for 5 min was observed. The difference in the activity, before and after drug administration, was noted. The percentage decrease in motor activity was calculated.
Central versus Peripheral Skeletal Muscle Relaxant Property
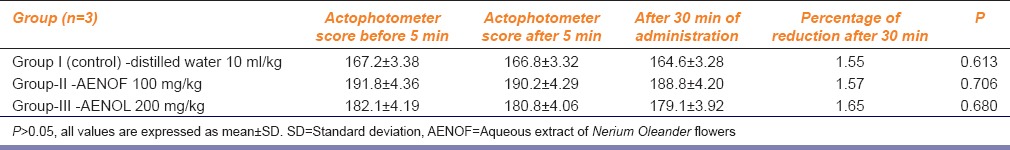
To elucidate exact site of action, three groups with three rats in each was selected and AENOF 100 mg/kg and 200 mg/kg was injected directly into the thigh muscles of the above said two groups and control group received distilled water intramuscularly. The locomotor activity was observed with actophotometer before 5 min and 30 min after injection.
Statistical Analysis
The results were expressed as a mean ± standard deviation. Statistical analysis was carried out by using the analysis of variance followed by Dunnett's multiple comparison tests using a Primer of Biostatistics (Stanton A, Glantz; Primer for Windows. McGraw-Hill, Inc., Version 5.0) (2011). P < 0.05 was considered significant.
Results
Phytochemical Screening
The preliminary phytochemical analysis of the flower extracts of N. oleander showed the presence of carbohydrates, phenols, saponins, tannins, and alkaloids but devoid of steroids. The phytochemical results of N. oleander were in conformity with other studies[19,20] who pointed this species as one of the most poisonous plants, which contain numerous toxic compounds such as alkaloids and triterpenes. All the extracts were stored in a clean glass bottles for further pharmacological studies.
Toxicity Study
A preliminary acute oral toxicity study, the flower extract produced no adverse effects at dose 2000 mg/kg and did not cause any death up to a dose of 3000 mg/kg in mice.
Rotarod Test
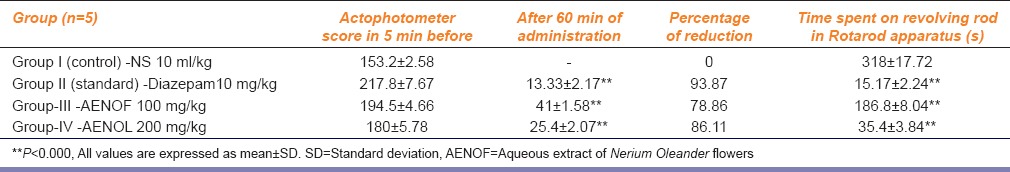
For muscle relaxation, in this test, (AENOF 100 mg/kg and 200 mg/kg) showed highly significant reduction in the time spent by the animals on the revolving rod when compared to the control (P < 0.000). The standard drug (diazepam) also showed a highly significant effect when compared to the control (P < 0.000). However, two different doses of AENOL (100 and 200 mg/kg p.o.,) showed a dose-dependent increase in muscle relaxation, that is, 186.8 ± 8.04** and 35.4 ± 3.84**, respectively, when compared to the control [Table 1]. Maximum muscle relaxation was observed with 200 mg/kg of aqueous extract of AENOF. The result from the Rotarod test showed that the extract significantly reduced the motor coordination of the tested animals.
Table 1.
Effect of AENOF on locomotor activity in actophotometer and muscle coordination on the Rotarod apparatus
Actophotometer
Test for locomotor activity: The percentage of reduction in the locomotor activity with diazepam (10 mg/kg, p.o.,) after 30 min was 93.87, that is, there was a highly significant (P < 0.000) decrease in locomotor activity compared to the control, whereas, two different doses of AENOF (100, and 200 mg/kg, p.o.,) showed a dose-dependent decrease in the locomotor activity, that is, 41 ± 1.58** and 25.4 ± 2.07**, respectively, when compared to the control. Maximum muscle relaxation was observed with 200 mg/kg of aqueous extract of AENOF. The values were highly significant (P < 0.000) [Table 1]. In second experiment, where the drugs were given directly into the muscle, there was no statistically significant decrease in the locomotor activity with two different doses of AENOF (100, and 200 mg/kg, i.m.) that is, 188.8 ± 4.2 and 179.1 ± 3.92, respectively when compared to control group (P > 0.05) [Table 2].
Table 2.
Effect of AENOF on locomotor activity in actophotometer after giving the extract directly into the muscle
Discussion
In recent years, the herbal medicines have been extensively used in various diseases because of their safety profile. N. oleander (family: Apocynaceae) also known as Kenner is one of them and used against various disorders in indigenous system of medicine, especially for arthritis. The leaves and the flowers are cardiotonic, diaphoretic and diuretic, emetic, expectorant, used in the treatment of scabies, and to reduce swellings. It has also being reported to have antibacterial and antidiabetic activities.[21] The major components of it are oleander, neriin, and oleandrin. The bark contains toxic glucosides, rosaginin and nerrin, volatile oil, fixed oil, etc.[11,22] The objective of the present study was to investigate the effect of aqueous extracts of the flowers of N. oleander on muscle relaxant activity in experimental animals like albino rats. Actophotometer is widely used screening method for evaluating the locomotor activity and antianxiety activity in rodents and Rotarod for muscle relaxation. Anxiety is associated with increased muscle tone along with the other symptoms such as restlessness or feeling keyed up or on edge, easily fatigued difficulty in concentration, irritability, increased muscle tension, and sleep disturbance. The reduction of spontaneous motor activity could be attributed to the sedative effect of the extract.[23] The present study showed a dose-dependent increase in muscle relaxation with different doses of AENOF. From the leaves of N. oleander four central nervous system (CNS) depressant cardenolides, neridiginoside, nerizoside, neritaloside, and odoroside-H, have been isolated.[24] Decrease in locomotion implies depression effect on the central nervous system and also it has been well-known that an augment in the concentration of gamma-aminobutyric acid (GABA) may lead to CNS depressant effect.[11] This suggests that AENOF may act via GABA receptors. Phytochemical analysis of N. oleander leaves revealed the presence of alkaloids, tannins, cardiac glycosides, steroids, terpenoids, flavonoids, reducing sugars, and saponins.[24] The muscle relaxant activity observed with AENOF may be due to the presence of flavonoids, alkaloids, and terpenoids in the plant extract. One study[25] on AENOF revealed the presence of various compounds such as alkaloids, saponins, flavones, triterpenoids, steroids, tannins, and amino acids. The results of present study suggest the muscle relaxant activity of alcoholic extract of AENOF at the doses of 100 and 200 mg/kg. The observed muscle relaxant effect of AENOF may be due to the agonistic effect on GABA/benzodiazepine receptor complex.[26] Studies on this species pointed, as one of the most poisonous plants, which contain numerous toxic compounds such as alkaloids and triterpenes.[19,20] The standard reference drug diazepam, which acts as an anxiolytic (at low doses), anticonvulsant and also produces sedation, and a myorelaxant effect at higher doses.[27] In this case, diazepam at a dose of 10 mg/kg body weight showed a significant lack in motor coordination and muscle relaxant activity in animals and animals treated with the extract showed muscle relaxation and reduced motor activity. These effects of AENOF could be due to the interaction of flavones, triterpenoids, steroids (chemical constituents of the plant) with the GABA/benzodiazepine receptor complex in the brain.[28] In another study[29] in Rotarod motor co-ordination test, AENOL at 100 mg/kg, i.p., significantly (P < 0.05–0.01) reduced the endurance. As the phyto constituents of AENOF and N. oleander leaves are same. Our study is in correlation with our previous study.[12] Moreover, also with another study.[30] However, further extensive phytochemical analysis and research is necessary to identify the exact constituents and elucidation of its possible mechanism of action underlying the myorelaxant activity of AENOF.
Conclusions
The study suggests that the aqueous extract of N. oleander flower has muscle relaxant action. As the comparison is done with centrally acting benzodiazepine group of drug diazepam, it is assumed that the muscle relaxation and reduced motor activity effects of AENOF could be due to the interaction of isoflavonoids of the plant with the GABA/benzodiazepine receptor complex in brain as the absence of muscle relaxation was observed when the extracts are given directly into the muscles. Still extensive research is needed to synthesize new molecule with muscle relaxation property from N. oleander.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Lokesh R, Barnalas L, Madhuri P, Saurav K, Sundar K. Larvicidal activity of Trigonella, foenum and Nerium oleander Linn. Curr Res J Biol Sci. 2010;2:154–60. [Google Scholar]
- 2.Brenzel, Norris K. Menlo Park: Sunset Pub Co; 1995. Sunset editors. Western Garden Book; pp. 606–7. [Google Scholar]
- 3. [Last retrieved on 2010 Oct 22]. Available from: http://www.arnoldia.arboretum.harvard.edu/pdf/articles/892.pdf .
- 4.New Delhi: Council of Scientific and Industrial Research; 1952. The Wealth of India. Publications and Information Directorate. Vol. III, Reprint 1988; p. 17. [Google Scholar]
- 5.Shanthi R, Lakshmi G, Priyadarshini AM, Anandaraj L. Phytochemical screening of Nerium oleander Linn. Leaves and Momordica charantia leaves. Int Res J Pharm. 2011;2:131–5. [Google Scholar]
- 6.Vaidyaratnam PS. Vol. 4. Chennai, India: Orient Longman; 1994. Varier's Arya Vaidya Sala – Indian Medicinal Plants. A Compendium of 500 Species; pp. 126–30. [Google Scholar]
- 7.Smith JA, Madden T, Vijjeswarapu M, Newman RA. Inhibition of export of fibroblast growth factor-2 (FGF-2) from the prostate cancer cell lines PC3 and DU145 by Anvirzel and its cardiac glycoside component, oleandrin. Biochem Pharmacol. 2001;62:469–72. doi: 10.1016/s0006-2952(01)00690-6. [DOI] [PubMed] [Google Scholar]
- 8.Zia A, Siddiqui BS, Begum S, Siddiqui S, Suria A. Studies on the constituents of the leaves of Nerium oleander on behavior pattern in mice. J Ethnopharmacol. 1995;49:33–9. doi: 10.1016/0378-8741(95)01300-8. [DOI] [PubMed] [Google Scholar]
- 9.Neumann W, Lindner W. Uber die stellung der oleanderglykoside in der digitallsgruppe. Arch Exp Pathol Pharmacol. 1937;185:630. [Google Scholar]
- 10.Nuki B. Folia. Pharmacol Jpn. 1949;45:134. [Google Scholar]
- 11.Erdemoglu N, Küpeli E, Yesilada E. Anti-inflammatory and antinociceptive activity assessment of plants used as remedy in Turkish folk medicine. J Ethnopharmacol. 2003;89:123–9. doi: 10.1016/s0378-8741(03)00282-4. [DOI] [PubMed] [Google Scholar]
- 12.Ubedulla S, Jayasree T, Chandra Sekhar N, Prakash M, samreen S, Shankar J, et al. Evaluation of skeletal muscle relaxant activity of aqueous extract of Nerium oleander leaves in albino rats. Int J Biol Pharm Res. 2014;5:191–5. [Google Scholar]
- 13.Kokate CK. 10th ed. New Delhi: Vallabh Prakashan; 1994. A Text Book of Practical Pharmacognosy; pp. 112–20. [Google Scholar]
- 14.Pathak S, Multani AS, Narayan S, Kumar V, Newman RA. Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anticancer Drugs. 2000;11:455–63. doi: 10.1097/00001813-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Jayaraman J. 1st ed. New Delhi: New Age International (p) Ltd; 1981. Laboratory Manual in Biochemistry; p. 13. [Google Scholar]
- 16.Ghosh MN. 2nd ed. Kolkata: Scientific Book Agency; 1984. Fundamentals of Experimental Pharmacology; p. 156. [Google Scholar]
- 17.Lipnick RL, Cotruvo JA, Hill RN, Bruce RD, Stitzel KA, Walker AP, et al. Comparison of the up-and-down, conventional LD50, and fixed-dose acute toxicity procedures. Food Chem Toxicol. 1995;33:223–31. doi: 10.1016/0278-6915(94)00136-c. [DOI] [PubMed] [Google Scholar]
- 18.Al-Naggar TB, Gómez-Serranillos MP, Carretero ME, Villar AM. Neuropharmacological activity of Nigella sativa L. extracts. J Ethnopharmacol. 2003;88:63–8. doi: 10.1016/s0378-8741(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 19.Harbone JB. 2nd ed. London: Academic Press; 1983. Phytochemical Methods. [Google Scholar]
- 20.Goetz RJ, Jordan TN, McCain W, Su J, Nancy Y. India: Cooperative Extension Service, Purdue University; 1998. “Oleander”. Indiana Plants Poisonous to Livestock and Pets. [Google Scholar]
- 21.Mostaqul Huq M, Jabbar A, Rashid MA, Hasan CM. A novel antibacterial and cardiac steroid from the roots of Nerium oleander. Fitoterapia. 1999;70:5–9. [Google Scholar]
- 22.Hadizadeh I, Peivastegan B, Kolahi M. Antifungal activity of nettle (Urtica dioica L.), colocynth (Citrullus colocynthis L. Schrad), oleander (Nerium oleander L) and konar (Ziziphus spina-christi L.) extracts on plants pathogenic fungi. Pak J Biol Sci. 2009;12:58–63. doi: 10.3923/pjbs.2009.58.63. [DOI] [PubMed] [Google Scholar]
- 23.Vogel HG, Vogel WH. 2nd ed. Berlin, Heidelberg, New York: Springer-Verlag; 2002. Drug Discovery and Evaluation. [Google Scholar]
- 24.Leewanich P, Tohda M, Matsumoto K, Subhadhirasakul S, Takayama H, Aimi N, et al. Behavioral studies on alkaloids extracted from the leaves of Hunteria zeylanica. Biol Pharm Bull. 1996;19:394–9. doi: 10.1248/bpb.19.394. [DOI] [PubMed] [Google Scholar]
- 25.Satti AA, Edriss AE. Phytochemical analyses of three sudanese plants for their constituents of bioactive compounds. Int J Sci Nat. 2013;4:169–73. [Google Scholar]
- 26.Vikas G, Payal M. Phytochemical and pharmacological potential of Nerium oleander: A review. IJPSR. 2010;1:21–7. [Google Scholar]
- 27.Thippeswamy BS, Mishra B, Veerapur VP, Gupta G. Anxiolytic activity of Nymphaea alba Linn. in mice as experimental models of anxiety. Indian J Pharmacol. 2011;43:50–5. doi: 10.4103/0253-7613.75670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar V. Varanasi: Banaras Hindu University; 2000. Neuropsychopharmacological studies on Indian Hypericum perforatum Linn. Ph.D. thesis. [Google Scholar]
- 29.Onaivi ES, Maguire PA, Tsai NF, Davies MF, Loew GH. Comparison of behavioral and central BDZ binding profile in three rat lines. Pharmacol Biochem Behav. 1992;43:825–31. doi: 10.1016/0091-3057(92)90414-b. [DOI] [PubMed] [Google Scholar]
- 30.Singhal KG, Gupta GD. Some central nervous system activities of Nerium oleander Linn (Kaner) flower extract. Trop J Pharm Res. 2011;10:455. [Google Scholar]


