Abstract
In the last five decades the role for lymphocytes in host immune response to tumors has been shown, at least in some patients, to be a critical component in disease prognosis. Also, the heterogeneity of lymphocytes has been documented including the existence of regulatory T cells that suppress the immune response. As the functions of lymphocytes have become better defined in terms of antitumor immunity, specific targets on lymphocytes have been uncovered. The appreciation of the role of immune-checkpoints has also led to therapeutic approaches that illustrate the effectiveness of blocking negative regulators of the antitumor immune response. In this Masters of Immunology article, we trace the evolution of our understanding of tumor-infiltrating lymphocytes and discuss their role in melanoma prognosis from the very basic observation of their existence to the latest manipulation of their functions with the result of improvement of the host response against the tumor.
Introduction
The importance of lymphocytes in tumors has been long recognized. At first, it was considered that they were causative of malignancy. Their possible role in prognosis in melanoma and other tumors has only been recognized in the last 40 to 50 years. This Masters of Immunology article relates, often through the lens of personal experience, the history of the discovery and the characterization of tumor-infiltrating lymphocytes and the elucidation of their possible role in the control of human melanoma.
Historical Aspects of Tumor-infiltrating Lymphocytes in Melanoma
The observation of lymphocytes and mononuclear cells associated with human cancer was first noted more than a century ago, and these infiltrates were thought to be causative of the disease (1). The concept of an immunologic response to malignant tumors in patients was probably first proposed by Paul Ehrlich in 1907 and later expanded in 1909 (2). Coley injected bacteria into tumors, so-called Coley’s toxin, with some tumor shrinkage recognized as a host response to tumors (3). Subsequently, studies of various cancers, including osteogenic sarcoma, neuroblastoma, carcinoma of the colon, and melanoma, have been performed indicating the relationship of host immune response to tumors and patient survival. Moore and Foote hypothesized for medullary carcinoma of the breast that the “rather characteristic lymphoid infiltrate indicates some maladjustment between tumor and host, and that the clinical behavior is a further indication thereof” (4). Already by the time of the publication of “Melanoma and Skin Cancer” in the 1972 Proceedings of the International Congress on Skin Cancer in Sydney, Australia, descriptions of host immune response to melanoma in the form of anti-melanoma antibodies (5) and lymphocytotoxicity (6) to human melanoma and mouse melanoma cells have been documented. The injection of minced melanoma cells into 26 patients with metastatic melanoma resulted in 2 patients with complete tumor regression and 5 patients with partial regression of tumors (7). In a similar study, Ryan and colleagues (8) reported a high index of lymphocytotoxicity in patients with metastases injected with irradiated autologous melanoma cells. They also reported regression of metastases after injections with Bacillus Calmette-Guerin (BCG). Histology of the regressing tumors exhibited marked infiltration with lymphocytes.
The term “tumor-infiltrating lymphocytes” (TIL) was utilized for the first time in the experience of one of us (MCM) during work with Wallace H. Clark, Jr. In the detailed study of malignant melanomas that was spearheaded by Dr. Clark, the anatomic levels of invasion were proposed (9). The epidermis, the papillary dermis, and the superficial vascular plexus were considered reactive sites where cutaneous inflammatory processes occurred. Dr. Clark considered these sites the sensitizing area of the skin and used the example of poison ivy to support his hypothesis. When one of us (MCM) began to study malignant melanomas (10), Dr. Clark was struck with the prominent inflammatory infiltrate (Fig. 1a) that accompanied the superficial spreading variant of malignant melanoma in the radial growth phase, and we both invoked the possibility of a contact-like reaction. He also noted that, in the lesion that we described as the precursor of melanoma, lentigo maligna, there was often no inflammation until the lesion became microinvasive. These observations and hypotheses led to the designation of level II as a microinvasive melanoma that was probably controlled by the host response through a sensitization of the host by the tumor cells (9). Another feature that was described both in superficial spreading melanoma and in lentigo maligna melanoma was regression in the radial growth phase (11). This phenomenon was identified by the presence of partial areas of fibrosis with lymphocytes and melanophages flanked on one or both sides by the tumor (Fig. 1b). This observation was considered to be evidence of a host immune response to the tumor. As far as the levels III, IV and V were concerned, they represented a new event in tumor progression that was associated with the acquisition of metastatic potential. However, at level III, the tumor was still confined to the papillary reticular dermal junction and also often had an inflammatory infiltrate, frequently at the base of the tumor. Levels IV and V were proposed to confer the most adverse prognosis, either because the tumor could overcome the barrier of the papillary dermal interface and successfully invade through the dermis and into the fat, or because the host response had failed. In 1967 a patient of the Massachusetts General Hospital Pigmented Lesion clinic presented with a metastatic nodule surrounded by a halo of hypopigmentation. A biopsy revealed a nodule of metastatic melanoma diffusely infiltrated by lymphocytes as observed by Dr. Clark who described this response as “tumor-infiltrating lymphocytes.” This was the first time in the recollection of one of us (MCM) that this description of TILs was applied to this type of host response in melanoma (Fig. 1c). Our hope was to someday find a way to enhance the patient’s lymphocyte response to these tumors.
Figure 1.
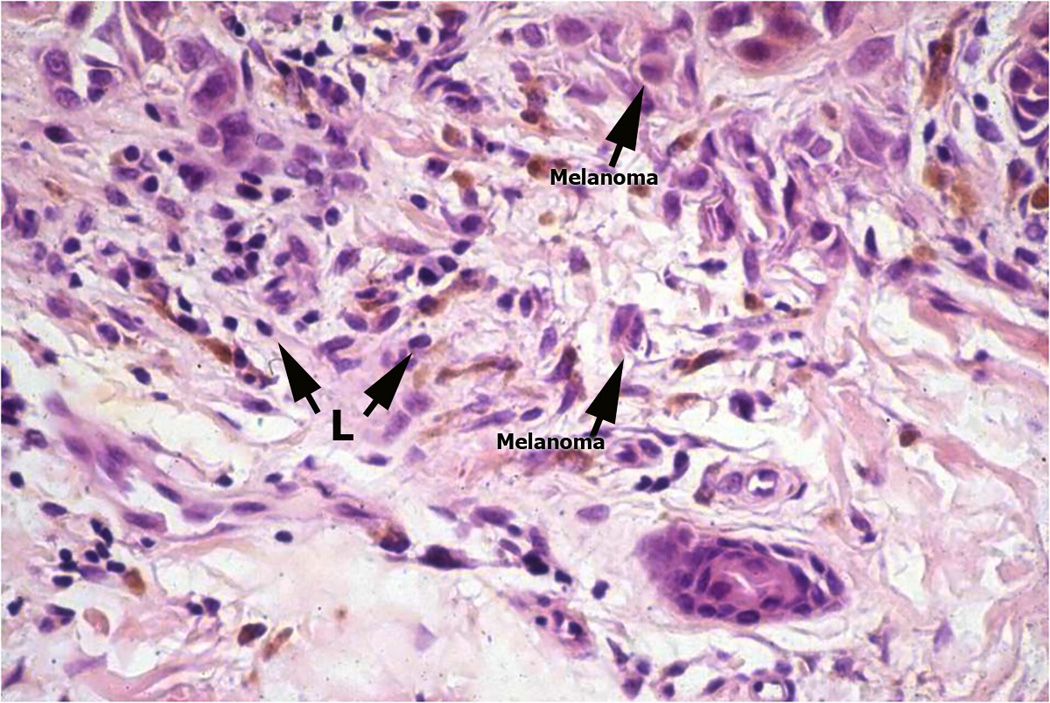
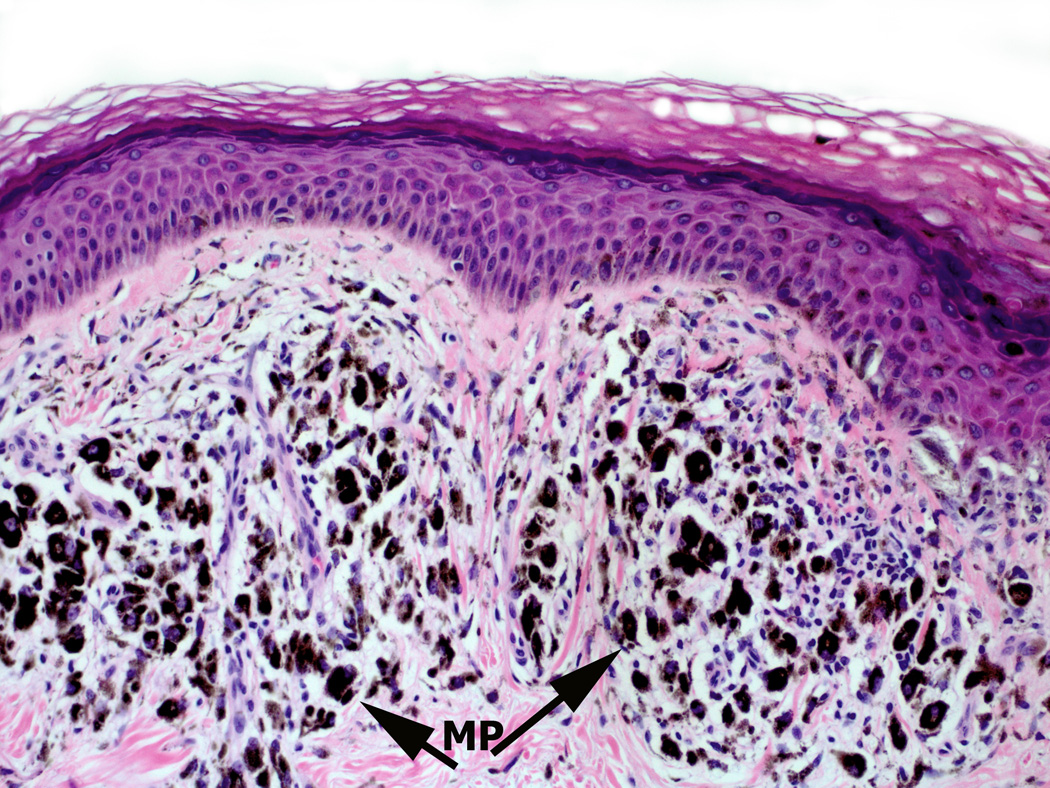
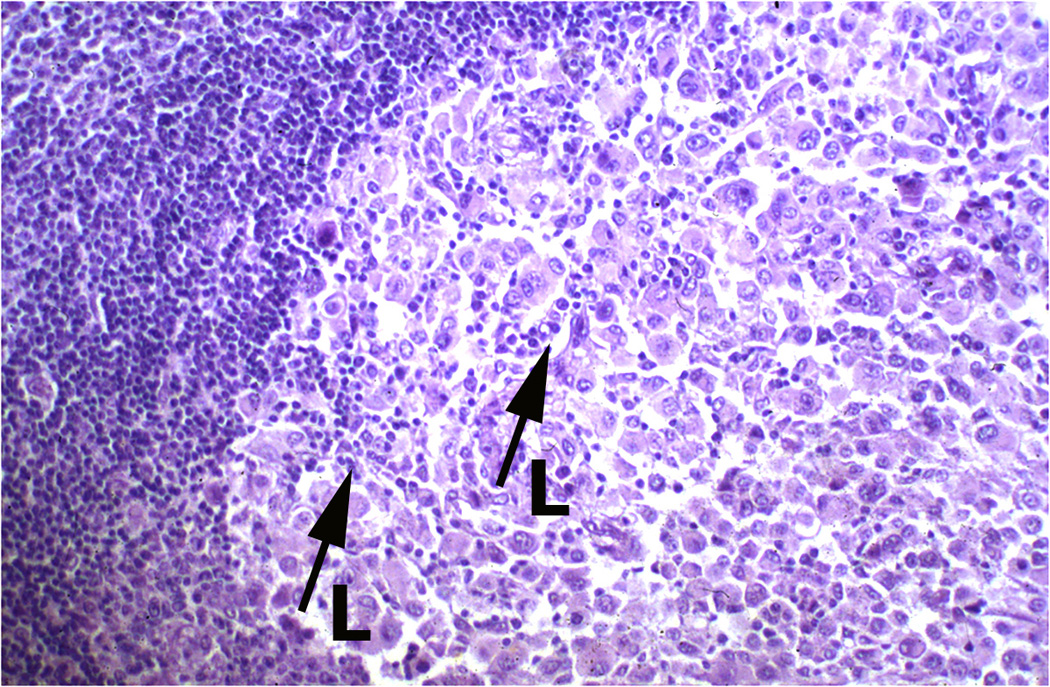
Melanoma infiltrated with various immune cell types. Panel A: Superficial spreading melanoma with multiple lymphocytes (arrow L) infiltrate amidst melanoma cells (arrow) in papillary dermis (Hematoxylin and eosin-stained light micrographs; 40x.) Panel B: Dense melanophages (MP) with scattered lymphocytes (blue) and fibrosis represent area of regression flanked by the tumor (magenta). Panel C: Nodule of metastatic melanoma with dense lymphoid infiltrate (L arrowhead). Note the striking interaction of lymphocytes with melanoma cells, and areas of melanoma cell nuclear pyknosis.
In the meantime Larsen and Grude (12) described increased survival in patients with a dense lymphocytic infiltrate in contrast to those with a weak response. The dense infiltrate was found predominantly in patients whose melanomas were confined to the papillary dermis. The more deeply invasive lesions had little host response and a worse prognosis. Hansen and McCarten reported that patient survival was related to the density of the lymphoid infiltrate at the advancing edge of the tumor. Favorable prognosis was associated with a dense response (13). Day and colleagues (14) reported a better survival in patients with primary melanoma that had a moderate to marked infiltrate of intratumoral and/or peritumoral lymphocytes compared to those with no infiltrate.
Meanwhile in Australia, Dr. Vincent McGovern and colleagues began to study lymphocytic infiltration in halo nevi and melanoma (15). Dr. P. Thompson reported an improved prognosis in patients with a prominent infiltrate at the advancing edge of the melanoma nodule in a very limited study (16). Dr. McGovern studied the evolution of regression and considered a dense infiltrate of lymphocytes in a nodule of melanoma to represent early regression (17). A panel of 11 pathologists and a surgeon, Dr.G.W.Milton, chaired by Dr. Vincent McGovern with one of us (MCM) as rapporteur was convened at the Sydney Conference in 1972 to formalize the classification of melanoma and its reporting. The group recognized that lymphoid infiltrates appeared important in primary melanoma but were not well enough studied to be included in the routine pathology report (18). In a subsequent review of prognosis in melanoma, McGovern reported on varying studies that described improved prognosis to be associated with lymphocytes, especially in the base of the tumor nodule (19).
In the early 1970's there was much interest in vaccination of metastases. In collaboration with Drs. M. L. Brow, A. B. Cosimi, and T.B. Fitzpatrick, we injected vaccinia virus into several melanoma nodules of the lower leg of a patient with multiple metastatic lesions in the right lower limb. The injected nodules had marked inflammatory reactions and some nodules regressed. All melanoma nodules, with or without vaccinia injection, reacted with at least some swelling and erythema. These changes were an example of the now recognized abscopal response. A biopsy of one of the injected lesions revealed histologically a striking infiltrates of mainly lymphocytes with some mononuclear cells and focal neutrophils. Direct interactions of the lymphocytes with melanoma cells were observed with pyknosis of melanoma cells. Roenigk and colleagues (20) reported on a similar response of vaccinia virus injections into metastatic melanoma nodules. These changes certainly reinforced earlier suggestions for developing a therapy that utilizes the lymphocyte response to control melanoma.
In the United States Dr. D.L. Morton pursued studies showing the importance of the immune response in melanomas that led him to publish a rationale for immunotherapy (21). One study showed significant sensitization of lymphocytes by melanoma antigens (22) and further studies with BCG vaccination of tumor nodules recorded the importance of the lymphocytes in the reaction (23).
In addition to the study of melanoma, one of us (MCM) formed a very active collaboration with Dr. Harold F. Dvorak in the study of delayed hypersensitivity reactions as a component of allergic and tumor responses. Because of the interest in hypersensitivity reactions, Dvorak and colleagues (24) studied four examples of radial growth-phase superficial spreading melanoma with electron microscopy and compared these to prior findings in human delayed hypersensitivity responses, including allergic contact dermatitis and human allograft skin rejection. This investigation revealed activation of lymphocytes and macrophages, prominent vascular changes, basophils, and mast cells with a partial loss of mast cell granule content, as seen in delayed-type hypersensitivity reactions. There was evidence for killing of melanoma cells through direct contact with lymphocytes, often with rosette-like formation or satellitosis (Fig. 2). The vascular changes included endothelial cell swelling as well as vessels manifesting necrosis of the endothelium. The basal lamina showed marked reduplication typical of recurrent injury such as chronic inflammation. While these changes, other than the lymphocyte-melanocyte satellitosis, were not specific, they were compatible with the findings in human cutaneous hypersensitivity responses (24).
Figure 2.
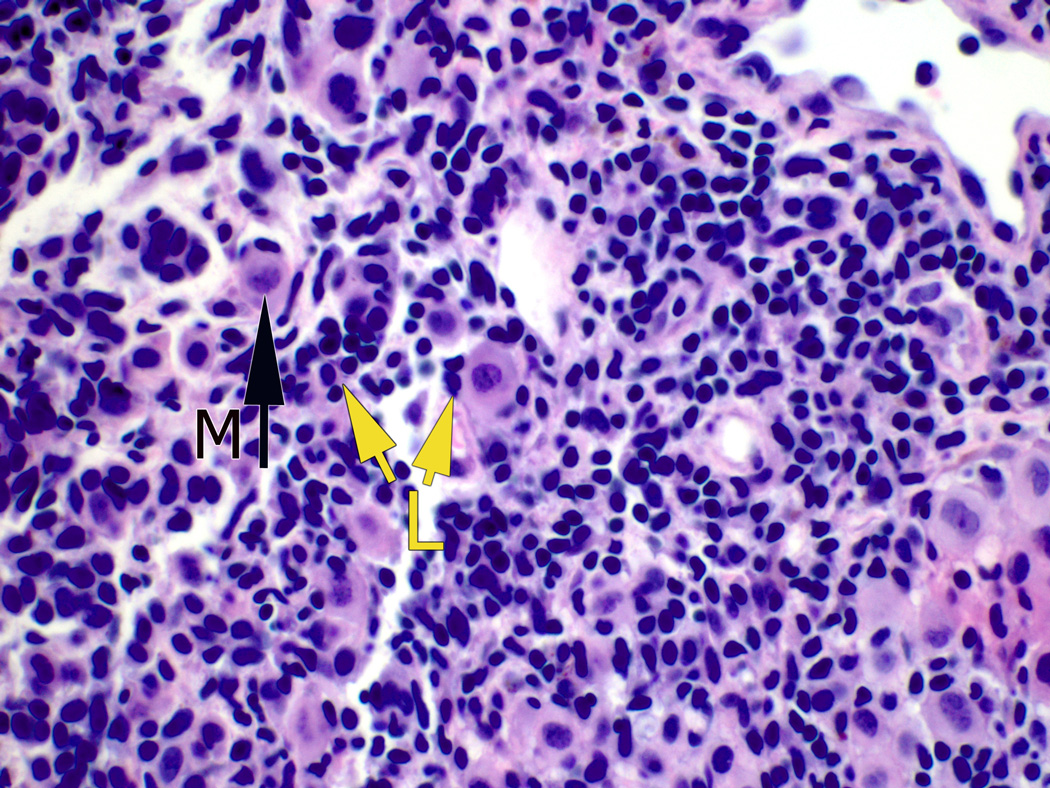
Vertical growth phase melanoma with striking lymphocyte (yellow L arrows)-melanoma cell satellitosis with evidence of degeneration of affected melanoma cells (arrow M).
During these years Dr. Clark, with Dr. Elder and other collaborators started to study the patterns of lymphocyte infiltration that resulted in the publication in 1989 of the most commonly used classification of TILs as brisk, non-brisk and absent (25). The study by Clemente and colleagues (26) in the WHO melanoma pathology group confirmed the Clark data, and demonstrated a highly significant survival advantage in patients with lesions with a brisk infiltrate, an intermediate survival for those with lesions with non-brisk infiltrates, and a poor prognosis for those with melanomas without an infiltrate (Fig. 3). However, neither study described the variation in the density of the infiltrating lymphocytes. The WHO group subsequently studied the presence of TILs in Clinical Stage II melanomas with positive lymph nodes from subjects treated on a trial of alpha-interferon (IFNα), some of whom did very well with a prolonged survival (27). These lymph node metastases were evaluated anonymously with the use of grading system of brisk, non-brisk and absent as applied to the primary lesion. To qualify as brisk, all the metastatic tumor deposits, whether in one or several lymph nodes, had to exhibit a diffuse infiltrate of lymphocytes interacting with tumor cells. The non-brisk category included those in which there was partial infiltration of the tumor by lymphocytes. The results indicated that patients with the brisk immune infiltrates had significantly better survival compared to those with absent inflammation or non-brisk inflammation. There was no evidence that the IFN therapy had any effect on the outcome. An additional study of these patients indicated a matched clonality of lymphocytes in the primary tumor and in the metastatic tumor with regard to T-cell receptor (TCR) usage; this finding suggested the potential specificity of the host response (28).
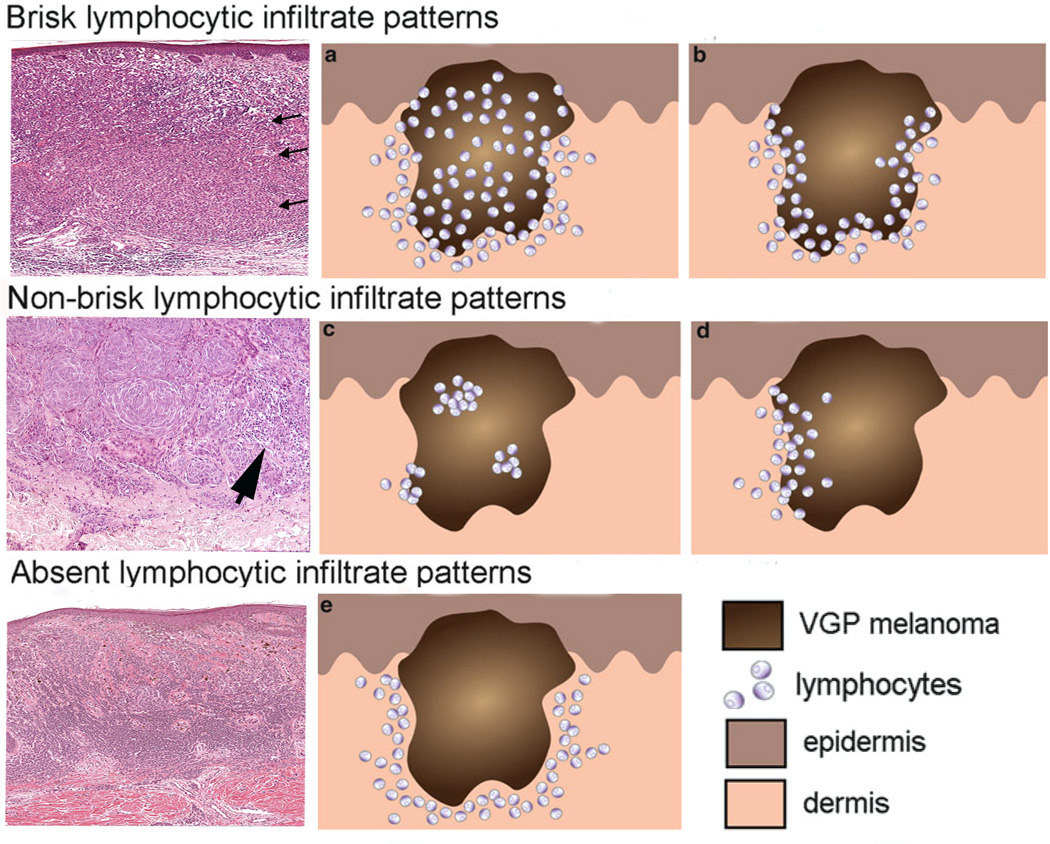
Figure 3.
Brisk, non-brisk, and absent lymphocytic infiltration in primary melanoma nodules. The three photomicrographs in the left column exhibit the brisk, non-brisk and absent patterns. The brisk pattern is defined by lymphocytes present throughout the tumor as shown by arrows in the top left panel. Non-brisk is defined by scattered, focal groups of lymphocytes (arrow). Panel 3: Absent is defined by no lymphocyte infiltration in the tumor. Panels (a, b, c, d, e) in the middle and right columns are schematic renderings of different types of TIL patterns in primary melanoma. The brisk lymphocytic response may involve the entire tumor nodule (panel a) or be present along the advancing edge of the entire peripheral nodule (panel b). The focal nature of the non-brisk pattern is demonstrated in panel c and d. In the rendering of the absent lymphocytic response, there are either no lymphocytes in the tumor, or if lymphocytes are present (panel e) they do not interact with melanoma cells. Panels a, b, c, d, e are adapted from figure 4 of chapter 16 by Schatton and colleagues, entitled “Tumor Infiltrating Lymphocytes and their Significance in Melanoma Prognosis,” in Molecular Diagnostics for Melanoma. Thurin M, Marincola FM eds; Humana Press. 2014, 287–324.
Over the last twenty years, several additional histopathology studies of TILs in melanoma have been published. Some supported the antitumor significance of TILs in melanoma and T cells in sentinel lymph nodes. Others failed to show significant correlation as reviewed by Schatton and colleagues (29). While the basis for these differences remains to be established, one factor might relate to the heterogeneity of T-cell subsets. The importance of T regulatory (Treg) cell inhibition of protective anti-melanoma host responses, for example, has been documented in murine studies (30). The importance of stimulatory and co-stimulatory molecules in determining whether lymphocyte activation or inhibition occurs has also been revealed. A more complete definition of melanoma antigens, including mutated neoepitopes, has been achieved as well. All of this new information will lead to a more comprehensive understanding of anti-melanoma immunity.
Present and Future Histopathology Studies of TILs in Melanoma
A recent article by Thomas and colleagues (31) described TILs in 2,845 patients with a 5-year follow up from the population-based Genes, Environment and Melanoma (GEM) Study. A definite survival benefit was found when comparing melanomas with brisk and non-brisk TILs versus those with absent TILs (p<0.001). These investigators used the criteria of Clark and colleagues (25) in evaluating the infiltrates and the report included information on Breslow thickness, mitoses, and ulceration, as well as the age and the site of the tumor. All cases included the American Joint Committee on Cancer (AJCC) tumor-staging features. The index cases were predominantly vertical growth-phase lesions but also included a small number of Stage T1b lesions according to the AJCC tumor determinations. Melanoma-specific death was 30% and 50% less in tumors with non-brisk and brisk TILs, respectively, compared to tumors with absence of TILs. The brisk infiltrates were found more commonly in tumors from young patients, with the absence of infiltrates found in tumors with increased mitoses and ulceration. Interestingly, in a larger population of patients from the GEM study, including the index cases, the radial growth-phase tumors were consistently associated with a brisk infiltrate. This finding harkens back to the studies of Clark and of Dvorak and colleagues discussed above. During these years, the Sydney Melanoma group, now formalized into the Melanoma Institute of Australia under the leadership of Drs. John Thompson, Jonathan Stretch, and Richard Scolyer, has engaged in numerous melanoma studies. A recent benchmark paper on TILs and their relationship to sentential lymph nodes (SLN) and on patient survival demonstrated that the presence of dense TILs in the primary tumor was associated with 5.6% SNL-positivity versus 27.8% positivity in tumors with absent TILs. Furthermore, there was 100% survival in patients with dense TILs infiltration (32).
Cintolo and colleagues (33) recently reported on 161 patients and determined the influence of TILs and tumor regression in patients evaluated by the standard parameters including the AJCC staging criteria. The tumors had a median thickness of 5.27 mm. The favorable prognostic factors included: female sex, site, non axial, positive TILs, age <60 years, no ulceration or histologic regression, no prior elective lymph node dissection, or no evidence of any regional nodal disease. The presence of TILs was prognostically significant, only when there was no radial growth-phase (RGP) regression. The TILs benefit was lost if RGP regression was present (P<0.001). These authors hypothesized that the presence of regression indicated partial success of host response to the radial growth-phase lesions but failure as far as the deeper tumor was concerned.
The Role of Chemokines, Mononuclear Dendritic Cells, and Immunosuppression in Melanoma Pathogenesis
The original concepts that lymphocytes could generate cancer have long been based on the fact that chronic inflammation can be associated with malignancy. One example is the association of the inflammatory infiltrate triggered by H. pylori infection and gastric malignancy, or the persistent inflammation of chronic prostatitis and prostate cancer. These observations pose the dilemma as to why some TILs in melanoma result in prolonged survival in some cases, whereas in other cases the same inflammatory infiltrate is associated with immunosuppression. The answer, in part, to these observations may be found in understanding the role of inflammatory chemokines in lymphocyte function, as well as understanding of the immunosuppressive aspects of certain mononuclear dendritic cells (DC) and the tumor milieu. There are at least 48 chemokines (in 4 groupings, i.e. CC, CXC, C, and CX3C) and at least 20 chemokine receptors that are involved in the various types of response to stimuli that result in inflammation and/or are important in homeostasis (34). A recent paper by Taube and colleagues has described how both melanoma cells and benign nevi, in the presence of immune host responses and secretion of IFNγ, express the programmed death ligand-1 (PD-L1), whose interaction with the PD-1 receptor leads to lymphocyte exhaustion. The lymphocyte is the apparent source of IFN that then results in rendering the TILs response effete (35). There are different types of DCs in the skin and in the lymph nodes. On exposure to antigen, some result in sensitization; others can lead to tolerance (36). Plasmacytoid DCs are abundant in positive sentinel lymph nodes and appear associated with immunosuppression (37). Finally, with regard to the issue of malignant transformation in the milieu of chronic inflammation, the medley of cytokines and chemokines that are expressed lead to altered DNA, angiogenesis, and inactivation of suppressor genes among others with resultant malignant change. Among the most active of cytokines are TNFα, IL1, IL6, and IL8 with support of stromal fibroblasts. In the latter, CXCL12, when reacting with CXCR4, can result in angiogenesis associated with tumor growth (38, 39). The future lies in unraveling the complexities of tumor-host and stromal interaction to better understand the role of TILs in relation to tumor progression, control and eradication as well as establishing the definitive role of the monocyte macrophages in enhancing and suppressing the immune response (40).
Ectopic Lymph Node-Like Structures or Tertiary Lymphoid Structures within Melanomas
The term “tumor-localized ectopic lymph node-like structures” (TL-ELNs) was introduced to one of us (JJM) for the first time in Seattle by Karl Erik and Ingegerd Hellstrom along with Ellsworth “Buster” Alvord during the studies of the capacity of immune lymphocytes to selectively accumulate in tumors in vivo (41, 42) (Fig. 4).
Figure 4.
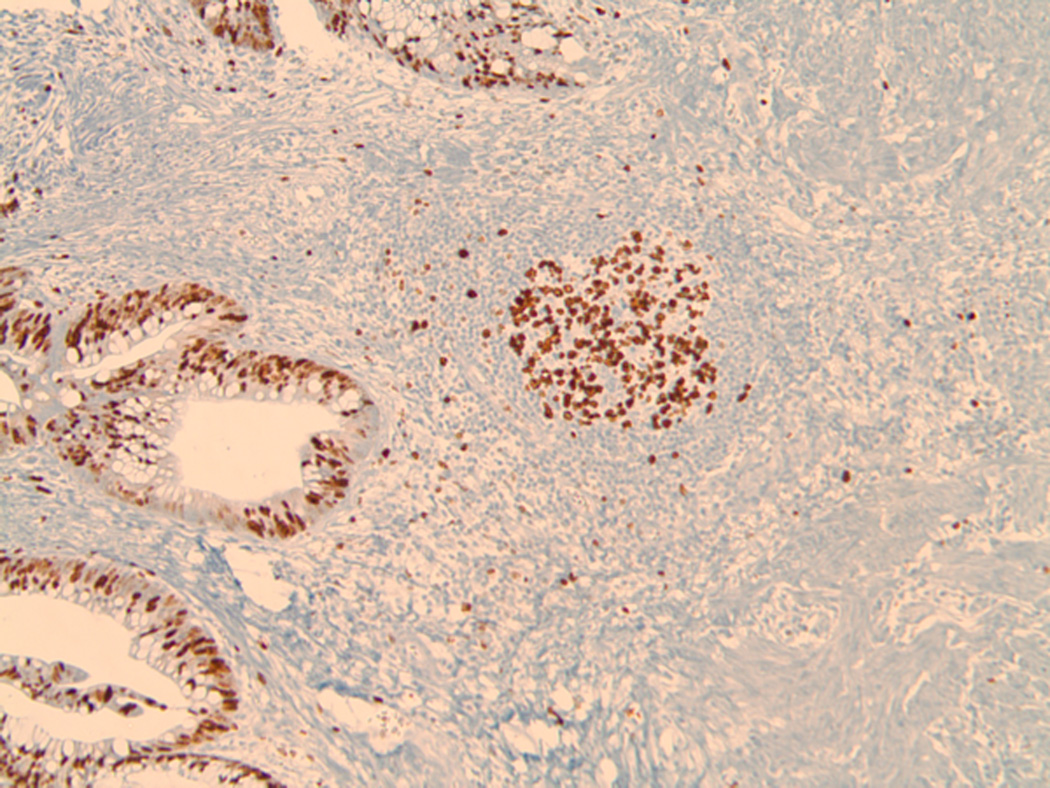
The image depicts in light microscopy the Ki-67 immunohistochemical staining of a section of a typical TL-ELN (center) within a stage IV (non-locoregional), visceral melanoma metastasis. The tissue was stained using the avidin-biotin complex method with retrieval under high pH. Prediluted antibody to Ki-67 [Ventana Medical System, Inc.; anti-Ki-67 (30–9) primary antibody is a rabbit monoclonal antibody (IgG) directed against C-terminal portion of Ki-67 antigen.] was used for the proliferative analysis of the TL-ELN. Lymphocytic proliferation within and surrounding the follicle (brown staining cells) was found to contain both B and T lymphocytes, suggesting newly formed and/or activated TL-ELNs within the tumor microenvironment. (Image was generated by Dr. Jane Messina of the Departments of Anatomic Pathology and Cutaneous Oncology at the Moffitt Cancer Center.
Years later, Messina and colleagues (43) interrogated a novel 12-chemokine gene expression signature (GES) on genomic arrays of stage IV melanoma metastases. They found this GES to accurately predict the degree and type of lymphoid infiltrate, organized as TL-ELNs (43). TL-ELNs had been identified earlier by others in human non-small cell lung cancers and in a few other solid tumors (reviewed in ref. 44). Harlin and colleagues (45) suggested that a lack of critical chemokines in a subset of melanoma metastases might limit the migration of activated T cells, which in turn could limit the effectiveness of antitumor immunity. Of interest, Hong and colleagues (46) reported that CXCR3 ligands and the CCL5 chemokine could determine T-cell infiltration into cutaneous melanomas. In addition, certain chemotherapy drugs could induce the expression of these chemokines in human melanoma cell lines. These chemotherapy-induced chemokines correlated with T-cell infiltration in melanomas, also resultant with tumor control and prolonged patient survival.
By immunohistochemistry, Messina and colleagues (43) demonstrated that TL-ELNs uniformly contained prominent CD20+ B-cell follicles surrounded by marginal zone areas of CD3+ T cells (comprising both CD4+ and CD8+ subsets). In addition, CD86+ follicular DCs, but not FoxP3+ Treg cells were present within these TL-ELNs. Stage IV melanoma metastases negative for the GES were uniformly found to have either an absence or a diffuse pattern of lymphoid infiltrate. There was a highly significant (p<0.0001) and consistent association between a marked increase in overall patient survival, the value of the mean score of the GES, and the presence of TL-ELNs in stage IV melanoma metastases. These findings suggested an ongoing adaptive immune response within the melanoma microenvironment. More recently, van Baron and colleagues (47) have confirmed lymphoid neogenesis in melanoma. Moreover, Cipponi and colleagues (48) performed an analysis of the repertoire of rearranged immunoglobulin genes in B cells of the microdissected follicles within melanoma metastases and demonstrated clonal amplification, somatic mutation and isotype switching, all of which suggested a local antigen-driven B-cell response. TL-ELNs have recently been described in primary cutaneous melanoma as well (49), but this has not been observed by others in a very limited number of specimens examined (48).
Future Directions
The recent progress in advanced melanoma therapy, both targeted and immune response-related, has emphasized the importance of the relationship of melanoma and the host response as well as the tumor microenvironment. The treatment of BRAFV600-mutated melanoma with the BRAF-specific inhibitor BRAFi not only results in cessation of tumor growth but also in diminution in tumor size. This effect is also associated with an increase of CD8+ TILs in the affected tumor nodule; the treated tumor cells show an increase in MART-1 expression (50–52). Thus, targeted therapy is associated with TIL infiltration as a marker of its effect.
Furthermore, a study of the checkpoint blockade antigenPD-1 with one of its ligands, PD-L1, results in effective blocking of host response to the tumor as a result of exhaustion of the PD-1-bearing lymphocytes. PD-L1 can be found on a variety of cell types including tumor cells, stromal cells and macrophages. Treatment of melanoma patients with a specific humanized mouse monoclonal antibody directed against PD-1, Pembrolizumab, or other antibodies directed against PD-L1 can result in freeing of the host response and effective tumor regression. Tumeh and colleagues (53) reported that melanomas that have a great number of CD8+ lymphocytes at the advancing tumor margin are the ones that respond to this treatment. Results from their study indicate that release of the blockade leads to a proliferation of the CD8+ cells that migrate into the tumor and induce apoptosis and necrosis of tumor cells. In contrast, melanomas without CD8+ lymphocytes at the advancing tumor edge do not show response, and the tumors progress. Once again antigen-specific TILs that infiltrate the tumor show prognostic and therapeutic significance. TILs, by their presence in both of these instances, have led to better understanding of the tumor-host interaction and, at least in part, to the therapeutic response. The future lies in more intense study of the various inhibitors of TILs and of better ways to enhance their interaction with not only melanomas but with other cancers as well. Special emphasis should be given to co-stimulatory and inhibitory molecules and the effect that TILs may have on enhancing or thwarting their positive or negative effects, respectively (53). Moreover, the phenomenon of the abscopal effect in melanoma patients following treatment with the combination of anti-CTLA-4 antibody and radiotherapy, although linked to the participation of the immune system, has not yet been thoroughly examined, particularly with respect to changes in the melanoma microenvironment as it pertains to TILs (54, 55).
The existence of a functional connection between TL-ELNs and identifying (and expanding) tumor-specific, therapeutic TILs is now being examined in melanoma. Laser-capture microdissection with DNA/RNA isolation and sequencing methodologies can also be employed to address questions of clonality and functional relationships of the resident T cells within the TL-ELNs, including in depth TCR transcript repertoire analyses. Further, the predictive nature of TL-ELNs in melanoma patients that have participated, or are participating, in TILs-based (56,57) and/or checkpoint antibody-based clinical immunotherapy trials is likely to be examined retrospectively. It will also be of interest to determine whether the presence of TL-ELNs reflects an underlying pathogen infection or is related to baseline gene mutational load of the particular melanoma.
In summary, we have traced TILs and their relationship to melanomas from their first identification under the microscope over a century ago to a progressive understanding of their relationship to tumor control and prognosis. In the molecular biology era, we find molecular-based therapies that directly affect patient survival. It is hoped that the great enthusiasm in the scientific community engendered by these discoveries, will lead not only to melanoma control, but to a possible understanding of melanoma initiation and eradication by immunotherapy alone or in combination with other promising therapeutics.
Acknowledgements
This work was supported in part by the NCI-NIH (1 R01 CA148995-01 to JJM), the V Foundation, and the Dr. Miriam and Sheldon G. Adelson Medical Research Foundation.
Contributor Information
Martin C. Mihm, Jr., Brigham and Women’s Hospital, Harvard Institute of Medicine, 41 Avenue Louis Pasteur, Room 317B, Boston, MA 02115, Phone: (617) 264-5910, mmihm@partners.org.
Dr. James J. Mulé, Associate Center Director, Translational Science, Michael McGillicuddy Endowed Chair, Melanoma Research and Treatment, Moffitt Cancer Center, 12902 Magnolia Drive, SRB-3, Room 23015, Tampa, FL 33612, Office Phone # (813) 745-1536, James.Mule@moffitt.org
References
- 1.Virchow R. Die Krankhaften Geschwulste: Dressig Vorlesungen, gehalten waehrend des Wintersemesters 1862–1863 an der Universitaet zu Berlin: Vorlesungen ueber Pathologie: Verlag von August Hirschwald. 1863. [Google Scholar]
- 2.Ehrlich P. Experimentelle studien an maestumoren. Zeitschrift f Krebsforschung. 1907;5:59–81. [Google Scholar]
- 3.Coley W. The treatment of malignant tumors by repeated inoculations of Erysipelas, with a report of ten original cases. Am J Med SCi. 1989;105:487–511. [PubMed] [Google Scholar]
- 4.Moore OS, Jr, Foote FW., Jr The relatively favorable prognosis of medullary carcinoma of the breast. Cancer. 1949;2:635–642. doi: 10.1002/1097-0142(194907)2:4<635::aid-cncr2820020411>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 5.Brownstein S, Sheikh KM, Lewis MG. Immunological studies in patients with malignant melanoma of the uvea. Can J Ophthalmol. 1977;12:16–23. [PubMed] [Google Scholar]
- 6.Nairn RC. Mechanisms, Detection and Significance of Immunity to Skin Cancer. In: McCarthy WH, editor. Melanoma and Skin Cancer: Proceedings of the International Cancer Conference. Sydney, Australia: V.C.N. Blight; 1972. pp. 257–271. [Google Scholar]
- 7.Krementz ET, Goodwin DP, Samuels MS, Hornung MO, Benes EN. Immunologic Approaches in the Managment of Malignant Melanoma. In: McCarthy WH, editor. Melanoma and Skin Cancer. Sydney, Australia: V.C.N. Blight; 1972. pp. 473–495. [Google Scholar]
- 8.Ryan R, Krementz E, Carter R. Melanoma and Skin Cancer: Proceedings of the International Cancer Conference. Sydney, Australia: V.C.N. Blight; 1972. Treatment of Malignant Melanoma: A Review of the Tulane Experience; pp. 461–472. [Google Scholar]
- 9.Clark WH, Jr, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Cancer Res. 1969;29:705–727. [PubMed] [Google Scholar]
- 10.Clark WH, Jr, Ainsworth AM, Bernardino EA, Yang CH, Mihm MC, Jr, Reed RJ. The developmental biology of primary human malignant melanomas. Semin Oncol. 1975;2:83–103. [PubMed] [Google Scholar]
- 11.Clark WH, Jr, Mihm MC., Jr Lentigo maligna and lentigo-maligna melanoma. Am J Pathol. 1969;55:39–67. [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen TE, Grude TH. A retrospective histological study of 669 cases of primary cutaneous malignant melanoma in clinical stage I. 3. The relation between the tumour-associated lymphocyte infiltration and age and sex, tumour cell type, pigmentation, cellular atypia, mitotic count, depth of invasion, ulceration, tumour type and prognosis. Acta Pathol Microbiol Scand A. 1978;86A:523–530. [PubMed] [Google Scholar]
- 13.Hansen MG, McCarten AB. Tumor thickness and lymphocytic infiltration in malignant melanoma of the head and neck. Am J Surg. 1974;128:557–561. doi: 10.1016/0002-9610(74)90275-x. [DOI] [PubMed] [Google Scholar]
- 14.Day CL, Jr, Lew RA, Mihm MC, Jr, Sober AJ, Harris MN, Kopf AW, et al. A multivariate analysis of prognostic factors for melanoma patients with lesions greater than or equal to 3.65 mm in thickness. The importance of revealing alternative Cox models. Ann Surg. 1982;195:44–49. doi: 10.1097/00000658-198201001-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGovern VJ, Lane Brown M. Regression of Nevi and Malignant Melanoma. In: Kugelmass IN, editor. The Nature of Melanoma. Springfield, IL: Charles C. Thomas; 1969. pp. 158–164. [Google Scholar]
- 16.Thompson P, editor. Proceedings of VIIIthe International Pigment Cell Conference. Sydney: Blight, Government Printer; 1972. The relationshiop of lymphocytic infiltration to prognosis in primary malignant melanoma of skin. [Google Scholar]
- 17.McGovern VJ. Malignant Melanoma: Clinical and Histological Diagnosis. New York, NY: Wiley & Sons Inc; 1976. Spontaneous Regression; pp. 86–149. [Google Scholar]
- 18.McGovern VJ, Mihm MC, Jr, Bailly C, Booth JC, Clark WH, Jr, Cochran AJ, et al. The classification of malignant melanoma and its histologic reporting. Cancer. 1973;32:1446–1457. doi: 10.1002/1097-0142(197312)32:6<1446::aid-cncr2820320623>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 19.McGovern VJ. Melanoma: Histoloigcal Diagnosis and Prognosis. Biopsy Intrepretation Series. New York, NY: Raven Press; 1983. Prognostic Significance of Histological Features of Malignant Melanoma; pp. 158–174. [Google Scholar]
- 20.Roenigk HH, Jr, Deodhar S, St. Jacques R, Burdick K. Immunotherapy of malignant melanoma with vaccinia virus. Arch Dermatol. 1974;109:668–673. [PubMed] [Google Scholar]
- 21.Morton DL, Eilber FR, Joseph WL, Wood WC, Trahan E, Ketcham AS. Immunological factors in human sarcomas and melanomas: a rational basis for immunotherapy. Ann Surg. 1970;172:740–749. doi: 10.1097/00000658-197010000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roth JA, Grimm EA, Morton DL. A rapid assay for stimulation of human lymphocytes by tumor-associated antigens. Cancer Res. 1976;36:3001–3010. [PubMed] [Google Scholar]
- 23.Eilber FR, Townsend CM, Jr, Morton DL. Results of BCG adjuvant immunotherapy for melanoma of the head and neck. Am J Surg. 1976;132:476–479. doi: 10.1016/0002-9610(76)90323-8. [DOI] [PubMed] [Google Scholar]
- 24.Dvorak AM, Mihm MC, Jr, Osage JE, Dvorak HF. Melanoma. An ultrastructural study of the host inflammatory and vascular responses. J Invest Dermatol. 1980;75:388–393. doi: 10.1111/1523-1747.ep12523627. [DOI] [PubMed] [Google Scholar]
- 25.Clark WH, Jr, Elder DE, Guerry Dt, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. 1989;81:1893–1904. doi: 10.1093/jnci/81.24.1893. [DOI] [PubMed] [Google Scholar]
- 26.Clemente CG, Mihm MC, Jr, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer. 1996;77:1303–1310. doi: 10.1002/(SICI)1097-0142(19960401)77:7<1303::AID-CNCR12>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 27.Mihm MC, Jr, Clemente CG, Cascinelli N. Tumor infiltrating lymphocytes in lymph node melanoma metastases: a histopathologic prognostic indicator and an expression of local immune response. Lab Invest. 1996;74:43–47. [PubMed] [Google Scholar]
- 28.Clemente C, Rao S, Lupetti R, Tragni G, Pisarra P, Bersani I, et al. Immunohistochemical analysis of the T-cell receptor beta-chain variable regions expressed by T lymphocytes infiltrating primary human melanoma. Lab Invest. 1998;78:619–627. [PubMed] [Google Scholar]
- 29.Schatton T, Scolyer RA, Thompson JF, Mihm MC., Jr Tumor-infiltrating lymphocytes and their significance in melanoma prognosis. Methods Mol Biol. 2014;1102:287–324. doi: 10.1007/978-1-62703-727-3_16. [DOI] [PubMed] [Google Scholar]
- 30.Cohen AD, Schaer DA, Liu C, Li Y, Hirschhorn-Cymmerman D, Kim SC, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PloS One. 2010;5:e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. 2013;31:4252–4259. doi: 10.1200/JCO.2013.51.3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. 2012;30:2678–2683. doi: 10.1200/JCO.2011.37.8539. [DOI] [PubMed] [Google Scholar]
- 33.Cintolo JA, Gimotty P, Blair A, Guerry D, Elder DE, Hammond R, et al. Local immune response predicts survival in patients with thick (t4) melanomas. Ann Surg Oncol. 2013;20:3610–3617. doi: 10.1245/s10434-013-3086-3. [DOI] [PubMed] [Google Scholar]
- 34.Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- 35.Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012;4:127ra37. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flacher V, Tripp CH, Mairhofer DG, Steinman RM, Stoitzner P, Idoyaga J, et al. Murine Langerin+ dermal dendritic cells prime CD8+ T cells while Langerhans cells induce cross-tolerance. EMBO Mol Med. 2014;6:1638. doi: 10.15252/emmm.201303283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shu S, Cochran AJ, Huang RR, Morton DL, Maecker HT. Immune responses in the draining lymph nodes against cancer: implications for immunotherapy. Cancer Metastasis Rev. 2006;25:233–242. doi: 10.1007/s10555-006-8503-7. [DOI] [PubMed] [Google Scholar]
- 38.Rollins BJ. Inflammatory chemokines in cancer growth and progression. Eur J Cancer. 2006;42:760–767. doi: 10.1016/j.ejca.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 39.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 40.Mantovani A, Locati M. Tumor-associated macrophages as a paradigm of macrophage plasticity, diversity, and polarization: lessons and open questions. Arterioscler Thromb Vasc Biol. 2013;33:1478–1483. doi: 10.1161/ATVBAHA.113.300168. [DOI] [PubMed] [Google Scholar]
- 41.Mule JJ, Jones FR, Hellstrom I, Hellstrom KE. Selective localization of radiolabeled immune lymphocytes into syngeneic tumors. J Immunol. 1979;123:600–606. [PubMed] [Google Scholar]
- 42.Mule JJ, Hellstrom I, Hellstrom KE. Cell surface phenotypes of radiolabeled immune long-lived lymphocytes that selectively localize in syngeneic tumours. Am J Pathol. 1982;107:142–149. [PMC free article] [PubMed] [Google Scholar]
- 43.Messina JL, Fenstermacher DA, Eschrich S, Qu X, Berglund AE, Lloyd MC, et al. 12-Chemokine gene signature identifies lymph node-like structures in melanoma: potential for patient selection for immunotherapy? Sci Rep. 2012;2:765.43. doi: 10.1038/srep00765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppola D, Mule JJ. Ectopic lymph nodes within human solid tumors. J Clin Oncol. 2008;26:4369–4370. doi: 10.1200/JCO.2008.17.6149. [DOI] [PubMed] [Google Scholar]
- 45.Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. 2009;69:3077–3085. doi: 10.1158/0008-5472.CAN-08-2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong M, Puaux AL, Huang C, Loumagne L, Tow C, Mackay C, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71:6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 47.van Baren N, Baurain JF, Coulie PG. Lymphoid neogenesis in melanoma: What does it tell us? Oncoimmunology. 2013;2:e22505. doi: 10.4161/onci.22505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cipponi A, Mercier M, Seremet T, Baurain JF, Theate I, van den Oord J, et al. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012;72:3997–4007. doi: 10.1158/0008-5472.CAN-12-1377. [DOI] [PubMed] [Google Scholar]
- 49.Ladanyi A, Sebestyen T, Mohos A, Liszkay G, Somlai B, Toth E, et al. Ectopic lymphoid structures in primary cutaneous melanoma. Pathol Oncol Res. 2014;20:981–985. doi: 10.1007/s12253-014-9784-8. [DOI] [PubMed] [Google Scholar]
- 50.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res. 2012;18:1386–1394. doi: 10.1158/1078-0432.CCR-11-2479. [DOI] [PubMed] [Google Scholar]
- 51.Wilmott JS, Scolyer RA, Long GV, Hersey P. Combined targeted therapy and immunotherapy in the treatment of advanced melanoma. Oncoimmunology. 2012;1:997–999. doi: 10.4161/onci.19865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Frederick DT, Piris A, Cogdill AP, Cooper ZA, Lezcano C, Ferrone CR, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res. 2013;19:1225–1231. doi: 10.1158/1078-0432.CCR-12-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimaldi AM, Simeone E, Giannarelli D, Muto P, Falivene S, Borzillo V, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology. 2014;3:e28780. doi: 10.4161/onci.28780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Postow MA, Callahan MK, Barker CA, Yamada Y, Yuan J, Kitano S, et al. Immunologic Correlates of the Abscopal Effect in a Patient with Melanoma. N Engl J Med. 2012;366:925–931. doi: 10.1056/NEJMoa1112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosenberg SA, Yang JC, Sherry RM, Kammula US, Hughes MS, Phan GQ, et al. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pilon-Thomas S, Kuhn L, Ellwanger S, Janssen W, Royster E, Marzban, et al. Efficacy of adoptive cell transfer of tumor infiltrating lymphocytes after lymphopenia induction for metastatic melanoma. J Immunother. 2012;35:615–620. doi: 10.1097/CJI.0b013e31826e8f5f. [DOI] [PMC free article] [PubMed] [Google Scholar]