Abstract
Parkinson’s disease (PD) is a movement disorder whose cardinal motor symptoms arise due to the progressive loss of dopamine. Although this dopamine loss typically progresses slowly over time, currently there are very few animal models that enable incremental dopamine depletion over time within the same animal. This type of gradual dopamine depletion model would be useful in studies aimed at the prodromal phase of PD, when dopamine levels are pathologically low but motor symptoms have not yet presented. Utilizing the highly characterized neurotoxin 6-hydroxydopamine (6-OHDA), we have developed a paradigm to gradually deplete dopamine levels in the striatum over a user-defined time course – spanning weeks to months – in C57BL/6 mice. Dopamine depletions were achieved by administration of five low dose injections (0.75 µg) of 6-OHDA through an implanted intracranial bilateral cannula targeting the medial forebrain bundle. Levels of dopamine within the striatum declined linearly with successive injections, quantified using tyrosine hydroxylase immunostaining and high-performance liquid chromatography. Behavioral testing was carried out at each time point to study the onset and progression of motor impairments as a function of dopamine loss over time. We found that spontaneous locomotion, measured in an open field, was robust to loss of dopamine until ~70% of striatal dopamine was lost. Beyond this point, additional dopamine loss caused a sharp decline in motor performance, reaching a final level comparable to that of acutely depleted mice. Similarly, although rearing behavior was more sensitive to dopamine loss and declined linearly as a function of dopamine levels, it eventually declined to levels similar to that seen in acutely depleted mice. In contrast, motor coordination, measured on a vertical pole task, was only moderately impaired in gradually depleted mice, despite severe impairments observed in acutely depleted mice. These results demonstrate the importance of the temporal profile of dopamine loss on the magnitude and progression of behavioral impairments. Our gradual depletion model thus establishes a new paradigm with which to study how circuits respond and adapt to dopamine loss over time, information which could uncover important cellular events during the prodromal phase of PD that ultimately impact the presentation or treatability of behavioral symptoms.
Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by a progressive loss of dopamine neurons in the substantia nigra pars compacta (SNc) (Fearnley and Lees 1991, Morrish, Sawle et al. 1996, Damier, Hirsch et al. 1999). This results in decreased dopamine signaling in the striatum, a major input nucleus of the basal ganglia involved in the control of voluntary movement (Marsden and Obeso 1994, Mink 1996, Olanow and Tatton 1999, Dauer and Przedborski 2003). Motor symptoms such as tremors, rigidity, bradykinesia, freezing, and balance instability typically do not become overt enough to diagnose PD until dopaminergic loss exceeds 70% in the striatum (Bernheimer, Birkmayer et al. 1973, Riederer and Wuketich 1976, Betarbet, Sherer et al. 2002, Deumens, Blokland et al. 2002, Fahn 2003). Unfortunately by this time, patients have likely been living with chronically low levels of dopamine for years and dysfunction in neural circuits may have already passed a point of no return. It has been proposed that the pre-symptomatic phase of PD, called the prodromal phase, is an ideal time to begin therapies (Schapira and Tolosa 2010, Olanow and Obeso 2012). Treatments administered prior to complete dopamine loss may prevent further neurodegeneration or delay the onset of motor deficits. In addition, further understanding of how and when motor systems begin to break down during this phase may lead to techniques for early detection and more effective therapies aimed at restoring circuit function (Little and Brown 2014).
Current standard animal models of PD typically involve acute, rapid degeneration of dopamine neurons which does not recapitulate PD disease progression (for reviews see (Betarbet, Sherer et al. 2002, Schober 2004, Bove, Prou et al. 2005, Terzioglu and Galter 2008, Hisahara and Shimohama 2010)). These models preclude the development of pathogenic mechanisms and prevent studies of various stages of PD. This need for chronic models of dopamine degeneration has led to an increase in the number and availability of genetic models of PD, but only 5% of human PD cases are inherited and these models come with their own set of limitations, discussed at length in a number of reviews (Betarbet, Sherer et al. 2002, Dauer and Przedborski 2003, Chesselet, Fleming et al. 2008, Meredith, Sonsalla et al. 2008, Terzioglu and Galter 2008, Dawson, Ko et al. 2010, Potashkin, Blume et al. 2010). In contrast to genetic strategies, well-characterized neurotoxin models, and more recently, AAV-induced overexpression of α- synuclein (Decressac, Mattsson et al. 2012, Decressac, Mattsson et al. 2012, Lundblad, Decressac et al. 2012), have been adapted to create alternative chronic models of dopamine depletion (Greenamyre, Betarbet et al. 2003, Fleming, Delville et al. 2005, Meredith, Sonsalla et al. 2008, Goldberg, Haack et al. 2011, Goldberg, Fields et al. 2012). However, models using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) require increased safety precautions when handling mice which has proven to be a deterrent for widespread application of these models. Furthermore MPTP has had questionable toxicity in various mouse strains and does not always result in persistent and progressive motor symptoms (Bezard, Dovero et al. 1997, Schwarting, Sedelis et al. 1999, Przedborski, Jackson-Lewis et al. 2001, Betarbet, Sherer et al. 2002, Meredith, Totterdell et al. 2002, McNaught, Perl et al. 2004, Schober 2004, Blume, Cass et al. 2009, Blesa, Juri et al. 2010). Other adapted models utilize 6-hydroxydopamine (6-OHDA), the neurotoxin used in the first animal model of PD associated with SNc dopaminergic neurodegeneration (Ungerstedt 1968). In most models utilizing 6-OHDA, animals are dopamine depleted unilaterally or given various acute doses to model different stages (Przedborski, Levivier et al. 1995, Schwarting and Huston 1996, Schwarting and Huston 1996, Kirik, Rosenblad et al. 1998, Ferro, Bellissimo et al. 2005, Fleming, Delville et al. 2005, Truong, Allbutt et al. 2006). While dopamine loss in human PD can be asymmetrical, it ultimately results in dopamine loss in both hemispheres (Deumens, Blokland et al. 2002, Simola, Morelli et al. 2007). There are also concerns regarding contralateral compensation in unilateral depletions and a lack of compensatory mechanisms in using acute doses to model different stages of the disease (Schwarting and Huston 1996, Meredith and Kang 2006, Potashkin, Blume et al. 2010). Thus, there is a lack of behavioral data available for the prodromal phase, where dopamine is being depleted bilaterally and gradually within the same animal.
In this study, we adapt the traditional 6-OHDA model to produce a gradual, bilateral dopamine loss over a user-defined time course. By administering low doses of 6-OHDA bilaterally through an intracranial cannula targeting the medial forebrain bundle (MFB), we gradually depleted dopamine levels within the same animal over 2–7 weeks rather than 2–3 days. Using this technique, we were able to slowly deplete dopamine over a prolonged time course to study the effects of gradual vs. acute depletion on the onset and progression of motor impairments. We find that certain aspects of motor behavior are altered differentially as dopamine is depleted. Furthermore, some behaviors were differentially affected in gradually vs. acutely depleted mice, suggesting the engagement of compensatory plasticity during gradual depletion that is not engaged with the more traditional, acute paradigm. This study demonstrates that the time course of dopamine loss can influence the final behavioral state of the animal and provides a paradigm with which to study how motor systems adapt to chronically low levels of dopamine over time.
Materials and Methods
Animals
Experiments were conducted in accordance with the guidelines from the National Institutes of Health and with approval from Carnegie Mellon University Institutional Animal Care and Use Committee. Male and female P30–P80 day old mice on a C57BL/6J background were used for experiments. Acutely depleted animals were P42–P59 at time of injection and were P47–P81 when they completed their last behavioral testing. Gradual(3day) depleted animals and littermate saline controls were P34–P61 at the time of their first injection and were P44–P80 when they completed their last behavioral testing. Gradual(7day) depleted animals and littermate controls were P41 at the time of their first injection and were P90 when they completed their last behavioral testing. After surgical implantation of the cannula, animals were housed separately to prevent damage to the cannula. Animals were provided with dishes of crushed high fat food pellets moistened with water, additional hard food pellets on the floor of the cage, as well as access to a water bottle. All cages were placed half on/half off heating pads following surgery and each subsequent infusion of 6-OHDA. Cages remained on heating pads unless animals were observed resting mainly in the unheated portion of the cage. Each infusion of saline or 6-OHDA was performed while animals were lightly anesthetized on a heating pad, and all animals were injected with 0.1 cc of saline i.p. before being returned to their home cage. Animal’s weights were tracked regularly and extra i.p. saline and softened food or trail mix were provided to encourage weight gain and proper hydration when appropriate.
Implantation of bilateral cannulas and 6-OHDA injection in the MFB
Under ketamine/xylazine (100 mg/kg: 30 mg/kg, i.p.) anesthesia, the animals were placed on a stereotaxic frame (David Kopf Instruments) and maintained throughout surgery using 1–2% isoflurane. Bilateral internal cannulas (Plastics One) cut to target ±1.1 mm lateral and −5.0 mm ventral were implanted 0.45 mm posterior to bregma and secured using superglue. 6-hydroxydopamine was prepared at a concentration of 5 µg/µL in 0.9% NaCl for acute depletions and diluted further with 0.9% NaCl to 0.75 µg/µL for gradual depletions (Sigma-Aldrich H116 6-Hydroxydopamine hydrobromide). Injections were performed using a 33-gauge cannula (Plastics One) attached to a 10 µL Hamilton syringe within a syringe pump (GenieTouch; Kent Scientific) running at 0.5µL/min, to a total volume of 1µL/side. The injection cannula was left in place for 5 min following the injection. Control animals received the same volume of vehicle (0.9%NaCl), following the same procedure. 6-OHDA and vehicle were administered every 3 or 7 days, respectively, for gradual depletions and controls. Animals were sacrificed 72–80 h following the last given injection.
Behavioral Assessment
Following initial surgery and subsequent injections, animals were exposed to the following sequential behavioral tests: open field, rearing, and pole task. The minimum interval between two consecutive procedures was 30 min.
Open Field
To determine overall spontaneous mobility, mice were placed in a 24 cm diameter clear cylindrical open field chamber with video monitoring from above. Mice were in the arena for a total of 20 m, with 10 m for acclimation, and 10 m for data acquisition. Positions of nose, tail, and center of mass of each mouse were tracked using EthoVision 9.0 software (Noldus). Ambulation was defined as periods when the velocity of the animal’s center point averaged more than 2.00 cm/s until the velocity was reduced to 1.75 cm/s. Two blind observers validated accuracy of this definition, and it excluded other fine movements such as rearing and sniffing. Immobility was defined as continuous periods of time (at least 1 s) during which the average pixel change of the entire video image is less than 0.5%. This definition is very strict, such that any movement of the head, limbs, or tail would not be scored as immobility. Total time spent mobile, total time spent immobile, distance traveled, and average velocities were calculated using EthoVision 9.0. The arena was cleaned with 50% ethanol between each animal.
Rearing
To assess spontaneous vertical activity, mice were placed in a standard 1000 mL glass beaker with video monitoring from the side for a total of 10 min. The number of full extension rears was manually scored post-hoc by observers blind to treatment. The beaker was cleaned with 50% ethanol between each animal.
Pole Task
To evaluate coordination and bradykinesia, mice were placed head-upward at the top of a vertical gauze-wrapped circular wooden pole (diameter = 1 cm; height = 55 cm) with video monitoring from the side. To encourage descent, a 60-watt lamp was aimed at the top of the pole. Prior to surgery/testing mice were trained for 3–5 trials or until descent took < 30 s under testing conditions to ensure each mouse was able to perform the task with ease before dopamine depletion. The latency to turn downward (turn down latency = TDL), time from orientation downward until all four paws reached the ground (traverse time), and total time spent on the pole (total) was recorded with a maximum duration of 120 s for three trials. All measurements were manually scored offline by observers blind to treatment. Even if the mouse fell part way into its descent, the behavior was scored until it reached the ground. When the mouse was unable to turn downward and/or instead dropped from the pole, all latencies were recorded as 120 s (default value) because of the severity of motor dysfunction.
Immunohistochemistry
Degree of dopamine denervation was assessed at 72 h post-injection in 6-OHDA-treated mice by tyrosine hydroxylase staining. Following decapitation, brains were surgically removed and drop-fixed with 4% paraformaldehyde in phosphate buffered saline at 4°C for 24 h. After rinsing with phosphate buffered saline, brains were transferred to 30% sucrose in phosphate buffered saline and stored at 4°C for at least 24 h prior to sectioning. Immunohistochemistry was carried out in free-floating coronal frozen sections (30 µm). Tissue was sectioned using a freezing microtome (Microm HM 430; Thermo Scientific), blocked with 10% normal donkey serum, and permeabilized with 0.5% Triton X-100 for 1 h. Primary antibody incubations were performed at room temperature for 24 h using rabbit anti-TH (1:500; Pel-Freez). Primary antibodies were detected with Alexa Fluor 647-conjugated donkey anti-rabbit (1:500, Vector Laboratories), incubated for 90 min at room temperature.
Fluorescence Quantification
Epifluorescent images (10× magnification) were taken from bilateral dorsal striatum in one coronal section between 0.62 mm and 1.10 mm Bregma (according to Paxinos second edition Mouse Brain in Stereotaxic Coordinates). Pixel intensity over a 75 × 75 µm area (5625 µm2) from each hemisphere was measured using the pixel intensity measuring tool in ImageJ and normalized to the pixel intensities measured in saline control mice processed and imaged in parallel.
High Performance Liquid Chromatography
Levels of dopamine neurotransmitter were assessed at 72 h post-injection in 6-hydroxydopamine-treated mice by high performance liquid chromatography (HPLC). Following decapitation, brains were quickly placed in a Vibrotome and a 700 µm coronal section containing striatum was removed. Dorsal striatum from each side was dissected and flash frozen on dry ice immediately. These samples were numbered and shipped on dry ice to CMN/KC Neurochemistry Core Laboratory at Vanderbilt University in Nashville, TN for analysis. In order to control for variability of dopamine, data from each animal was normalized to a same sex, same litter saline control.
Statistical Analysis
All data sets were tested for normality with the Shapiro-Wilk test prior to any statistical analysis. Data are expressed as mean ± standard error of mean (SEM) unless otherwise indicated. Statistical analysis was performed using Kruskal-Wallis analysis of variance nonparametric test (KW) and any differences were further investigated by Kruskal-Wallis pairwise comparison between percentage dopamine remaining or injection number and saline controls with a Bonferroni correction for number of comparisons. Bar graphs comparing performance between saline, gradual, and acute depletions were also tested using KW and pairwise comparisons included gradual vs. acute. In Figure 1F comparison of dorsal and ventral dopamine was analyzed with a Student’s t-test. Dopamine metabolites in Figure 2 were analyzed using a one-way analysis of variance (ANOVA) followed by a Dunnett T3 post hoc test (HVA, DOPAC, 3-MT) or a Dunnett t (2-sided) post hoc test (Nor, 5-HT). A p-value of 0.05 was considered statistically significant. All statistical procedures were performed using IBM SPSS Statistics, version 22.
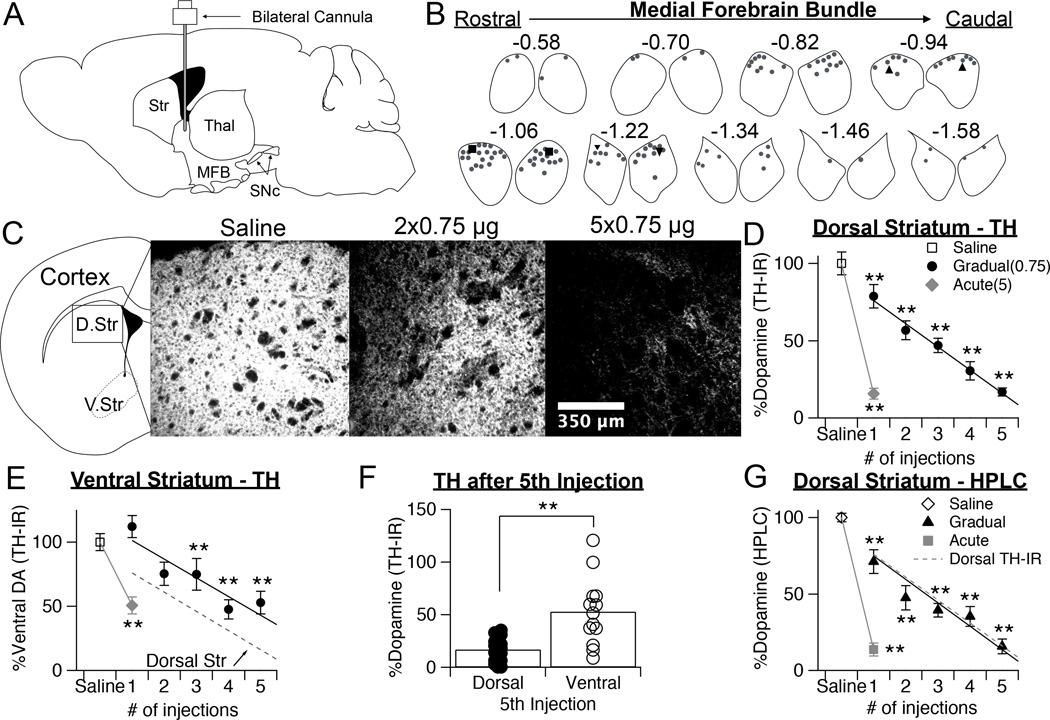
Figure 1.
Gradual depletion paradigm linearly reduces dopamine over time. A, Schematic of sagittal section of mouse brain showing the position of the infusion cannula in the MFB. Str = striatum, Thal = thalamus, MFB = medial forebrain bundle, SNc = substantia nigra pars compacta. B, Cannulae placements in the MFB, confirmed postmortem. Left and right MFBs from coronal sections spanning the cannulae placements are shown, and Bregma coordinates are notated. Representative bilateral cannulae placements from three representative mice are indicated with triangles, squares, and inverted triangles. C, Representative images of TH immunofluorescence (TH-IR), taken from the dorsal striatum of mice treated with saline, two doses of 0.75 µg 6-OHDA, or five doses of 0.75 µg 6-OHDA. D, Quantification of TH-IR in the dorsal striatum, normalized to saline controls, following repeated injections of 0.75 µg or a single injection of 5 µg 6-OHDA. Values are expressed as percentage of dopamine remaining. Throughout figure, error bars are SEM. KW, χ2(6) = 106.218, p < 0.0001, pairwise, **p < 0.005 from saline. E, Quantification of TH-IR in the ventral striatum, normalized to saline controls, following repeated injections of 0.75 µg or a single injection of 5 µg 6-OHDA. Values are expressed as percentage of dopamine remaining. KW, χ2(6) = 51.744, p < 0.0001, pairwise, **p < 0.005 from saline. F, Quantification of TH-IR in the dorsal and ventral striatum following the 5th injection of 0.75 µg of 6-OHDA. Data from each mouse, as well as population averages are shown. Student’s t-test, t = −3.896, **p = 0.002. G, Quantification of HPLC detected levels of dopamine in the dorsal straitum, normalized to saline controls, following repeated injections of 0.75 µg or a single injection of 5 µg 6-OHDA. Values are expressed as percentage of dopamine remaining. Fit line from the graph of TH-IR levels is overlaid for comparison. KW, χ2(6) = 69.554, p < 0.0001, pairwise, **p < 0.005 from saline.
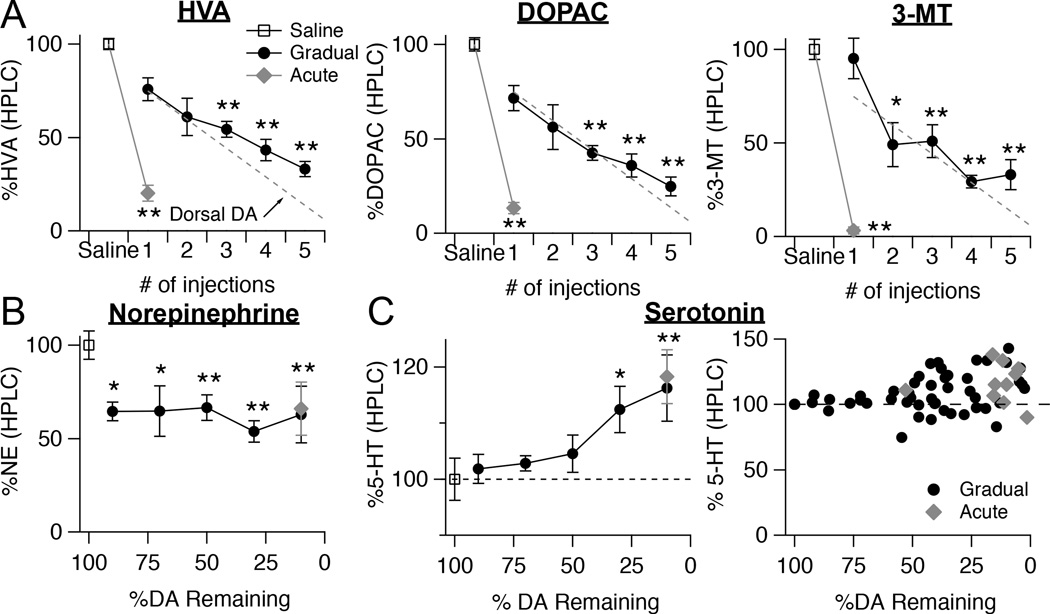
Figure 2.
Levels of dopamine metabolites and other monoamines measured with HPLC during gradual dopamine depletion paradigm. A, Quantification of HPLC detected levels of the dopamine metabolites homovanillic acid (HVA), 3,4-Dihydroxyphenylacetic acid (DOPAC), and 3-methoxytyramine (3-MT). Tissue samples were taken from the dorsal striatum of mice who received repeated injections of 0.75 µg 6-OHDA or a single injection of 5 µg of 6-OHDA. Values are normalized to saline controls for each time point. Throughout figure, error bars are SEM. Fit line from the graph of HPLC dopamine levels is overlaid for comparison. ANOVA, HVA: F[6,73] = 51.085; DOPAC: F[6,73] = 55.560; 3-MT: F[6,73] = 34.572, all p < 0.0001, Dunnett T3, *p < 0.05 from saline; **p < 0.005 from saline. B, Quantification of HPLC detected levels of norepinephrine in the dorsal striatum at all time points of the gradual depletion paradigm and in acutely depleted mice. Values are normalized to saline controls. ANOVA, F[6,73] = 9.709, p < 0.0001, Dunnett t, *p < 0.05 from saline; **p < 0.005 from saline. C, Quantification of HPLC detected levels of serotonin in the dorsal striatum at all time points of the gradual depletion paradigm and in acutely depleted mice. Values are normalized to saline controls. ANOVA, F[6,73] = 4.083, p = 0.01, Dunnett t, *p < 0.05 from saline; **p < 0.005 from saline. Serotonin levels in the dorsal striatum from each mouse, normalized to saline controls, plotted as a function of striatal dopamine levels.
Results
Injections Of 6-OHDA Over Time Result in Graded Depletion of Striatal Dopamine
To develop a depletion paradigm where the rate of dopamine loss can be controlled over time in the same animal, we administered multiple, low doses of 6-OHDA through bilateral internal guide cannulas implanted in the MFB (Fig. 1A). Bilateral cannulas cut to target the MFB at ± 1.1 mm medial/lateral and −5.0 mm ventral were implanted 0.45 mm posterior to bregma. Targeting was confirmed post-hoc by analyzing Nissl-stained sections of tissue. Summaries of paired cannula locations within the MFB are shown in Figure 1B.
To determine an optimal concentration of 6-OHDA to use for our paradigm, we first tested the acute effects of different concentrations on striatal dopamine levels, assessed with immunostaining for tyrosine hydroxylase (TH) (Table 1). We chose to use a dose of 0.75 µg because this reduced striatal dopamine by ~20% (TH-IR loss = 21.3 ± 7.5 %, n = 8) and should therefore permit 4–5 injections to be administered before reaching full dopamine depletion. For the majority of experiments, injections were administered once every three days, but in Figure 6 we will show that the time in between doses can be increased to deplete dopamine over an even longer period.
Table 1.
Quantification of TH immunoreactivity (TH-IR) and high performance liquid chromatography (HPLC) detected levels of dopamine in the striatum normalized to saline controls following an injection of 0.5, 0.75, 1, and 5 µg of 6-OHDA. Values are expressed as average percentage of dopamine remaining ± standard deviation.
| Dose of 6-OHDA | % TH-IR Remaining (avg ± std dev) |
% DA-HPLC Remaining (avg ± std dev) |
|---|---|---|
| 0.5 µg | 88.14 ± 19.57 | 90.64 ± 12.11 |
| 0.75 µg | 78.70 ± 21.18 | 71.20 ± 20.37 |
| 1 µg | 54.15 ± 23.44 | 43.46 ± 26.06 |
| 5 µg | 15.80 ± 12.99 | 13.70 ± 13.90 |
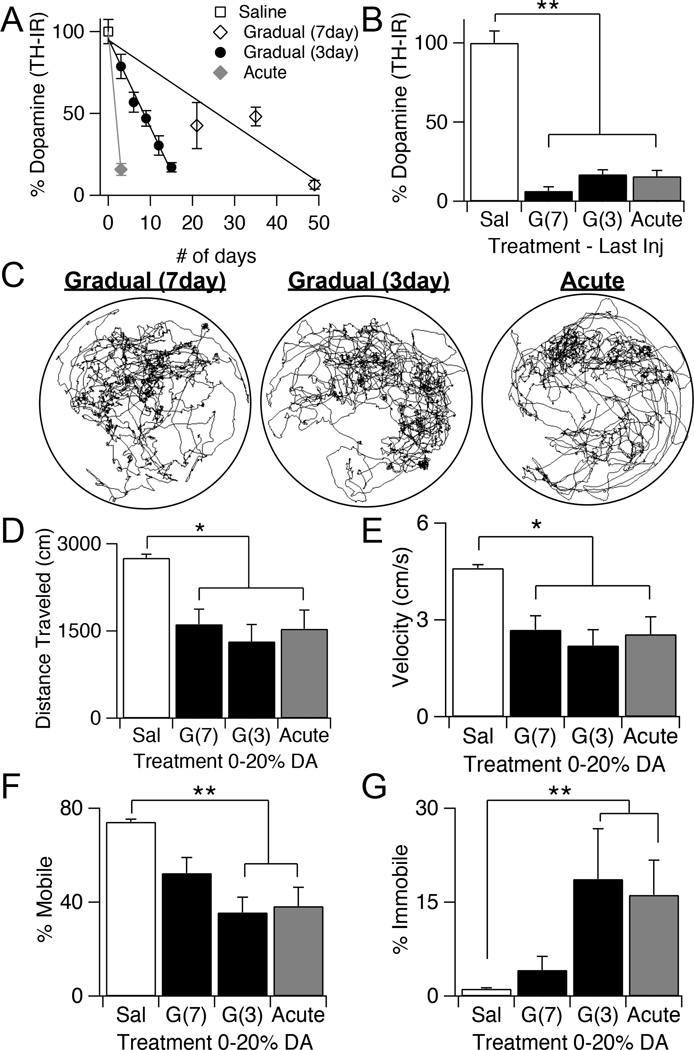
Figure 6.
Time course of gradual depletions can be varied and extended over a month. A, Quantification of TH-IR in the dorsal striatum normalized to saline controls following repeated injections of 0.75 µg 6-OHDA, administered every seven days, every three days, or a single injection of 5 µg of 6-OHDA. Values are normalized to saline controls and expressed as percentage of dopamine remaining. Throughout the figure, error bars are SEM. B, Quantification of TH-IR in the dorsal striatum, normalized to saline controls, following the last injection of the gradual seven-day, gradual three-day, and acute paradigms. KW, χ2(3) = 110.653, p < 0.0001, pairwise, **p < 0.005. C, Example plot tracks from a gradually depleted mouse that has received seven injections of 0.75 µg 6-OHDA every seven days, a gradually depleted mouse that has received five injections of 0.75 µg every three days, and an acutely depleted mouse that has received one injection of 5 µg. D, Total distance traveled during 10 minutes in the open field arena by saline control animals compared to mice with 0-20% dopamine remaining that were depleted with the seven-day gradual, three-day gradual, or acute dopamine depletion paradigms. KW, χ2(3) = 36.831, p < 0.0001, pairwise, *p < 0.05. E, Average velocity of saline control animals compared to animals with 0-20% dopamine that were depleted with the seven-day gradual, three-day gradual, or acute dopamine depletion paradigms. KW, χ2(3) = 36.881, p < 0.0001, pairwise, *p < 0.05. F, Percentage of time spent mobile in saline control animals and animals with 0-20% dopamine that received 0.75 µg every 7 days, 0.75 µg every 3 days, or a single injection of 5 µg of 6-OHDA during open field. KW, χ2(3)= 40.548, p < 0.0001, pairwise, **p < 0.005. G, Percentage of time spent immobile in saline control animals and animals with 0–20% dopamine that received 0.75 µg every 7 days, 0.75 µg every 3 days, or a single injection of 5 µg of 6-OHDA during open field. KW, χ2(3)= 34.129, p < 0.0001, pairwise, **p < 0.005.
Over the course of our protocol, a subset of animals were sacrificed after each injection to analyze dopamine levels within the striatum. Dopamine levels within the dorsal striatum were quantified with two distinct methods: TH immunostaining to estimate the degree of dopaminergic innervation, and high performance liquid chromatography (HPLC) to measure levels of dopamine neurotransmitter. Histological analysis of TH immunostaining revealed that repeated injections of 0.75 µg resulted in a gradual and linear depletion (r2 = 0.857, p < 0.0001) of dopamine levels within the striatum over the course of 5 injections, spanning 15 days (KW pairwise, p < 0.005 from saline) (Fig. 1C–D). By the fifth injection, the degree of dopamine depletion was similar to that observed with the traditional acute method (5 µg, 1 injection). Both the gradual and acute paradigms resulted in a more pronounced loss of dopamine in the dorsal striatum compared to the ventral striatum (Fig. 1E–F). Although dopamine levels declined somewhat in the accumbens over the course of our depletions, this was significantly less than the dopamine loss observed in the dorsal striatum (Student’s t-test, D. Str. = 16.9 ± 2.5 %, n = 20; V. Str. = 52.8 ± 8.8 %, n = 13, p = 0.002).
TH immunoreactivity often correlates tightly with dopamine levels, but this relationship may break down under dopamine depleted conditions (Zigmond, Acheson et al. 1984), so dopamine levels were also measured directly with HPLC. HPLC analysis of dorsal striatum confirmed that levels of dopamine neurotransmitter were also gradually and linearly depleted (r2 = 0.819, p < 0.0001), in strong accordance with our measurements of TH immunofluorescence (KW pairwise, p < 0.005 from saline) (Fig. 1G). Combined, these results show that repeated injections of 0.75 µg of 6-OHDA into the MFB gradually depletes dopamine to the same endpoint achieved with the traditional 5 µg acute model, although over a much longer time course.
Effects Of Gradual Depletion On Dopamine Metabolites And Monoamines
To test whether our gradual depletion paradigm produced any compensatory changes in dopamine metabolism, we used HPLC to measure levels of additional compounds in the tissue. Levels of the dopamine metabolites homovanillic acid (HVA), 3,4-Dihydroxyphenylacetic acid (DOPAC), and 3-methoxytyramine (3-MT) all showed similarly linear declines (HVA: r2 = 0.762, p < 0.0001; DOPAC: r2 = 0.776, p < 0.0001; 3-MT: r2 = 0.602, p < 0.0001) to that of dopamine during our gradual depletion paradigm, but did not reach quite the same end stage values as seen during acute depletion (Fig. 2A). This suggests there may be a compensatory mechanism to slow dopamine metabolism that is engaged by gradual dopamine depletion, but this mechanism is not sufficient to boost dopamine levels by a significant amount (see Fig. 1G).
Because 6-OHDA can also kill noradrenergic neurons, a population of neurons that is also depleted in PD (Rommelfanger and Weinshenker 2007), we used HPLC to measure norepinephrine (NE) levels in the striatum. NE levels dropped by 40% (NE loss = 40.2 ± 9.1 %, n = 7, ANOVA with Dunnett t, p = 0.04 from saline) after the first injection, but then remained constant across subsequent injections, ending at the same level of depletion as seen in acutely depleted mice (Gradual NE = 52.4 ± 8.7 %, n = 9 vs. Acute NE = 41.1 ± 6.7 %, n = 8) (Fig. 2B). Finally, we examined levels of serotonin in our tissue. Although serotonin neurons are not directly affected by 6-OHDA, there have been conflicting reports about changes in serotonin levels in the dorsal striatum following dopamine depletion (Breese, Baumeister et al. 1984, Commins, Shaughnessy et al. 1989, Karstaedt, Kerasidis et al. 1994, Frechilla, Cobreros et al. 2001, Balcioglu, Zhang et al. 2003, Carta, Lindgren et al. 2006, Rylander, Parent et al. 2010) and this has been hypothesized to play a role in L-dopa induced dyskinesias (Nagatsua and Sawadab 2009, Rylander, Parent et al. 2010). Interestingly, serotonin levels remained constant until ~60% of dopamine had been lost, then suddenly increased by 10–20% (ANOVA with Dunnett t, p < 0.05 from saline). The magnitude of serotonin increase was similar in both gradually and acutely depleted animals (Fig. 2C). These results suggest that monoamine levels are altered to the same extent in both gradually and acutely depleted animals, however, the trajectories of their changes differed compared to those for dopamine.
Open Field Locomotor Deficits Develop Suddenly During Gradual Depletions
The behavioral effects of our gradual dopamine depletion paradigm were first assessed by examining spontaneous locomotion in an open field arena (Ferro, Bellissimo et al. 2005, Kreitzer and Malenka 2007, Simola, Morelli et al. 2007, Kravitz, Freeze et al. 2010). Mice were placed in a clear walled, circular arena and allowed to habituate for 10 minutes. After the habituation period, their spontaneous behavior was monitored for an additional 10 minutes with overhead and side-mounted video cameras (Fig. 3A). For all of the behavioral tasks, our questions were two-fold: At what stage of dopamine loss would behavioral impairments first become apparent, and, would end-stage motor deficits differ between gradually and acutely depleted mice?
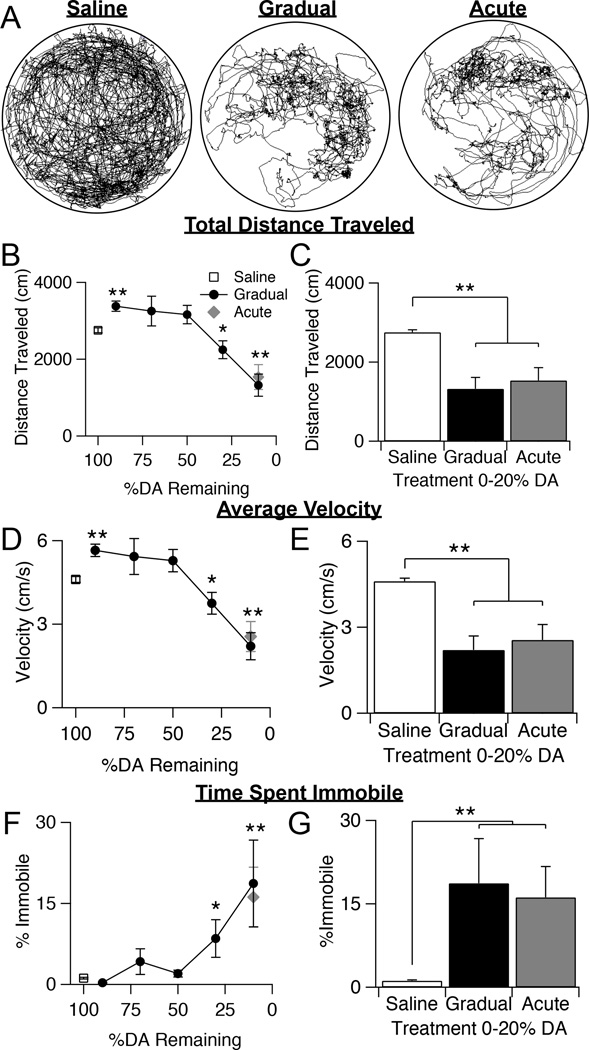
Figure 3.
Effects of gradual dopamine depletion on spontaneous locomotion in an open field. A, Example plot tracks from a saline control mouse, a gradually depleted mouse that has received 5 injections of 0.75 µg 6-OHDA, and an acutely depleted mouse that has received 1 injection of 5 µg 6-OHDA. B, Total distance traveled over 10 minutes in saline control animals, gradually depleted animals, and acutely depleted animals. Throughout the figure, error bars are SEM. KW, χ2(6) = 49.674, p < 0.0001, pairwise, *p = 0.012 from saline; **p < 0.005 from saline. C, Total distance traveled by saline control animals compared to gradually and acutely depleted mice with 0–20% dorsal striatal dopamine remaining. KW, χ2(2) = 31.091, p < 0.0001, pairwise, **p < 0.005. D, Average velocity of saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 49.642, p < 0.0001, pairwise, *p = 0.012 from saline; **p < 0.005 from saline. E, Average velocity of saline control animals compared to gradually and acutely depleted mice with 0–20% striatal dopamine remaining. KW, χ2(2) = 31.141, p < 0.0001, pairwise, **p < 0.005. F, Percentage of time spent immobile by saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 40.429, p < 0.0001, pairwise, *p < 0.05 from saline; **p < 0.005 from saline. G, Percentage of time spent immobile in saline control animals compared to gradually and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2(2) = 31.679, p < 0.0001, pairwise, **p < 0.005.
Using EthoVision behavioral tracking software (Noldus), we quantified movement parameters such as total distance traveled, average velocity, and time spent immobile (Fig. 3B–G). We measured parameters that would capture both mobility and immobility because these different aspects of motor function are believed to be controlled by distinct pathways within the basal ganglia and may be differentially affected by dopamine depletion (Albin, Young et al. 1989, DeLong 1990, Graybiel, Aosaki et al. 1994, Schwarting and Huston 1996, Schwarting and Huston 1996, Day, Wang et al. 2006, Mallet, Ballion et al. 2006, Galvan and Wichmann 2007, Kreitzer and Malenka 2007, Gittis, Hang et al. 2011). Gradually depleted mice were grouped into five groups, based on percent dopamine remaining (89.8 ± 2.4%, n = 10; 73.9 ± 1.8%, n = 9; 51.8 ± 1.3%, n = 22; 30.1 ± 1.4%, n = 21; 14.1 ± 1.7 %, n = 12). Dopamine levels decreased linearly with increasing number of injections of 0.75 µg 6-OHDA received (r2 = 0.594, p<0.0005). Saline-injected mice moved an average of 2756 ± 63 cm (n = 28) in 10 minutes. At early stages of gradual dopamine depletion (<20%), locomotion was actually increased (3381 ± 132 cm, n = 10, KW pairwise, p = 0.006 from saline) (Fig. 3B). As our gradual depletion paradigm progressed, mice continued to show robust movement around the arena until dopamine levels had dropped by ~70%, after which distance traveled sharply declined. At end-stage dopamine levels (dopamine loss >80%), locomotion had decreased by about 2-fold compared to saline and was similarly impaired in both gradually and acutely depleted mice (Gradual = 1323 ± 29 cm, n=12; Acute = 1534 ± 324 cm, n=14, KW pairwise, p = 1) (Fig. 3C).
Average velocity during the 10-minute period followed a similar pattern. The average velocity of saline-treated mice was 4.6 ± 0.1 cm/s and increased slightly after the initial injection, before declining sharply once dopamine loss exceeded ~70% (Fig. 3D). At end-stage dopamine levels, both gradually and acutely depleted animals showed a similar 2-fold decrease in velocity compared to saline control animals (Gradual = 2.2 ± 0.4 cm/s; Acute = 2.5 ± 0.5 cm/s, KW pairwise, p = 1) (Fig. 3E). Finally, we also observed that mice spent relatively little time during this task immobile, until gradual depletions reached ~70%, after which immobility increased significantly (KW pairwise, p < 0.05 from saline) (Fig. 3F). Immobility was defined such that any movement of the head, limbs, or tail did not count towards time spent immobile. By the time dopamine depletions were >80%, gradually depleted mice spent 18.7 ± 8.0% of their time immobile, similar to the 16.1 ± 5.6% of time acutely depleted mice were immobile (KW pairwise, p = 1) (Fig. 3G). These results suggest that both mobile and immobile aspects of general locomotion remain intact until >70% dopamine is lost. Furthermore, animals that have lost >80% of their dopamine, either through gradual or the acute depletion paradigms, show the same degree of locomotor deficits on this task.
Rearing Deficits Progress Gradually As Dopamine Is Depleted
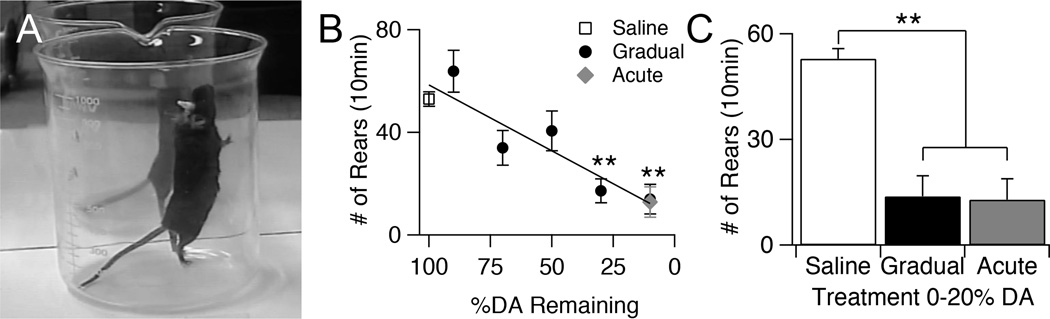
Although movement in the open field can detect general deficits in locomotion, this task is very broad and does not reveal deficits to any particular system. In contrast, vertical movement, assessed with rearing tasks, has been suggested to correlate strongly with dorsolateral striatal function (Jicha and Salamone 1991, Drago, Gerfen et al. 1994, Schwarting, Sedelis et al. 1999). To assess how rearing behavior changed over the course of our gradual dopamine depletion, mice were placed in a 1000 mL beaker for 10 minutes, and the number of rears were counted over this period (Fig. 4A).
Figure 4.
Effects of gradual dopamine depletion on rearing behavior. A, Example of a full extension rear. B, Average number of rears of saline control animals, gradually depleted animals across all dopamine levels, and acutely depleted animals during 10 minutes in beaker. Throughout the figure, error bars are SEM. KW, χ2(6) = 42.623, p < 0.0001, pairwise, **p < 0.005 from saline. C, Average number of rears of saline control animals, gradually depleted animals with 0–20% dorsal striatal dopamine, and acutely depleted animals with 0–20% dorsal striatal dopamine. KW, χ2(2) = 23.977, p < 0.0001, pairwise, **p < 0.005. Figure 5. Effects of gradual dopamine depletion on motor coordination assessed with a vertical pole task. A, Image of mouse descending pole and schematic of timed parameters of pole task. B, Average time needed to complete the entire task for saline control animals, gradually depleted animals, and acutely depleted animals. Throughout the figure, error bars are SEM. KW, χ2(6) = 52.592, p < 0.0001, pairwise, **p < 0.005 from saline. C, Average total time of saline control animals compared to gradually and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2(2) = 39.537, p < 0.0001, pairwise, *p < 0.05. D, Average turn down latency (TDL) of saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 37.667, p < 0.0001, pairwise, **p < 0.005 from saline. E, Average TDL of saline control animals compared to gradually and acutely depleted animals with 0–20% dorsal striatal dopamine, and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2 (2) = 29.908, p < 0.0001, pairwise, **p < 0.005. F, Average traversal time of saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 58.834, p < 0.0001, pairwise, *p < 0.05 from saline; **p < 0.005 from saline. G, Average traversal time of saline control animals compared to gradually and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2(2)= 43.068, p < 0.0001, pairwise, *p < 0.05.
The number of rears in gradually depleted mice decreased linearly (r2 = 0.314, p < 0.0001) with decreasing levels of dopamine (Fig. 4B). At end-stage dopamine levels (depletion >80%), acute and gradually depleted mice showed similar reductions in rearing frequency compared to saline controls that had been tested a similar number of times (Saline = 52.9 ± 2.8, n = 24; Gradual = 13.8 ± 5.7, n = 10; Acute = 12.8 ± 5.9, n = 13, KW pairwise, saline vs. gradual and acute, p < 0.006, gradual vs. acute, p = 1) (Fig. 4C).
Motor Coordination is Differentially Affected In Gradually vs. Acutely Depleted Mice
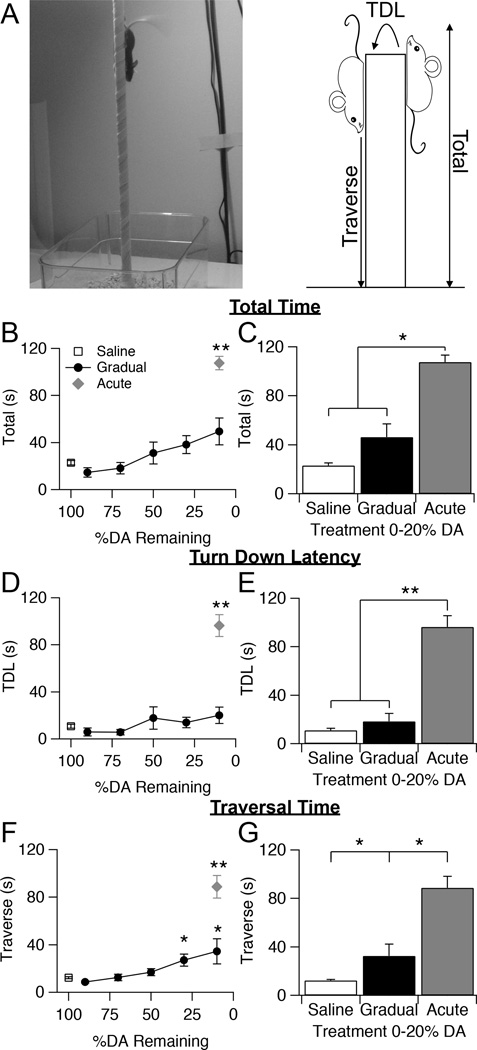
Both open field and rearing tasks assess different aspects of spontaneous gross motor function in mice, but neither provides a measurement of fine movement or coordination, behaviors which detect early motor deficits in some mouse models of disease (Ogawa, Hirose et al. 1985, Matsuura, Kabuto et al. 1997, Sedelis, Schwarting et al. 2001, Fernagut, Chalon et al. 2003, Fleming, Salcedo et al. 2004). To assess fine motor coordination in gradually depleted mice, animals were subjected to a pole task. While less commonly utilized than open field and rearing, the pole task is a well-established technique used to assess motor skills in many animal models of diseases that result in motor dysfunction (Matsuura, Kabuto et al. 1997, Hickey, Kosmalska et al. 2008, Credle, George et al. 2014). The task involves placing an animal face upward on a tall vertical pole with small diameter (1 cm) and measuring the time it takes for the animal to turn facing downward (Turn down latency = TDL), the time from turning downward to finish traversing the pole (Traverse), and the total time spent on the pole (Total) (Fig. 5A).
Figure 5.
Effects of gradual dopamine depletion on motor coordination assessed with a vertical pole task. A, Image of mouse descending pole and schematic of timed parameters of pole task. B, Average time needed to complete the entire task for saline control animals, gradually depleted animals, and acutely depleted animals. Throughout the figure, error bars are SEM. KW, χ2(6) = 52.592, p < 0.0001, pairwise, **p < 0.005 from saline. C, Average total time of saline control animals compared to gradually and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2(2) = 39.537, p < 0.0001, pairwise, *p < 0.05. D, Average turn down latency (TDL) of saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 37.667, p < 0.0001, pairwise, **p < 0.005 from saline. E, Average TDL of saline control animals compared to gradually and acutely depleted animals with 0–20% dorsal striatal dopamine, and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2 (2) = 29.908, p < 0.0001, pairwise, **p < 0.005. F, Average traversal time of saline control animals, gradually depleted animals, and acutely depleted animals. KW, χ2(6) = 58.834, p < 0.0001, pairwise, *p < 0.05 from saline; **p < 0.005 from saline. G, Average traversal time of saline control animals compared to gradually and acutely depleted animals with 0–20% striatal dopamine remaining. KW, χ2(2)= 43.068, p < 0.0001, pairwise, *p < 0.05.
Intriguingly, although acutely depleted mice were severely impaired on this task, gradually depleted mice maintained a level of performance similar to that of saline controls (KW pairwise, p > 0.12 from saline) (Fig. 5B). The total time gradually depleted animals spent on the pole increased slightly with decreasing levels of striatal dopamine, but even at end-stage dopamine levels, gradually depleted mice significantly outperformed acutely depleted mice (KW pairwise, p = 0.00495), and their time spent on the pole was not significantly different from that of saline controls (KW pairwise, p = 0.102) (Saline = 23.0 ± 2.0, n = 26; Gradual =46.3 ± 10.7, n = 11; Acute = 107.4 ± 5.7, n = 15) (Fig. 5C).
To differentiate between the two different components of this task: orienting downward after being placed at the top of the pole, and descending to the bottom, we separately analyzed the time needed to complete these two phases of the task. Turn down latency of gradually depleted mice remained similar to that of saline controls throughout dopamine depletion (KW pairwise, p > 0.318 from saline) (Fig. 5D–E). In contrast, acutely depleted mice displayed a significantly increased turn down latency (Saline = 11.0 ± 1.7; Gradual =18.4 ± 6.5; Acute = 96.3 ± 9.4; KW pairwise, p < 0.005 from saline and gradual). In many cases, acutely depleted animals either could not turn downward on their own, or it took them over half of the total time allotted for the task. Gradually depleted mice showed a significant impairment on this task during the traverse phase, where the amount of time it took them to walk to the bottom was significantly greater than that of saline controls (KW pairwise, p = 0.012), but still much faster than that of acutely depleted mice (KW pairwise, p = 0.0012) (Saline = 12.3 ± 0.7; Gradual =32.4 ± 9.6; Acute = 88.7 ± 9.6) (Fig. 5F–G). In many cases, acutely depleted animals either fell from the pole, unable to complete the task, or circled around the pole many times as they descended, resulting in traversal times over half of the allotted time for the task. Gradually depleted animals also circled around the pole as they descended; however they were still significantly faster than acutely depleted animals.
These results suggest that certain aspects of an animal’s behavior may adapt differently when dopamine is depleted gradually rather than acutely. Gradually depleted animals show little or no deficits on the pole task, whereas acutely depleted animals show severe deficits on the same task.
Time Course of Gradual Depletions Can Be Varied And Extended
The gradual paradigm used for this study involved injections every three days for a total of 15 days. This is five times longer than the traditional acute depletion, however this is still a relatively short time span compared to the slow progression of PD in humans. Therefore, we wanted to determine whether dopamine could be fully depleted using a longer time course. We therefore spaced our injections of 0.75 µg 6-OHDA every seven days, instead of every three days, for a total of 35 days. Subsets of animals were sacrificed after three injections and five injections to analyze TH levels in the dorsal striatum (Fig. 6A). In contrast to our findings with injections every three days, five injections spaced every seven days was not sufficient to fully deplete dopamine from the striatum (Fig. 6A). However, increasing the number of injections to seven, for a total of 49 days, resulted in full striatal dopamine depletion (KW pairwise, p < 0.0001 from saline) (Fig. 6B).
At end-stage dopamine depletions (>80%), spontaneous locomotion of seven-day gradually depleted mice in the open field was compared to that of three-day gradually depleted and acutely depleted mice (Fig. 6C). Distance traveled and velocity were decreased in seven-day depleted animals compared to saline (KW pairwise, p = 0.018 from saline), to a similar extent as that seen in three-day gradual and acutely depleted mice (KW pairwise, p < 0.002 from saline, p = 1 from 7day) (Fig. 6D–E). Although immobility tended to increase in seven-day depleted mice compared to saline, this increase was not as dramatic as that seen with the acute and three-day gradual paradigm (KW pairwise, p = 0.24 from saline), suggesting there may be additional compensatory mechanisms engaged during the longer seven-day depletion paradigm resulting in a smaller reduction in mobility (Saline = 1.1 ± 0.1 %, n = 40; Gradual(7day) = 4.1 ± 2.1 %, n = 4; Gradual(3day) = 18.6 ± 8.0 %, n =12; Acute = 16.1 ± 5.5 %, n = 14) (Fig. 6F–G). Percentage of time spent performing fine movements was also analyzed, however there were no differences between mice that underwent the seven-day paradigm, three-day paradigm, or acute paradigm (data not shown).
Discussion
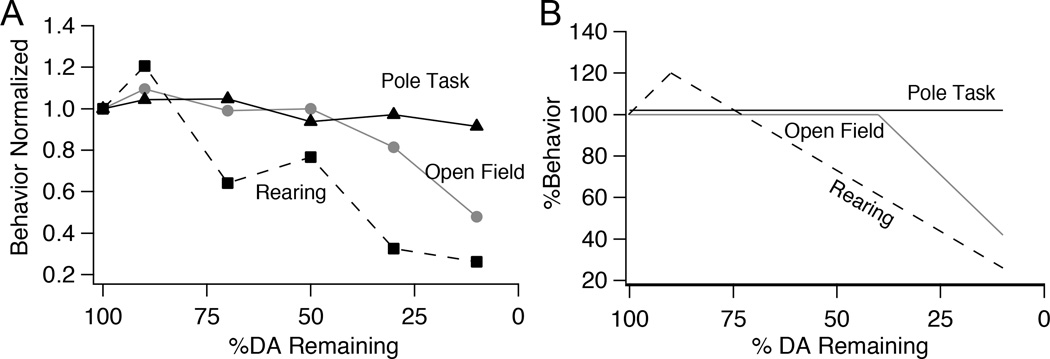
Using a progressive model of dopamine depletion, we show that the time course of dopamine loss can impact the severity of motor symptoms. Furthermore, progressive dopamine loss reveals dissociable rates of behavioral impairment for different types of motor tasks (Fig. 7A–B). Spontaneous locomotion in the open field remained normal until rapidly declining with depletions >70%; rearing behavior declined steadily as a function of decreasing dopamine levels; and performance on the pole task remained robust in gradually depleted mice, despite severe deficits on this task in acutely depleted mice. Taken together, these data suggest that our gradual depletion paradigm provides an experimental platform to study circuit changes during early dopamine loss, including mechanisms of compensatory plasticity that may be precluded in traditional acute models.
Figure 7.
Differential progression motor deficits in gradually depleted mice. A, Normalized behavior of gradually depleted animals plotted as a function of striatal dopamine levels. Percentage of time spent mobile normalized to saline controls is plotted as a representative of open field activity, total number of rears normalized to saline controls is plotted for rearing, and turn down latency normalized to saline controls is plotted as a representative of pole task performance. B, Schematic showing the differential degradation of behavior of animals whose dopamine was gradually depleted.
A major challenge in treating PD is that the hallmark motor symptoms of the disease typically do not present until the majority of dopamine (>70%) has been depleted from the system (Bernheimer, Birkmayer et al. 1973, Riederer and Wuketich 1976, Deumens, Blokland et al. 2002, Fahn 2003, Olanow and Obeso 2012). Therefore, by the time of diagnosis, physiological progression of the disease may have already reached a point where it cannot be reversed (Bezard and Gross 1998, Bezard, Gross et al. 2003, Schapira and Tolosa 2010). This early phase of the disease, when dopamine levels have started to decline but overt motor symptoms have not yet presented, is called the prodromal phase, and is likely an ideal window in which to begin therapy (Bezard, Gross et al. 2003, Tolosa, Gaig et al. 2009). However, our understanding of the physiological changes occurring during prodromal PD are sparse, and there is no clear understanding of when and where to best intervene during this period to maximize therapeutic outcome.
This knowledge gap is exacerbated by the fact that most mouse models of PD are not suitable for study of the prodromal phase because they rely on administration of an acute dose of 6-OHDA, or other neurotoxins, to produce a certain degree of depletion (Przedborski, Levivier et al. 1995, Schwarting and Huston 1996, Schwarting and Huston 1996, Kirik, Rosenblad et al. 1998, Ferro, Bellissimo et al. 2005, Truong, Allbutt et al. 2006, Meredith, Sonsalla et al. 2008). Although the acute 6-OHDA model has unveiled many differences in the brain following full dopamine depletion (Jeon, Jackson-Lewis et al. 1995, Schwarting and Huston 1996, Schober 2004, Iancu, Mohapel et al. 2005, Day, Wang et al. 2006, Mallet, Ballion et al. 2006, Kreitzer and Malenka 2007, Taverna, Ilijic et al. 2008, Kravitz, Freeze et al. 2010, Gittis, Hang et al. 2011), this technique fails to capture what is happening to the behavior of an animal as dopamine is gradually and consistently depleted within the same system, similar to the pathology of human PD (Bernheimer, Birkmayer et al. 1973, Savitt, Dawson et al. 2006, Meredith, Sonsalla et al. 2008, Potashkin, Blume et al. 2010). One promising progressive model involves AAV-induced overexpression of α-synuclein. This model results in considerable loss of dopamine, as well as gradual motor impairments and proteinaceous inclusions (Decressac, Mattsson et al. 2012, Decressac, Mattsson et al. 2012, Lundblad, Decressac et al. 2012). These vector injections may shed more light on the role of α-synuclein inclusions in the degeneration of dopamine and appearance of motor deficits, however optimization and characterization of this model is still ongoing (see (Lindgren, Lelos et al. 2012).
By administering repeated, low doses of 6-OHDA to the MFB, we were able to linearly deplete dopamine levels over a user-defined time course of 15–49 days, based on the spacing of injections. Using this approach, we induced behavioral deficits at end-stages of dopamine depletion (>80%), that were comparable to that of acutely depleted mice in both locomotor open field and rearing tasks. The only difference we observed between gradually and acutely depleted mice on these tasks was that mice who had been gradually depleted over 49 days showed less immobility in the open field, largely because they spent more of their time moving, which was more similar to the behavioral patterns observed in saline-treated mice. The fact that we observed a greater difference in immobility than mobility, suggests that there may be different degrees of compensatory plasticity within separate pathways of the basal ganglia that differentially control these distinct aspects of movement (Smith, Bevan et al. 1998, DeLong and Wichmann 2007, Kravitz, Freeze et al. 2010, Gerfen and Surmeier 2011). The lack of immobility in seven-day depleted mice compared to three-day depleted mice may indicate compensatory mechanisms that are engaged over weeks rather than days.
Through assessment of exploratory rearing, we found that impairments in this behavior appear even at early stages of dopamine loss, and continue to progress as a direct function of dopamine levels. This linear decrease in rearing has been noted in previous studies using genetic or graded toxin models (Fleming, Salcedo et al. 2004, Goldberg, Haack et al. 2011). This behavior relies heavily on dorsolateral striatal function and therefore suggests that dorsolateral striatum is being progressively impaired over the course of our depletion protocol. The dorsolateral striatum is thought to be the source for many motor symptoms of PD – because it is the nucleus most directly affected by the loss of dopaminergic neurons in the SNc – and is critically involved in motor behaviors (Albin, Young et al. 1989, DeLong 1990, Bolam, Hanley et al. 2000). The different trajectories of behavioral impairments on the open field vs. rearing tasks could be due to a number of reasons. While rearing appears to be most directly related to decreasing levels of striatal dopamine, mobility in the open field may be temporarily preserved due to compensation or adaptation in the dopamine system (Zigmond, Acheson et al. 1984, Zigmond and Hastings 1998, Zigmond, Hastings et al. 2002), altered activity of nuclei within the basal ganglia (Maneuf, Mitchell et al. 1994, Bezard, Boraud et al. 1997, Bezard, Boraud et al. 1999, Qiu, Chen et al. 2014), or compensation outside of the basal ganglia (Brooks 1999, Bezard, Gross et al. 2003, Brotchie and Fitzer-Attas 2009, Schroll, Vitay et al. 2014).
An intriguing discovery of our study is that performance on the pole task, which relies on fine motor control and coordination, was dramatically different in severely dopamine depleted animals (>80%), depending on whether mice were gradually or acutely depleted. Previous studies using other models of dopamine depletion have shown severe deficits in performing this task (Ogawa, Hirose et al. 1985, Matsuura, Kabuto et al. 1997, Fleming, Salcedo et al. 2004, Fleming, Salcedo et al. 2006). Consistent with these previous studies, acutely depleted animals either could not perform the task, or took longer than half the allotted time for the task. In contrast, gradually depleted animals showed almost no deficits when compared to saline controls. This suggests that the improved performance on the pole task is due specifically to the time course of dopamine depletion. These results lend further support to the notion that the brain compensates or adapts differently to decreased levels of dopamine when dopamine loss occurs gradually over weeks instead of acutely over days. While the pole task may not be a good test of basal ganglia function, another possibility is that performance on this task can be regulated by a number of interacting motor systems (Thullier, Lalonde et al. 1997, Brooks and Dunnett 2009), some of which can compensate when basal ganglia function is impaired. Many studies have shown the cerebellum as well as the supplementary motor area (SMA) may contribute to motor coordination and fine motor skills and show different activity patterns in human PD patients (Thach, Goodkin et al. 1992, Rascol, Sabatini et al. 1997, Debaere, Swinnen et al. 2001, Ramnani, Toni et al. 2001, Bezard, Gross et al. 2003, Wu and Hallett 2005, Yu, Sternad et al. 2007, Bostan and Strick 2010, Wu and Hallett 2013, Rosin, McAllister et al. 2014). These outside brain areas may be recruited only when dopamine is gradually depleted from the system and could account for the differences seen on the pole task. This difference in pole task performance highlights the importance of developing and analyzing models where dopamine is depleted gradually within the same animal in a manner that is more consistent with PD pathology.
Although toxin models do not replicate all symptoms of human PD, most notably Lewy body inclusions, they are critical tools for gaining better mechanistic insights into circuit changes underlying the motor symptoms of the disease. In particular, toxin models are useful for studies directed at cell-specific mechanisms of circuit dysfunction, especially in mice where genetic labeling and manipulation of cells and circuits is unparalleled compared to any other mammalian system. By developing toxin paradigms that better replicate the chronic dopamine loss seen in human PD, we can gain a better understanding of how the temporal progression of dopamine loss alters neural circuits and ultimately motor function. A thorough understanding of how neural circuits adapt to decreasing levels of dopamine could lead to more effective therapies targeted at restoring circuit function in addition to restoration of dopamine levels.
In summary, we have shown that 6-OHDA can be utilized to gradually deplete dopamine within the same animal over time. This model has allowed us to uncover differential trajectories of motor degradation due to gradual dopamine depletion. Furthermore, this gradual model does not show the same extent of motor deficits seen in traditional acute models of dopamine depletion, suggesting that compensation may have a bigger impact on behavior when dopamine is gradually reduced within the same animal over time. This gradual depletion model can be manipulated to further uncover important aspects of how the brain adapts during the prodromal phase of PD and may lead to better methods of diagnosing and treating this neurodegenerative disease.
Highlights.
Development of an experimental platform for prodromal PD using gradual, bilateral dopamine depletion with 6-OHDA
Progressive dopamine loss reveals dissociable rates of behavioral impairment for different types of motor tasks
Rearing declines linearly with dopamine depletion
Open field locomotion remains robust until striatal dopamine levels decrease by >70%
Pole task performance remains intact, suggesting compensation during the slow depletion paradigm.
Acknowledgements
The authors would like to thank Michael Zigmond and Sandy Castro for helpful discussion. We also thank Amira Millette and Alexis Willis for their assistance with immunohistochemistry, behavioral scoring, and imaging. This work was supported by a NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation, National Institutes of Health Grants R00 NS076524 and T32 NS007433, and a grant from the Disruptive Healthcare Technology Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12(10):366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Balcioglu A, Zhang K, Tarazi FI. Dopamine depletion abolishes apomorphine- and amphetamine-induced increases in extracellular serotonin levels in the striatum of conscious rats: a microdialysis study. Neuroscience. 2003;119(4):1045–1053. doi: 10.1016/s0306-4522(03)00219-7. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24(4):308–318. doi: 10.1002/bies.10067. [DOI] [PubMed] [Google Scholar]
- Bezard E, Boraud T, Bioulac B, Gross CE. Compensatory effects of glutamatergic inputs to the substantia nigra pars compacta in experimental parkinsonism. Neuroscience. 1997;81(2):399–404. doi: 10.1016/s0306-4522(97)00226-1. [DOI] [PubMed] [Google Scholar]
- Bezard E, Boraud T, Bioulac B, Gross CE. Involvement of the subthalamic nucleus in glutamatergic compensatory mechanisms. Eur J Neurosci. 1999;11(6):2167–2170. doi: 10.1046/j.1460-9568.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- Bezard E, Dovero S, Bioulac B, Gross CE. Kinetics of nigral degeneration in a chronic model of MPTP-treated mice. Neurosci Lett. 1997;234(1):47–50. doi: 10.1016/s0304-3940(97)00663-0. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE. Compensatory mechanisms in experimental and human parkinsonism: towards a dynamic approach. Prog Neurobiol. 1998;55(2):93–116. doi: 10.1016/s0301-0082(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Bezard E, Gross CE, Brotchie JM. Presymptomatic compensation in Parkinson's disease is not dopamine-mediated. Trends Neurosci. 2003;26(4):215–221. doi: 10.1016/S0166-2236(03)00038-9. [DOI] [PubMed] [Google Scholar]
- Blesa J, Juri C, Collantes M, Penuelas I, Prieto E, Iglesias E, Marti-Climent J, Arbizu J, Zubieta JL, Rodriguez-Oroz MC, Garcia-Garcia D, Richter JA, Cavada C, Obeso JA. Progression of dopaminergic depletion in a model of MPTP-induced Parkinsonism in non-human primates. An (18)F-DOPA and (11)C-DTBZ PET study. Neurobiol Dis. 2010;38(3):456–463. doi: 10.1016/j.nbd.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Blume SR, Cass DK, Tseng KY. Stepping test in mice: a reliable approach in determining forelimb akinesia in MPTP-induced Parkinsonism. Exp Neurol. 2009;219(1):208–211. doi: 10.1016/j.expneurol.2009.05.017. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196(Pt 4):527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan AC, Strick PL. The cerebellum and basal ganglia are interconnected. Neuropsychol Rev. 2010;20(3):261–270. doi: 10.1007/s11065-010-9143-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J, Prou D, Perier C, Przedborski S. Toxin-induced models of Parkinson's disease. NeuroRx. 2005;2(3):484–494. doi: 10.1602/neurorx.2.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Baumeister AA, McCown TJ, Emerick SG, Frye GD, Crotty K, Mueller RA. Behavioral differences between neonatal and adult 6-hydroxydopamine-treated rats to dopamine agonists: relevance to neurological symptoms in clinical syndromes with reduced brain dopamine. J Pharmacol Exp Ther. 1984;231(2):343–354. [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ. Functional imaging of Parkinson's disease: is it possible to detect brain areas for specific symptoms? J Neural Transm Suppl. 1999;56:139–153. doi: 10.1007/978-3-7091-6360-3_8. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user's guide. Nat Rev Neurosci. 2009;10(7):519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- Brotchie J, Fitzer-Attas C. Mechanisms compensating for dopamine loss in early Parkinson disease. Neurology. 2009;72(7 Suppl):S32–S38. doi: 10.1212/WNL.0b013e318198e0e9. [DOI] [PubMed] [Google Scholar]
- Carta M, Lindgren HS, Lundblad M, Stancampiano R, Fadda F, Cenci MA. Role of striatal L-DOPA in the production of dyskinesia in 6-hydroxydopamine lesioned rats. J Neurochem. 2006;96(6):1718–1727. doi: 10.1111/j.1471-4159.2006.03696.x. [DOI] [PubMed] [Google Scholar]
- Chesselet MF, Fleming S, Mortazavi F, Meurers B. Strengths and limitations of genetic mouse models of Parkinson's disease. Parkinsonism Relat Disord. 2008;14(Suppl 2):S84–S87. doi: 10.1016/j.parkreldis.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins DL, Shaughnessy RA, Axt KJ, Vosmer G, Seiden LS. Variability among brain regions in the specificity of 6-hydroxydopamine (6-OHDA)-induced lesions. J Neural Transm. 1989;77(2–3):197–210. doi: 10.1007/BF01248932. [DOI] [PubMed] [Google Scholar]
- Credle JJ, George JL, Wills J, Duka V, Shah K, Lee YC, Rodriguez O, Simkins T, Winter M, Moechars D, Steckler T, Goudreau J, Finkelstein DI, Sidhu A. GSK-3beta dysregulation contributes to parkinson's-like pathophysiology with associated region-specific phosphorylation and accumulation of tau and alpha-synuclein. Cell Death Differ. 2014 doi: 10.1038/cdd.2014.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson's disease: mechanisms and models. Neuron. 2003;39(6):889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Ko HS, Dawson VL. Genetic animal models of Parkinson's disease. Neuron. 2010;66(5):646–661. doi: 10.1016/j.neuron.2010.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9(2):251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Debaere F, Swinnen SP, Beatse E, Sunaert S, Van Hecke P, Duysens J. Brain areas involved in interlimb coordination: a distributed network. Neuroimage. 2001;14(5):947–958. doi: 10.1006/nimg.2001.0892. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Bjorklund A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson's disease. Exp Neurol. 2012;235(1):306–315. doi: 10.1016/j.expneurol.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45(3):939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13(7):281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Arch Neurol. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- Deumens R, Blokland A, Prickaerts J. Modeling Parkinson's disease in rats: an evaluation of 6-OHDA lesions of the nigrostriatal pathway. Exp Neurol. 2002;175(2):303–317. doi: 10.1006/exnr.2002.7891. [DOI] [PubMed] [Google Scholar]
- Drago J, Gerfen CR, Lachowicz JE, Steiner H, Hollon TR, Love PE, Ooi GT, Grinberg A, Lee EJ, Huang SP, et al. Altered striatal function in a mutant mouse lacking D1A dopamine receptors. Proc Natl Acad Sci U S A. 1994;91(26):12564–12568. doi: 10.1073/pnas.91.26.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S. Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci. 2003;991:1–14. doi: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114(Pt 5):2283–2301. doi: 10.1093/brain/114.5.2283. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Chalon S, Diguet E, Guilloteau D, Tison F, Jaber M. Motor behaviour deficits and their histopathological and functional correlates in the nigrostriatal system of dopamine transporter knockout mice. Neuroscience. 2003;116(4):1123–1130. doi: 10.1016/s0306-4522(02)00778-9. [DOI] [PubMed] [Google Scholar]
- Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson's disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148(1):78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Delville Y, Schallert T. An intermittent, controlled-rate, slow progressive degeneration model of Parkinson's disease: antiparkinson effects of Sinemet and protective effects of methylphenidate. Behav Brain Res. 2005;156(2):201–213. doi: 10.1016/j.bbr.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Fernagut PO, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Early and progressive sensorimotor anomalies in mice overexpressing wild-type human alpha-synuclein. J Neurosci. 2004;24(42):9434–9440. doi: 10.1523/JNEUROSCI.3080-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SM, Salcedo J, Hutson CB, Rockenstein E, Masliah E, Levine MS, Chesselet MF. Behavioral effects of dopaminergic agonists in transgenic mice overexpressing human wildtype alpha-synuclein. Neuroscience. 2006;142(4):1245–1253. doi: 10.1016/j.neuroscience.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frechilla D, Cobreros A, Saldise L, Moratalla R, Insausti R, Luquin M, Del Rio J. Serotonin 5-HT(1A) receptor expression is selectively enhanced in the striosomal compartment of chronic parkinsonian monkeys. Synapse. 2001;39(4):288–296. doi: 10.1002/1098-2396(20010315)39:4<288::AID-SYN1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Galvan A, Wichmann T. GABAergic circuits in the basal ganglia and movement disorders. Prog Brain Res. 2007;160:287–312. doi: 10.1016/S0079-6123(06)60017-4. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gittis AH, Hang GB, LaDow ES, Shoenfeld LR, Atallah BV, Finkbeiner S, Kreitzer AC. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71(5):858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg NR, Fields V, Pflibsen L, Salvatore MF, Meshul CK. Social enrichment attenuates nigrostriatal lesioning and reverses motor impairment in a progressive 1-methyl-2-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson's disease. Neurobiol Dis. 2012;45(3):1051–1067. doi: 10.1016/j.nbd.2011.12.024. [DOI] [PubMed] [Google Scholar]
- Goldberg NR, Haack AK, Lim NS, Janson OK, Meshul CK. Dopaminergic and behavioral correlates of progressive lesioning of the nigrostriatal pathway with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2011;180:256–271. doi: 10.1016/j.neuroscience.2011.02.027. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265(5180):1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Greenamyre JT, Betarbet R, Sherer TB. The rotenone model of Parkinson's disease: genes, environment and mitochondria. Parkinsonism Relat Disord. 2003;9(Suppl 2):S59–S64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- Hickey MA, Kosmalska A, Enayati J, Cohen R, Zeitlin S, Levine MS, Chesselet MF. Extensive early motor and non-motor behavioral deficits are followed by striatal neuronal loss in knock-in Huntington's disease mice. Neuroscience. 2008;157(1):280–295. doi: 10.1016/j.neuroscience.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisahara S, Shimohama S. Toxin-induced and genetic animal models of Parkinson's disease. Parkinsons Dis. 2010;2011:951709. doi: 10.4061/2011/951709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res. 2005;162(1):1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- Jeon BS, Jackson-Lewis V, Burke RE. 6-Hydroxydopamine lesion of the rat substantia nigra: time course and morphology of cell death. Neurodegeneration. 1995;4(2):131–137. doi: 10.1006/neur.1995.0016. [DOI] [PubMed] [Google Scholar]
- Jicha GA, Salamone JD. Vacuous jaw movements and feeding deficits in rats with ventrolateral striatal dopamine depletion: possible relation to parkinsonian symptoms. J Neurosci. 1991;11(12):3822–3829. doi: 10.1523/JNEUROSCI.11-12-03822.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karstaedt PJ, Kerasidis H, Pincus JH, Meloni R, Graham J, Gale K. Unilateral destruction of dopamine pathways increases ipsilateral striatal serotonin turnover in rats. Exp Neurol. 1994;126(1):25–30. doi: 10.1006/exnr.1994.1039. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152(2):259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Kravitz AV, Freeze BS, Parker PR, Kay K, Thwin MT, Deisseroth K, Kreitzer AC. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–626. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreitzer AC, Malenka RC. Endocannabinoid-mediated rescue of striatal LTD and motor deficits in Parkinson's disease models. Nature. 2007;445(7128):643–647. doi: 10.1038/nature05506. [DOI] [PubMed] [Google Scholar]
- Lindgren HS, Lelos MJ, Dunnett SB. Do alpha-synuclein vector injections provide a better model of Parkinson's disease than the classic 6-hydroxydopamine model? Exp Neurol. 2012;237(1):36–42. doi: 10.1016/j.expneurol.2012.05.022. [DOI] [PubMed] [Google Scholar]
- Little S, Brown P. Focusing brain therapeutic interventions in space and time for Parkinson's disease. Curr Biol. 2014;24(18):R898–R909. doi: 10.1016/j.cub.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad M, Decressac M, Mattsson B, Bjorklund A. Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci U S A. 2012;109(9):3213–3219. doi: 10.1073/pnas.1200575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of parkinsonian rats. J Neurosci. 2006;26(14):3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneuf YP, Mitchell IJ, Crossman AR, Brotchie JM. On the role of enkephalin cotransmission in the GABAergic striatal efferents to the globus pallidus. Exp Neurol. 1994;125(1):65–71. doi: 10.1006/exnr.1994.1007. [DOI] [PubMed] [Google Scholar]
- Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117(Pt 4):877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- Matsuura K, Kabuto H, Makino H, Ogawa N. Pole test is a useful method for evaluating the mouse movement disorder caused by striatal dopamine depletion. J Neurosci Methods. 1997;73(1):45–48. doi: 10.1016/s0165-0270(96)02211-x. [DOI] [PubMed] [Google Scholar]
- McNaught KS, Perl DP, Brownell AL, Olanow CW. Systemic exposure to proteasome inhibitors causes a progressive model of Parkinson's disease. Ann Neurol. 2004;56(1):149–162. doi: 10.1002/ana.20186. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Kang UJ. Behavioral models of Parkinson's disease in rodents: a new look at an old problem. Mov Disord. 2006;21(10):1595–1606. doi: 10.1002/mds.21010. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK, Chesselet MF. Animal models of Parkinson's disease progression. Acta Neuropathol. 2008;115(4):385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 2002;956(1):156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Morrish PK, Sawle GV, Brooks DJ. An [18F]dopa-PET and clinical study of the rate of progression in Parkinson's disease. Brain. 1996;119(Pt 2):585–591. doi: 10.1093/brain/119.2.585. [DOI] [PubMed] [Google Scholar]
- Nagatsua T, Sawadab M. L-dopa therapy for Parkinson's disease: past, present, and future. Parkinsonism Relat Disord. 2009;15(Suppl 1):S3–S8. doi: 10.1016/S1353-8020(09)70004-5. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Hirose Y, Ohara S, Ono T, Watanabe Y. A simple quantitative bradykinesia test in MPTP-treated mice. Res Commun Chem Pathol Pharmacol. 1985;50(3):435–441. [PubMed] [Google Scholar]
- Olanow CW, Obeso JA. The significance of defining preclinical or prodromal Parkinson's disease. Mov Disord. 2012;27(5):666–669. doi: 10.1002/mds.25019. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Tatton WG. Etiology and pathogenesis of Parkinson's disease. Annu Rev Neurosci. 1999;22:123–144. doi: 10.1146/annurev.neuro.22.1.123. [DOI] [PubMed] [Google Scholar]
- Potashkin JA, Blume SR, Runkle NK. Limitations of animal models of Parkinson's disease. Parkinsons Dis. 2010;2011:658083. doi: 10.4061/2011/658083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przedborski S, Jackson-Lewis V, Naini AB, Jakowec M, Petzinger G, Miller R, Akram M. The parkinsonian toxin 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): a technical review of its utility and safety. J Neurochem. 2001;76(5):1265–1274. doi: 10.1046/j.1471-4159.2001.00183.x. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Levivier M, Jiang H, Ferreira M, Jackson-Lewis V, Donaldson D, Togasaki DM. Dose-dependent lesions of the dopaminergic nigrostriatal pathway induced by intrastriatal injection of 6-hydroxydopamine. Neuroscience. 1995;67(3):631–647. doi: 10.1016/0306-4522(95)00066-r. [DOI] [PubMed] [Google Scholar]
- Qiu MH, Chen MC, Huang ZL, Lu J. Neuronal activity (c-Fos) delineating interactions of the cerebral cortex and basal ganglia. Front Neuroanat. 2014;8:13. doi: 10.3389/fnana.2014.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnani N, Toni I, Passingham RE, Haggard P. The cerebellum and parietal cortex play a specific role in coordination: a PET study. Neuroimage. 2001;14(4):899–911. doi: 10.1006/nimg.2001.0885. [DOI] [PubMed] [Google Scholar]
- Rascol O, Sabatini U, Fabre N, Brefel C, Loubinoux I, Celsis P, Senard JM, Montastruc JL, Chollet F. The ipsilateral cerebellar hemisphere is overactive during hand movements in akinetic parkinsonian patients. Brain. 1997;120(Pt 1):103–110. doi: 10.1093/brain/120.1.103. [DOI] [PubMed] [Google Scholar]
- Riederer P, Wuketich S. Time course of nigrostriatal degeneration in parkinson's disease. A detailed study of influential factors in human brain amine analysis. J Neural Transm. 1976;38(3–4):277–301. doi: 10.1007/BF01249445. [DOI] [PubMed] [Google Scholar]
- Rommelfanger KS, Weinshenker D. Norepinephrine: The redheaded stepchild of Parkinson's disease. Biochem Pharmacol. 2007;74(2):177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- Rosin JM, McAllister BB, Dyck RH, Percival CJ, Kurrasch DM, Cobb J. Mice lacking the transcription factor SHOX2 display impaired cerebellar development and deficits in motor coordination. Dev Biol. 2014 doi: 10.1016/j.ydbio.2014.12.013. [DOI] [PubMed] [Google Scholar]
- Rylander D, Parent M, O'Sullivan SS, Dovero S, Lees AJ, Bezard E, Descarries L, Cenci MA. Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol. 2010;68(5):619–628. doi: 10.1002/ana.22097. [DOI] [PubMed] [Google Scholar]
- Savitt JM, Dawson VL, Dawson TM. Diagnosis and treatment of Parkinson disease: molecules to medicine. J Clin Invest. 2006;116(7):1744–1754. doi: 10.1172/JCI29178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Tolosa E. Molecular and clinical prodrome of Parkinson disease: implications for treatment. Nat Rev Neurol. 2010;6(6):309–317. doi: 10.1038/nrneurol.2010.52. [DOI] [PubMed] [Google Scholar]
- Schober A. Classic toxin-induced animal models of Parkinson's disease: 6-OHDA and MPTP. Cell Tissue Res. 2004;318(1):215–224. doi: 10.1007/s00441-004-0938-y. [DOI] [PubMed] [Google Scholar]
- Schroll H, Vitay J, Hamker FH. Dysfunctional and compensatory synaptic plasticity in Parkinson's disease. Eur J Neurosci. 2014;39(4):688–702. doi: 10.1111/ejn.12434. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50(2–3):275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Huston JP. Unilateral 6-hydroxydopamine lesions of mesostriatal dopamine neurons and their physiological sequelae. Prog Neurobiol. 1996;49(3):215–266. doi: 10.1016/s0301-0082(96)00015-9. [DOI] [PubMed] [Google Scholar]
- Schwarting RK, Sedelis M, Hofele K, Auburger GW, Huston JP. Strain-dependent recovery of open-field behavior and striatal dopamine deficiency in the mouse MPTP model of Parkinson's disease. Neurotox Res. 1999;1(1):41–56. doi: 10.1007/BF03033338. [DOI] [PubMed] [Google Scholar]
- Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson's disease. Behav Brain Res. 2001;125(1–2):109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- Simola N, Morelli M, Carta AR. The 6-hydroxydopamine model of Parkinson's disease. Neurotox Res. 2007;11(3–4):151–167. doi: 10.1007/BF03033565. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86(2):353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Taverna S, Ilijic E, Surmeier DJ. Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease. J Neurosci. 2008;28(21):5504–5512. doi: 10.1523/JNEUROSCI.5493-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzioglu M, Galter D. Parkinson's disease: genetic versus toxin-induced rodent models. FEBS J. 2008;275(7):1384–1391. doi: 10.1111/j.1742-4658.2008.06302.x. [DOI] [PubMed] [Google Scholar]
- Thach WT, Goodkin HP, Keating JG. The cerebellum and the adaptive coordination of movement. Annu Rev Neurosci. 1992;15:403–442. doi: 10.1146/annurev.ne.15.030192.002155. [DOI] [PubMed] [Google Scholar]
- Thullier F, Lalonde R, Cousin X, Lestienne F. Neurobehavioral evaluation of lurcher mutant mice during ontogeny. Brain Res Dev Brain Res. 1997;100(1):22–28. doi: 10.1016/s0165-3806(97)00010-2. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Gaig C, Santamaria J, Compta Y. Diagnosis and the premotor phase of Parkinson disease. Neurology. 2009;72(7 Suppl):S12–S20. doi: 10.1212/WNL.0b013e318198db11. [DOI] [PubMed] [Google Scholar]
- Truong L, Allbutt H, Kassiou M, Henderson JM. Developing a preclinical model of Parkinson's disease: a study of behaviour in rats with graded 6-OHDA lesions. Behav Brain Res. 2006;169(1):1–9. doi: 10.1016/j.bbr.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5(1):107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128(Pt 10):2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M. The cerebellum in Parkinson's disease. Brain. 2013;136(Pt 3):696–709. doi: 10.1093/brain/aws360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Sternad D, Corcos DM, Vaillancourt DE. Role of hyperactive cerebellum and motor cortex in Parkinson's disease. Neuroimage. 2007;35(1):222–233. doi: 10.1016/j.neuroimage.2006.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond MJ, Acheson AL, Stachowiak MK, Stricker EM. Neurochemical compensation after nigrostriatal bundle injury in an animal model of preclinical parkinsonism. Arch Neurol. 1984;41(8):856–861. doi: 10.1001/archneur.1984.04050190062015. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Hastings TG. Neurochemical responses to lesions of dopaminergic neurons: implications for compensation and neuropathology. Adv Pharmacol. 1998;42:788–792. doi: 10.1016/s1054-3589(08)60865-0. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Hastings TG, Perez RG. Increased dopamine turnover after partial loss of dopaminergic neurons: compensation or toxicity? Parkinsonism Relat Disord. 2002;8(6):389–393. doi: 10.1016/s1353-8020(02)00019-6. [DOI] [PubMed] [Google Scholar]