Abstract
Objectives:
The brain of mammals contains two major form of cholinesterase enzymes, acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE). The dual inhibition of these enzymes is considered as a promising strategy for the treatment of neurological disorder such as Alzheimer's disease (AD), senile dementia, ataxia, and myasthenia gravis. The present study was undertaken to explore the anticholinesterase inhibition property of allicin.
Materials and Methods:
An assessment of cholinesterase inhibition was carried out by Ellman's assay.
Results:
The present study demonstrates allicin, a major ingredient of crushed garlic (Allium sativum L.) inhibited both AChE and BuChE enzymes in a concentration-dependent manner. For allicin, the IC50 concentration was 0.01 mg/mL (61.62 μM) for AChE and 0.05 ± 0.018 mg/mL (308.12 μM) for BuChE enzymes.
Conclusions:
Allicin shows a potential to ameliorate the decline of cognitive function and memory loss associated with AD by inhibiting cholinesterase enzymes and upregulate the levels of acetylcholine (ACh) in the brain. It can be used as a new lead to target AChE and BuChE to upregulate the level of ACh which will be useful in alleviating the symptoms associated with AD.
KEY WORDS: Acetylcholinesterase, allicin, Alzheimer's diseases, butyrylcholinesterase
Introduction
Alzheimer's disease (AD) is the most common cause of dementia in the elderly people is characterized by disturbance of multiple cortical functions, including memory loss, judgement, orientation, comprehension, learning capacity, and language deficit.[1,2] The major symptoms of all type of dementia are presumed to be related to progressive deterioration of widespread and dense cholinergic innervation of the human cerebral cortex, which contributes to the cognitive and behavioral disturbances in AD, and is also associated with the decreased levels of the neurotransmitter, acetylcholine (ACh), choline acetyltransferase, and acetylcholinesterase (AChE).[3] Apart from AChE, ACh is also hydrolytically destroyed in the brain by another enzyme, butyrylcholinesterase (BuChE).[4] In the healthy brain, AChE is the most important enzyme regulating the levels of ACh, while BuChE plays a minor role. In patient with AD, the levels of AChE activity declines and the activity of BuChE increases and the ratio between BuChE and AChE can change from 0.6 in the normal brain to as high as 11 in the cortical areas affected the disease.[5] These observations lead to dual inhibition strategy of these enzymes has been proposed to increase the efficacy of treatment and broaden the indications.
The medicinal use of garlic and several Indian medicinal plants were demonstrated by more than 1,300 research articles during the past 100 years.[6,7] This background and the fact that garlic is also described as having immunomodulatory, anti-inflammatory, and antioxidant effects. This focused our interest on the question of whether the known constituents of processed garlic specifically allicin ([R, S]-diallyl disulfide-S-oxide) on inhibition of AChE and BuChE, two important enzymes involved in the breakdown of ACh in the human brain.[8] In the present study, we investigated the inhibitory effects of allicin on AChE and BuChE enzymes.
Materials and Methods
Chemicals/Reagents
AChE (EC 3.1.1.7) and BuChE (EC 3.1.1.8) from bovine erythrocytes, acetylthiocholine iodide (ATChI), butyrylthiocholine iodide, 5:5-dithiobis-2-nitrobenzoic acid (DTNB), and sodium bicarbonate were purchased from Sigma, UK. Allicin (Triveni Ltd). The extraction procedure was carried out as described in a previously published study.[9]
Cholinesterase Assays
An assessment of cholinesterase inhibition was carried out in vitro in flat-bottom 96-well microtiter plates using a Ellman's colorimetric method.[10,11] A typical run consisted of 5 μL of bovine AChE/BuChE solution, at final assay concentrations of 0.03 U/mL; 200 μL of 0.1 M phosphate buffer pH 8; 5 μL of DTNB at a final concentration of 0.3 mM prepared in 0.1 M phosphate buffer pH 7 with 0.12 M of sodium bicarbonate; and 5 μL of the test solution. The reactants were mixed and preincubated for 15 min at 30°C. The reaction was initiated by adding 5 μL of ATChI/BuChI at final concentrations of 0.5 mM. As a control, the inhibitor solution was replaced with buffer. The control and test samples were assayed in triplicate. To monitor any nonenzymatic hydrolysis in the reaction mixture, two blanks for each run were prepared in triplicate. One blank consisted of buffer replacing enzyme and a second blank had buffer replacing substrate. Change in absorbance at 405 nm was measured on a molecular device M2, 96-well plate reader for a period of 6 min at 30°C.
Results
Estimation of IC50 Value of Allicin
The result showed that allicin inhibited the AChE in a concentration-dependent manner at different concentrations tested (0.003 mg/mL to 0.1 mg/mL) [Figure 1], a concentration of 0.1 mg/mL showed maximum inhibition which was 95%. The IC50 value was 0.01 ± 0.009 mg/mL (61.62 μM) derived from equation of Figure 1. This result demonstrated that allicin was a potent AChE inhibitor. In contrast to AChE inhibition, allicin also inhibited BuChE enzyme, but the inhibition was not as strong compared with AChE. The concentration of allicin tested in the range of 0.003–0.1mg/mL, showed percentage inhibition ranging from 16% to 56%, respectively. The maximum inhibition was observed at the concentration of 0.1 mg/mL (56%) [Figure 2]. The IC50 concentration calculated was 0.05 ± 0.018 mg/mL (308.12 μM) from the equation of Figure 2. The results showed that allicin was a weak BuChE inhibitor.
Figure 1.
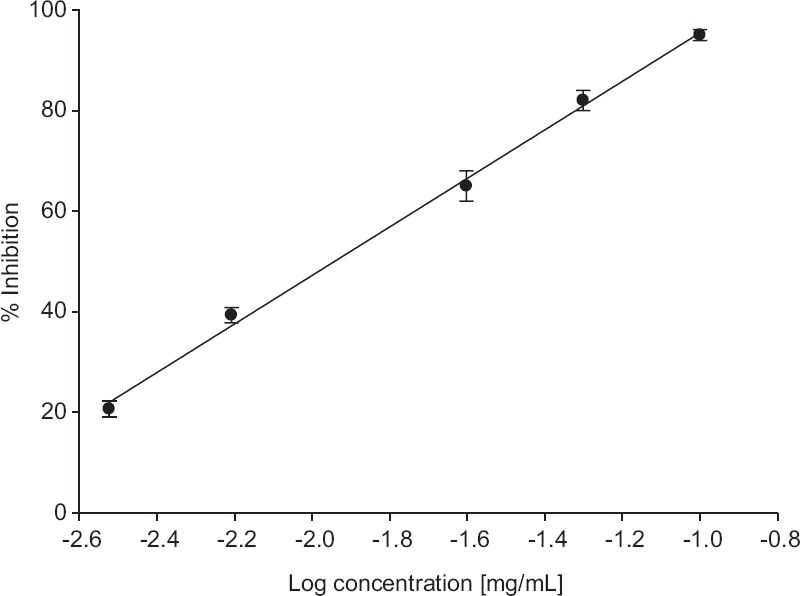
Inhibition of bovine acetylcholinesterase by allicin. Values are expressed as ± standard error of the mean (n = 6). The equation for the line is y = 48.66x + 144.22; R2 = 0.9973
Figure 2.
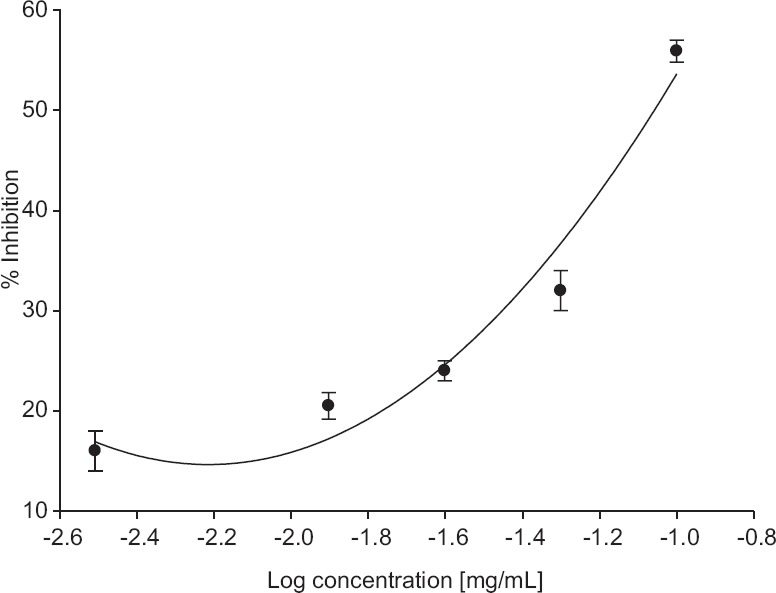
Inhibition of bovine butyrylcholinesterase by different concentration of allicin. Values are expressed as ± standard error of the mean (n = 6). The equation of the line is y = 18.949x2 + 86.651x + 114.59; R2 = 0.9872
Statistical Analysis
Data obtained were subjected to Windows 7 (Microsoft office) version 2007, mean, ± standard error of the mean, and IC50 was calculated using Windows 7 (Microsoft Excel software).
Discussion
Garlic (Allium sativum) has been used worldwide by Egyption, Chinese, and Indians as a spice, food, and folk medicine for centuries. It has been used to treat various ailments such as heart disease, arthritis, pulmonary complaints, cancer, diarrhea, and worm infestation.[12] Several investigators have reported different medicinal uses of garlic, one of these is its anti-oxidative properties, which not only protect against oxidative damage but also lower the risk of injury to vital molecules and to varying degree may help prevent the onset and progression of age-related neurodegenerative diseases.[13,14] Studies of senile dementia model in mice showed that aged garlic extract prevented atrophic changes in the frontal brain, improved learning abilities and memory retention, and increased longevity in the senescence-accelerated mouse.[15,16]
Allicin is reported to have most active lipid-soluble volatile organosulfur biological compound present in the freshly crushed garlic.[17] It was demonstrated earlier that Torpedo californiac AChE when incubated with allicin produced rapid inactivation which was time and concentration dependent.[18] Our results which showed concentration-dependent inhibition of bovine AChE by allicin complementing the previous finding. According to our knowledge, we are for the first time reporting the concentration-dependent inhibition of BuChE by allicin. Although several cholinesterase inhibitors such as tacrine, rivastigmine, and donepezil are being used for management of conditions associated with AD, their side effects have become increasingly noticeable.[19,20] Therefore, the therapy with these newer derivatives obtained from the natural product that have dual function and lesser adverse effects could be beneficial for AD patients.
In summary, it's demonstrated that allicin strongly inhibit AChE but weakly inhibit BuChE in a concentration-dependent manner. These results may provide an interesting basic contribution with regard to the beneficial effects claimed for garlic and may be of therapeutic value in AD. However, further study on the pharmacological and in vivo behavioral test is needed. Thus, this active compound has a potential to ameliorate the decline of cognitive function and memory loss associated with AD.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Desgranges B, Baron JC, de la Sayette V, Petit-Taboué MC, Benali K, Landeau B, et al. The neural substrates of memory systems impairment in Alzheimer's disease. A PET study of resting brain glucose utilization. Brain. 1998;121:611–31. doi: 10.1093/brain/121.4.611. [DOI] [PubMed] [Google Scholar]
- 2.Grosse DA, Gilley DW, Wilson RS. Episodic and semantic memory in early versus late onset Alzheimer's disease. Brain Lang. 1991;41:531–7. doi: 10.1016/0093-934x(91)90172-w. [DOI] [PubMed] [Google Scholar]
- 3.Lane RM, Potkin SG, Enz A. Targeting acetylcholinesterase and butyrylcholinesterase in dementia. Int J Neuropsychopharmacol. 2006;9:101–24. doi: 10.1017/S1461145705005833. [DOI] [PubMed] [Google Scholar]
- 4.Giacobini E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol Res. 2004;50:433–40. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Greig NH, Lahiri DK, Sambamurti K. Butyrylcholinesterase: An important new target in Alzheimer's disease therapy. Int Psychogeriatr. 2002;14:77–91. doi: 10.1017/s1041610203008676. [DOI] [PubMed] [Google Scholar]
- 6.Agarwal KC. Therapeutic actions of garlic constituents. Med Res Rev. 1996;16:111–24. doi: 10.1002/(SICI)1098-1128(199601)16:1<111::AID-MED4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Brijeshlata, Dixit S. Screening of Indian spices for inhibitory activity of acetylcholinesterase and butyrlcholinesterase enzymes. Int J Pharma Bio Sci. 2012;3:59–65. [Google Scholar]
- 8.Reuter HD, Koch HP, Lawson LD. Therapeutic effects and applications of garlic and its preparations. In: Koch HP, Lawson LD, editors. Garlic: The Science and Therapeutic Application of Allium sativum L. Baltimore: Williams and Wilkins; 1996. pp. 135–13. [Google Scholar]
- 9.Block E, Naganathan S, Putman D, Zhao SH. Allium chemistry: HPLC analysis of thiosulfinates from onion, garlic, wild garlic (ramsoms), leek, scallion, shallot, elephant (great-headed) garlic, chive, and chinese chive. Uniquely high allyl to methyl ratios in some garlic samples. J Agric Food Chem. 1992;40:2418. [Google Scholar]
- 10.Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 11.Kumar S, Seal CJ, Okello EJ. Kinetics of acetylcholinesterase inhibition by an aqueous extract of Withania somnifera roots. Int J Pharma Sci Res. 2011;2:1188–92. [Google Scholar]
- 12.Rivlin RS. Historical perspective on the use of garlic. J Nutr. 2001;131:951S–4. doi: 10.1093/jn/131.3.951S. [DOI] [PubMed] [Google Scholar]
- 13.Borek C. Antioxidant and cancer. Sci Med. 1997;4:51–62. [Google Scholar]
- 14.Gutteridge JM. Free radicals in disease processes: A compilation of cause and consequence. Free Radic Res Commun. 1993;19:141–58. doi: 10.3109/10715769309111598. [DOI] [PubMed] [Google Scholar]
- 15.Nishiyama N, Moriguchi T, Saito H. Beneficial effects of aged garlic extract on learning and memory impairment in the senescence-accelerated mouse. Exp Gerontol. 1997;32:149–60. doi: 10.1016/s0531-5565(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 16.Moriguchi T, Saito H, Nishiyama N. Anti-ageing effect of aged garlic extract in the inbred brain atrophy mouse model. Clin Exp Pharmacol Physiol. 1997;24:235–42. doi: 10.1111/j.1440-1681.1997.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 17.Ali M, Thomson M, Afzal M. Garlic and onions: Their effect on eicosanoid metabolism and its clinical relevance. Prostaglandins Leukot Essent Fatty Acids. 2000;62:55–73. doi: 10.1054/plef.1999.0124. [DOI] [PubMed] [Google Scholar]
- 18.Millard CB, Shnyrov VL, Newstead S, Shin I, Roth E, Silman I, et al. Stabilization of a metastable state of Torpedo californica acetylcholinesterase by chemical chaperones. Protein Sci. 2003;12:2337–47. doi: 10.1110/ps.03110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inglis F. The tolerability and safety of cholinesterase inhibitors in the treatment of dementia. Int J Clin Pract Suppl. 2002;127:45–63. [PubMed] [Google Scholar]
- 20.Jann MW, Shirley KL, Small GW. Clinical pharmacokinetics and pharmacodynamics of cholinesterase inhibitors. Clin Pharmacokinet. 2002;41:719–39. doi: 10.2165/00003088-200241100-00003. [DOI] [PubMed] [Google Scholar]


