Abstract
This review examines the current level of knowledge and techniques available for the study of laryngeal reflexes. Overall, the larynx is under constant control of several systems (including respiration, swallowing and cough) as well as sensory-motor reflex responses involving glossopharyngeal, pharyngeal, laryngeal and tracheobronchial sensory receptors. Techniques for the clinical assessment of these reflexes are emerging and need to be examined for sensitivity and specificity in identifying laryngeal sensory disorders. Quantitative assessment methods for the diagnosis of sensory reductions as well as sensory hypersensitivity may account for laryngeal disorders such as chronic cough, paradoxical vocal fold disorder and muscular tension dysphonia. The development of accurate assessment techniques could improve our understanding of the mechanisms involved in these disorders.
THE LARYNGEAL MUSCLES
Intrinsic Muscles
The larynx serves as a valve in the upper airway with multiple life supporting functions such as: maintaining opening of the vocal folds (previously referred to as vocal cords) for air exchange for respiration and closing during swallowing to help prevent aspiration of food and liquid into the trachea and lungs. Activation of the laryngeal musculature is continuous throughout life as the vocal folds are constantly being held open to varying degrees for air exchange for respiration, closed for airway protection during swallowing (Ekberg, 1982) or for chest stabilization during lifting by breath holding during exertion.
The upper airway is dilated for inspiration (Amis, Brancatisano and Tully, 1995) and relaxes on expiration. The only muscle producing vocal fold opening in the larynx is the posterior cricoarytenoid (Tully, Brancatisano, Loring, et al., 1991). The muscle is attached to the muscular process of the arytenoid cartilage and inserts on the middle and lateral part of the posterior cricoid. When it contracts it pulls the arytenoid backwards and rocking the vocal process upward and laterally (Figure 1). It is constantly active with greater increases in tone during inspiration for vocal fold opening (Poletto, Verdun, Strominger, et al., 2004). This muscle is essential to life support (Zealear, Billante, Courey, et al., 2003); with bilateral paralysis of the posterior cricoarytenoid the inspiratory flow of the air results in a negative pressure between the folds sucking them into the midline and obstructing inspiratory air flow.
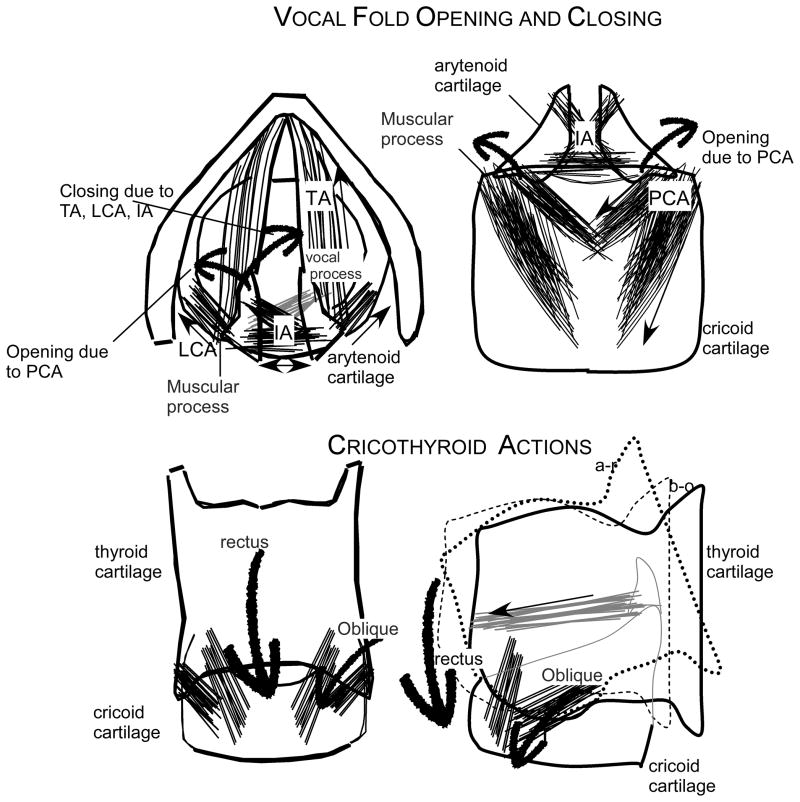
Figure 1.
Schematic illustration of the laryngeal muscles, LCA is the lateral cricoarytenoid, TA is the thyroarytenoid, IA is the inter-arytenoid and the PCA is the posterior cricoarytenoid, as well as the rectus and the oblique compartments of the cricothyroid muscle. The location of the arytenoid cartilage with the muscular and vocal processes, the cricoid cartilage and the thyroid cartilage. In the top left diagram the motion of the vocal process forward and downwards during closing of the vocal folds due to the actions of the adductor TA, LCA and IA muscles and the trajectory of the vocal process upwards and lateral with contraction of the PCA. In the bottom 2 illustrations, the effects of contraction of the rectus and oblique compartments of the cricothyroid muscle pull the thyroid cartilage forward (oblique) and downwards (rectus) to lengthen the vocal fold. This is adapted from Figure 1 of Ludlow (Ludlow, 2005).
During expiration, muscles which close the vocal folds are partly active to modulate air flow (Kuna, Insalaco and Woodson, 1988). The closing muscles include the thyroarytenoid which is the body of the vocal fold extending from the arytenoid cartilage, enveloping the vocal process, and inserting into the anterior inner commissure of the thyroid cartilage. As the thyroarytenoid contracts it pulls the vocal process downwards and to the midline, acting to shorten the muscle and close the vocal folds as it contracts (Figure 1). The muscle actively closing the vocal folds is the lateral cricoarytenoid which connects from the muscular process of the arytenoid to the rim and lateral side of the cricoid cartilage. When this muscle contracts, it pulls the muscular process forward and thus rocks the vocal process inward and downwards to the midline, effectively closing the vocal folds. The lateral cricoarytenoid is most active for voice and swallowing (Hillel, 2001). The other adductor muscle is the inter-arytenoid muscle which stabilizes the two arytenoids side by side in the back of the larynx, preventing them from collapsing into the glottis space between the folds when the other adductors contract. This is the only muscle bilaterally innervated by the recurrent laryngeal nerve from both sides and therefore less affected by unilateral recurrent laryngeal injury (Kawakita, Aibara, Kawamura, et al., 1998).
For voice, the vocal folds must be held in the midline with adequate tension and closure for expiratory air flow to induce vibration. Vibration is induced biomechanically by airflow between the vocal folds. With rapid flow between the folds, the pressure between the folds is lowered allowing for closure at the lower edge of the folds. Consequently after vocal fold closure, the subglottal pressure increases and assists opening the bottom part of the folds. Subsequently a wave of mucosal opening moves from the inferior to superior and lateral direction increasing the flow between the folds. This is followed by closing in the inferior position as the pressure drops. Thus for voice to be produced, the laryngeal muscles play a role in bringing the vocal folds to the midline with precise length-tension characteristics to enable vibration while adequate expiratory flow is provided from the lungs. Thus the role of the laryngeal muscles during voicing for speech is to set the vocal folds into an appropriate posture for phonation onset and offset. This depends upon adequate contraction of the thyroarytenoid muscle, shortening the vocal fold and lowering the fold into the midline adding to closure and opposing lengthening action of the cricothyroid. The ratio of contraction levels of these muscles is important in changing fundamental frequency of vibration and the voice pitch (Titze, Luschei and Hirano, 1989).
The only muscle not innervated by the recurrent laryngeal nerve is the cricothyroid, innervated by the external branch of the superior laryngeal nerve. This muscle lengthens the vocal fold as it moves the thyroid cartilage forwards and down over the cricoid cartilage in the front (Figure1). By lengthening the vocal fold, the cricothyroid increases tension in the vocal fold. The cricothyroid also increases opening during forceful inspiration (Kianicka, Diaz, Renolleau, et al., 1998). Alternatively when the vocal folds are brought to the midline for voice production and the cricothyroid increases in activation, the lengthening increases tension in tandem with opposing shortening action of the thyroarytenoid increasing the fundamental frequency and raising the pitch of the voice.
Extrinsic Muscles
The external laryngeal muscles, sometime referred to as the strap muscles, control the position of the larynx in the neck. The actions of these muscles are particularly important for swallowing when hyoid and laryngeal are elevated by 10–20 mm. Hyo-laryngeal elevation aids in descent of the epiglottis to cover the vestibule and assists in vestibule closure to prevent liquid and food from entering the trachea and being aspirated into the lungs. The elevators for the hyoid bone are the geniohyoid, mylohyoid, anterior digastric and hyoglossus, while the thyrohyoid muscle pulls the larynx up to the hyoid. All are active during the pharyngeal phase of swallowing when the food or liquid move through the pharynx and must enter the upper esophagus without spilling into the larynx and through the vocal folds into the trachea (Pearson, Hindson, Langmore, et al., 2013).
During voice production the larynx also moves up due to the action of the thyrohyoid muscle in particular. As the larynx is moved upwards, the angle between the cricoid and thyroid cartilages changes adding to lengthening of the vocal folds. This change is due to the angle of the spine as was demonstrated by Honda and associates (Honda, Hirai, Masaki, et al., 1999).
SYSTEMS DRIVING THE LARYNGEAL MUSCULATURE
The larynx is constantly under the control of life supporting systems such as breathing, swallowing and coughing as well as by reflexes or responses that are specific to the larynx. In addition, the laryngeal muscles are involved in the production of voice for speech where the pitch and loudness of the voice are controlled as well as rapid voice onsets and offsets is used to distinguish between voiced and voiceless speech sounds such as /b/ and /p/.
Central Control of laryngeal Sensory Responses
The sensory receptors in the larynx are innervated by the internal branch of the superior laryngeal nerve with cell bodies in the nodose ganglion. These sensory neurons project to the interstitial subnucleus of the nucleus tractus solitarius (Ambalavanar, Tanaka, Selbie, et al., 2004). Activation in the nucleus tractus solitarius may affect several of the brainstem systems such as cough and swallow. Some studies have shown that swallow can be activated by strong rapid electrical stimulation of the internal branch of the superior laryngeal nerve (Chi-Fishman, Capra and Mccall, 1994; Kitagawa, Nakagawa, Hasegawa, et al., 2009) while cough can be elicited by less intense and slower electrical stimulation of the same nerve (Gestreau, Bianchi and Grelot, 1997). Very intense stimulation of the internal branch of the superior laryngeal nerve can suppress respiration (Abu-Shaweesh, Dreshaj, Haxhiu, et al., 2001; Bongianni, Corda, Fontana, et al., 1988) as well as produce laryngospasm (Odom, 1993). Which type of responses, cough, swallowing, laryngospasm or suppression of expiration may depend upon which types of receptors in the superior laryngeal nerve are activated or the intensity or frequency of stimulation (Miller and Loizzi, 1974). This review will address the role of the larynx in these systems (cough, swallow, respiration) before addressing specific laryngeal reflexes (the laryngeal adductor or closure reflex, the glossopharyngeal closure reflex and the laryngeal expiratory reflex). Patterning of the laryngeal muscles for speech, an acquired system, will also be addressed.
Respiration
The larynx is constantly driven by the respiratory system to maintain its function as a valve above the trachea. During inspiration, the larynx is one of a chain of muscles activating in sequence to dilate the upper airway starting in the nasal cavity and extending to the larynx (Strohl, Hensley, Hallett, et al., 1980). During expiration in tidal breathing the amount of laryngeal muscle activity is low involving the thyroarytenoid, lateral cricoarytenoid and interarytenoid as the vocal folds have only partial adduction (Kuna, Insalaco and Villeponteaux, 1991). However, when thyroarytenoid motor units were studied during quiet breathing in normal adults, different patterns of unit firing were found; some units were phasic and active only during inspiration, others were only during expiration and others were active for both inspiration and expiration (Chanaud and Ludlow, 1992). As the stress on the respiratory system due to hypoxia or hypercapnia increases, the laryngeal muscles become more active in supporting air flow intake with greater posterior cricoarytenoid activation for vocal fold opening and greater adductor muscle activation to reduce air expiratory flow (Insalaco, Kuna, Cibella, et al., 1990; Kuna, Vanoye, Griffin, et al., 1994; Kuna, Insalaco, Villeponteaux, et al., 1993). Finally, during vital capacity testing, both the posterior cricoarytenoid was active for inspiration while the laryngeal adductor muscles became increasingly active close to the end of forced expiration when the vocal folds became further adducted (Kuna and Vanoye, 1994).
Sniff
Sniffing rapidly opens the vocal folds with posterior cricoarytenoid activation during a forceful inspiration (Poletto, Verdun, Strominger, et al., 2004). Sniffing usually occurs for olfaction and produces a series of rapid inspirations with optimal vocal fold opening on each inspiration. This can be used to assess the integrity of vocal fold opening due to posterior cricoarytenoid muscle activation, as the abductor or opening laryngeal muscles are reflexively activated during sniff. Clinically, sniff is used as a task during laryngeal viewing with a flexible nasoendoscope. The integrity of posterior cricoarytenoid function on each side of the larynx can be evaluated during sniff (Hirano and Bless, 1993). Although cricothyroid activation may also occur during sniff, it cannot fully abduct the vocal fold when there is posterior cricoarytenoid paralysis (Woodson, Sant'ambrogio, Mathew, et al., 1989).
Cough
Coughing also involves a complex pattern of respiratory and laryngeal actions. Cough starts with a rapid inspiration to expand lung volume, followed by closure of both the ventricular or false vocal folds and the vocal folds, and a forceful rapid air expulsion to open the vocal folds and expel substances form the trachea. This tightly coordinated pattern of vocal fold closure and forceful air expulsion can be repeated after the initial inspiration. Sequences of vocal fold closure and forceful expiration are repeated at intervals of 300–500 ms to continue to expire substances from the trachea after the initial inspiration. During cough the forceful repeated closure of the vocal folds is effected by the adductor muscles (thyroarytenoid, lateral cricoarytenoid and inter-arytenoid), while the opening phase is accompanied by posterior cricoarytenoid (Hillel, 2001) and cricothyroid activation (Poletto, Verdun, Strominger, et al., 2004).
Clinicians listen to a patient coughing to assess the adequacy of vocal fold closing. If the sound is muffled and sluggish and not sharp and clear then unilateral recurrent laryngeal nerve injury might be suspected. Nasoendoscopy is not accurate for assessing vocal fold closure for cough as closure can frequently occur in the ventricular or false folds above the vocal folds obscuring the view of the vocal fold closure during cough.
Sensory receptors for triggering cough are likely primarily in the trachea and pulmonary system. Unmyelinated C fibers can be activated by capsaicin when sprayed as in an aerosol into the upper airway and is a powerful stimulus for assessing the integrity of cough (Addington, Stephens and Gilliland, 1999). Further, this test has been shown to be accurate for detecting sensory hyper-reactivity to airway stimulation in chronic cough; good sensitivity and specificity has been demonstrated for diagnosing chronic cough due to sensory hyper-reactivity (Pullerits, Ternesten-Hasseus, Johansson, et al., 2014).
The role of unmyelinated C fiber stimulation in the airway for cough elicitation may not pertain to cough as a result of aspiration of liquid or food into the trachea. Clinically, patients being evaluated using videofluoroscopy during a modified barium swallow test, usually only cough when food or liquid is aspirated into the trachea but not when food or liquid penetrate into the laryngeal vestibule (Rosenbek, Robbins, Roecker, et al., 1996). In fact, the absence of a cough in response to aspiration into the trachea is considered predictive of patients at greater risk of developing aspiration pneumonia as they do not respond with a cough to expel substances reaching the trachea (Robbins, Coyle, Rosenbek, et al., 1999).
Cough elicitation either by aspiration into the trachea and in response to aerosolized capsaicin may have different results because different receptors may be involved. Capsaicin testing may indicate the integrity of unmyelinated C fibers in the trachea and the upper airway which may be innervated by afferents in the superior laryngeal and the recurrent laryngeal nerves. Because an aerosol is involved, the region of the upper airway tested is unclear; unmyelinated C fibers in both the laryngeal vestibule and the hypopharynx as well as the trachea could be stimulated to elicit a cough. Other stimuli such as pressure to mechanoreceptors in the hypopharynx above the trachea, innervated by afferents form the glossopharyngeal nerve were found to be much less effective for eliciting a cough (Hegland, Pitts, Bolser, et al., 2011). Some have proposed that the capsaicin test might be predictive of risk of aspiration in patients with swallowing disorders post stroke (Addington, Stephens and Gilliland, 1999), however the sensitivity and specificity were not good (Addington, Stephens, Widdicombe, et al., 2005).
Dependent upon which afferents are activated in the airway, different motor patterns can be triggered (Table 1). Air pressure stimuli in the anterior facial area in the oral cavity activating glossopharyngeal afferents can elicit a swallow (Theurer, Bihari, Barr, et al., 2005) while an air puff to the mucosa in the laryngeal vestibule activating afferents in the internal branch of the superior laryngeal nerve will only elicit the laryngeal adductor or closure reflex and not a swallow (Bhabu, Poletto, Mann, et al., 2003). This reflex response will be discussed in greater detail below. In contrast, cough is elicited easily with an aerosol spray of capsaicin to the upper airway although cough responses to deposition in the intrapulmonary airways are much greater than those elicited by capsaicin deposition in the larynx (Hansson, Wollmer, Dahlback, et al., 1992)
Table 1.
Upper Airway Afferents and Types of Stimuli Activating Systems and Triggering Laryngeal Reflexes
| Systems or Reflexes | Upper Airway Afferents | Type of Stimulus |
|---|---|---|
| Swallowing | Glossopharyngeal mechanoreceptors | air puff stimuli to anterior oral faucial pillar |
| Swallowing | Internal Branch of the Superior Laryngeal nerve | Repeated electrical stimulation |
| Cough | Tracheal afferents innervated by the Recurrent nerve | Capsaicin to unmyelinated C fibers |
| Aspiration Reflex or Gasp | Pharyngeal afferents | Tactile stimulation in the pharynx |
| Laryngeal Adductor Reflex | Internal branch of the superior laryngeal nerve | Electrical stimulation to the internal branch |
| Laryngeal Adductor Reflex | Mechanoreceptors in mucosa overlying the arytenoids, ventricular folds | Air puff stimulation or electrical stimulation to the mucosa |
| Laryngeal Expiration Reflex | Internal branch of the superior laryngeal nerve or recent | Tactile stimulation of the vocal folds or tracheobronchial tree |
| Pharyngoglottal Closure Reflex | Glossopharyngeal receptors in the pharynx | Water stimulation in the hypopharynx |
Swallowing
Both cough and swallowing are upper airway responses to stimulation and involve both laryngeal and respiratory system functions in very different ways. Cough involves, inspiration, vocal fold closure, and forceful expiration, that is, an active pattern coordinating respiratory and laryngeal control. On the other hand, swallowing actively suppresses the respiratory system while the upper airway is closed to allow for movement of the food or liquid into the esophagus.
Afferents important for eliciting swallowing are mechanoreceptors in the larynx innervated by the internal branch of the superior laryngeal nerve and mechanoreceptors in the pharynx innervated by the glossopharyngeal nerve (Chi-Fishman, Capra and Mccall, 1994; Paterson, 1999 ). Electrical stimulation of either nerve will elicit fictive swallowing in anesthetized animals and both have been found to upregulate swallowing in humans. Taste can also upregulate swallowing; sour is particularly effective (Kajii, Shingai, Kitagawa, et al., 2002; Leow, Huckabee, Sharma, et al., 2007; Logemann, Pauloski, Colangelo, et al., 1995).
Laryngeal vocal fold closure during swallowing is one of three regions that protect the upper airway from aspiration along epiglottic inversion, vestibular closure between the arytenoids and the epiglottis, and closing the false or ventricular folds and the vocal folds (Kawasaki, Fukuda, Shiotani, et al., 2001). The respiratory apnea during a swallow is not an obstructive apnea due to closure of the upper airway but rather an active cessation usually on expiration and resetting of the respiratory rhythm by swallowing (Paydarfar, Gilbert, Poppel, et al., 1995).
The dependence of the swallowing system on superior laryngeal nerve feedback was demonstrated by bilateral chemical block of the internal branch of the superior laryngeal nerve with bupivacaine in healthy volunteers (Jafari, Prince, Kim, et al., 2003). Loss of superior laryngeal nerve feedback not only induced a sensation of not being able to initiate a swallow but also produced a dramatic increase in the occurrences of penetration and aspiration in normal adults. Thus the swallowing system required laryngeal sensory feedback for normal execution of the pharyngeal phase coordinating respiratory apnea, hyo-laryngeal elevation, vestibule closure, ventricular fold and vocal fold closure and opening of the upper esophageal sphincter for clearance of the bolus to prevent penetration and aspiration.
However, it has been contested whether laryngeal sensory deficits can predict if a patient is prone to developing a swallowing disorder as some have tried to suggest (Aviv, Martin, Sacco, et al., 1996). Swallowing disorders are multi-faceted and can involve impairments in tongue propulsion, reduced hyo-laryngeal elevation, delayed onset of the pharyngeal phase or a lack of opening of the upper esophageal sphincter or various combinations (Logemann, 1990; Lundy, Smith, Colangelo, et al., 1999). Laryngeal sensory deficits have not been found to differentiate between patients with and without aspiration during swallowing (Widdicombe and Addington, 2006).
Aspiration Reflex or Gasp
This reflex is most evident in healthy infants to rapidly increase oxygen intake during hypoxic periods or in mild asphyxia (Tomori, Donic, Benacka, et al., 2013), and can also be induced by pharyngeal stimulation (Fung, Tomori and St John, 1995). Along with a diaphragmatic burst, the larynx dilates rapidly during the aspiration reflex allowing rapid increases in airflow into the lungs (Tomori, Benacka and Donic, 1998). One study showed increased activity in the thyroarytenoid muscle immediately following inhibition during the gasp in anesthetized cats (Fung, Tomori and St John, 1995). No neurophysiological studies of this respiratory reflex in humans could be found in the literature.
Breath hold or Valsalva
The ventricular folds and the vocal folds are closed tightly during a breath hold, when the arytenoid cartilages tilt anteriorly towards the thyroid cartilage. This closure produces the highest pressures in the pharynx and involves the highest level of thyroarytenoid activation than any other task but usually requires subject training to be performed correctly.
Interactions between Systems Affecting Laryngeal Reflexes
Suppression or resetting of respiration by swallowing suggests that swallowing can over-ride the respiratory system (Paydarfar, Gilbert, Poppel, et al., 1995). Similarly, some use swallowing to suppress cough in treatment of chronic cough (Vertigan, Theodoros, Gibson, et al., 2006a). Further, the occurrence of a swallow was found to suppress the laryngeal adductor closure reflex for up to 5 seconds (Barkmeier, Bielamowicz, Takeda, et al., 2000). Thus when there is interaction between the systems of respiration, cough and swallow, the swallow pattern resets or over-rides the other systems or reflex responses.
LARYNGEAL REFLEXES
Laryngeal reflexes to be reviewed here are those that are specific to the larynx and do not include additional systems such as respiration, swallow or cough. Most of these are triggered by specific stimuli that are confined to a particular afferents in the upper airway (Table 1). Each will be described before the section on clinical techniques for their study.
Laryngeal Adductor Reflex or Laryngeal Closure Reflex
The laryngeal adductor or closure reflex was first identified by Sasaki and colleagues in the anesthetized cat (Ikari and Sasaki, 1980; Sasaki and Suzuki, 1976; Suzuki and Sasaki, 1977). They reported an ipsilateral thyroarytenoid spasm within 10 ms following electrical stimulation of the internal branch of the superior laryngeal nerve. The same reflex adductor response was identified in awake humans with an initial response around 16 ms in the ipsilateral thyroarytenoid and a subsequent bilateral response at around 60 ms after electrical stimulation to the internal branch of the superior laryngeal nerve (Ludlow, Vanpelt and Koda, 1992). With repeated stimulation in pairs with inter-stimulus intervals of less than 2 s there was conditioning of the late response (Ludlow, Schulz, Yamashita, et al., 1995). The characteristics of the laryngeal adductor response when elicited by electrical stimulation of the internal branch of the superior laryngeal nerve were similar in the conditioning effects to the blink response (Sanes and Ison, 1983). The late bilateral thyroarytenoid response occurred in awake humans but was rare in the anesthetized cat. A study of changes in the laryngeal closure response in patients at different anesthesia levels demonstrated that the bilateral response only occurred when the level of anesthesia was reduced (Sasaki, Jassin, Kim, et al., 2003). The late responses in the cat only occurred when ketamine was not administered demonstrating that this late component is a glutaminergic pathway (Ambalavanar, Purcell, Miranda, et al., 2002).
The elicitation of the laryngeal adductor response by air puffs presented to the mucosa overlying the arytenoid cartilage in the larynx in awake humans was first shown to elicit a bilateral vocal fold closure reflex in human by Aviv and colleagues (Aviv, Martin, Kim, et al., 1999). This reflex was similar to the late response elicited by electrical stimulation of the internal branch of the superior laryngeal nerve (Figure 2). The threshold for eliciting the laryngeal adductor response and subjects perception of the stimulation were closely related (Bhabu, Poletto, Mann, et al., 2003). In addition, rapid presentations of air puff stimuli to the laryngeal mucosa showed conditioning effects of responses when presented in pairs with short inter-stimuli intervals of 2 s or less (Kearney, Poletto, Mann, et al., 2005). In summary, the laryngeal adductor or glottic closure reflex can most easily be elicited by air pressure puffs to the laryngeal mucosa and correlates well with laryngeal sensation. The importance of this reflex for airway protection was demonstrated when responses to electrical stimulation of the internal branch of the superior laryngeal nerve were studied during quiet inspiration, prolonged vowels, humming, forced inhalation and effort closure (Valsalva) (Henriquez, Schulz, Bielamowicz, et al., 2007). The early ipsilateral R1 responses were not altered during any of the voice and breathing tasks while fewer late responses occurred only during effort closure and humming when the larynx was already closed. The results demonstrated that the laryngeal closure reflex was very robust and not altered during volitional tasks likely due to its importance in maintaining airway protection (Henriquez, Schulz, Bielamowicz, et al., 2007).
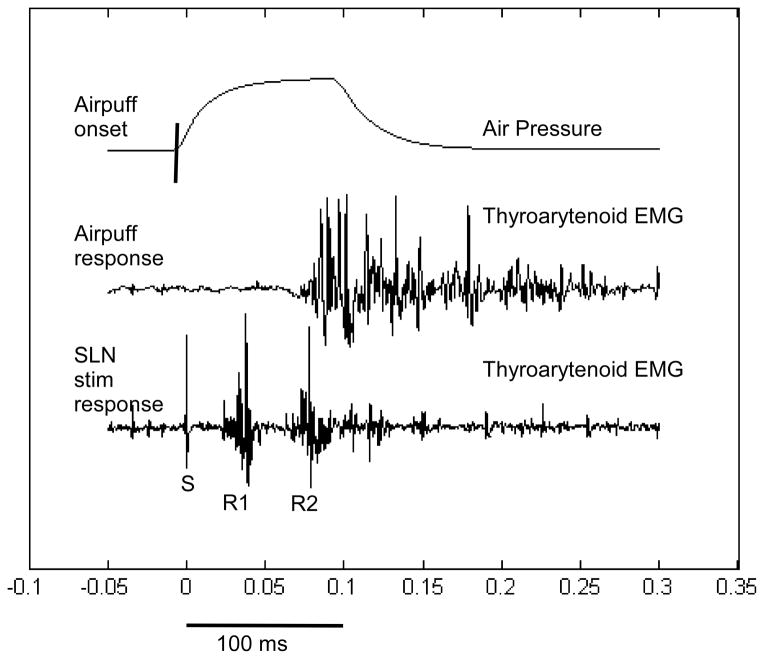
Figure 2.
The laryngeal adductor R1 and R2 responses in the ipsilateral thyroarytenoid muscle in response to an electrical stimulation of the ipsilateral internal branch of the superior laryngeal nerve overlaid on the same graph with the thyroarytenoid muscle response to an air puff on the mucosa overlying the arytenoid cartilage from Figure 3 of Ludlow (2005).
Laryngeal Expiration Reflex
The expiration reflex differs from cough in that it only involves a rapid expiration but does not include a preceding inspiration or vocal fold closure as occurs in cough (Tatar, Hanacek and Widdicombe, 2008). It can easily be elicited by stimulation at the larynx (Korpas and Jakus, 2000; Korpas, Misik and Kalocsayova, 1975) but may also be elicited by stimulation of the tracheobronchial tree (Tatar, Hanacek and Widdicombe, 2008) and is sometimes referred to as the “laryngeal cough”. The expiratory reflex is more likely to occur during high lung inflation and may be followed by multiple coughs with tracheobronchial stimulation (Tatar, Hanacek and Widdicombe, 2008). Thus the function of the laryngeal expiratory reflex is to prevent aspiration of subtances into the lower airways. Knowledge of this reflex is limited in humans as it is difficult to study. Animal studies suggest that this expiratory reflex is rapid and involves abdominal responses as early as 30 ms after laryngeal stimulation (Tomori and Stransky, 1973).
Pharyngoglottal closure reflex
The pharyngoglottal closure reflex is elicited by water emitted from a catheter in the pharynx about 2 cm above the upper esophageal sphincter, which produces a laryngeal closure and depends upon the afferents in the glossopharyngeal nerve for induction (Shaker, Medda, Ren, et al., 1998). The importance of this reflex is that it requires a higher volume of water in the pharynx for it to be elicited in older adults and that this reflex was absent in a small cohort of patients with dysphagia indicating that it may account for aspiration when it is absent (Shaker, Ren, Bardan, et al., 2003). Prospective studies are needed to determine if absence of this reflex can predict aspiration in patients.
Techniques for Study
Access to the laryngeal muscles for EMG and to the laryngeal sensory nerves for stimulation are difficult and not easily included in most clinical settings.
Laryngeal Electromyography
Surface recordings on the neck will reflect the muscles immediately beneath the skin including the platysma, with the sternohyoid beneath the platysma. The sternohyoid pulls the hyoid downwards towards the sternum during head flexion. Beneath the sternohyoid are the omohyoid, sternothyroid and the thyrohyoid, the first two muscles are activated similarly to the sternohyoid to pull the hyoid downwards. The thyrohyoid which is deep beneath the sternohyoid, pulls the larynx and hyoid closer together, and is very important for hyo-laryngeal elevation during swallowing. The cricothyroid muscle which passes from the inside of the thyroid cartilage to the outer surface of the cricoid is the only muscle not completely inside the larynx. However, this muscle is covered by the platysma, sternohyoid and sternothyroid. It is difficult to record from the cricothyroid muscle using a needle electrode as a) swallowing raises the hyo-larygeal complex by 1 to 2 cm often dislodging the electrode from the larynx, and b) contractions of the overlying muscles are often picked up on a concentric needle electrode in the cricothyroid providing an EMG signal that increases both for voice when raising the pitch and but also when the head is flexed forwards against resistance. Verifying gestures are necessary for determining which of the laryngeal muscles are being recorded (Table 2).
Table 2.
Verification Gestures for Identifying Laryngeal Muscles During EMG
| Muscle | Positive Identification | Interference |
|---|---|---|
| Thyroarytenoid | Activity during phonation High activity during effort closure Bursting activity on repeated glottal stops (i-i-i-i-i-i) Increased activity during high pitch |
High activity during sniff may be cricothyroid interference |
| Lateral Cricoarytenoid | Activity prior to onset of phonation and at offset of
phonation Activity during expiration |
High activity during sniff may be cricothyroid interference |
| Interarytenoid | Activity during phonation High activity during effort closure |
High activity during sniff may be posterior cricoarytenoid interference |
| Posterior Cricoarytenoid | Phasic activity on inspiration Burst of activity on sniff’ High levels of activity on “h” between vowels on (he-he-he) Increase activity at high pitch |
Activity during phonation may be interarytenoid
interference Activity during swallow may be cricopharyngeus interference |
| Cricothyroid | Increased activity during high pitch, reduced activity on
low pitch No activity during swallow |
Activity during swallow indicates thyroarytenoid
interfernce Activity during head raise or chin tuck indicates strap muscle interference |
Laryngeal EMG presents several difficulties limiting accurate techniques for accessing these muscles are as follows. First, laryngeal EMG requires needle and preferably hooked wire EMG as surface EMG on the neck overlying the larynx and the thyroid and cricoid cartilages will not reflect the intrinsic laryngeal muscles (thyroarytenoid, lateral cricoarytenoid, inter-arytenoid and posterior cricoarytenoid). Techniques for accessing the intrinsic laryngeal muscles as well as the cricothyroid muscle are very carefully described in a journal article (Hirano and Ohala, 1969). As needles are difficult to keep in one location due to swallowing, bipolar hooked wire electrodes are the preferred method for recording from the intrinsic laryngeal muscles as the hooked wires can maintain their position during swallowing if well placed into the muscles (Hirano and Ohala, 1969). Concentric needles are used for sampling motor units in different locations within a muscle, however, these are not selective given the small size of the laryngeal muscles and the close proximity between the laryngeal muscles. In particular to determine the degree of denervation due to recurrent laryngeal nerve injury when sampling the thyroarytenoid, fibers in the lower lateral region of this muscle insert on the interior thyroid cartilage in close proximity to the cricothyroid muscle which is innervated by the external branch of the superior laryngeal nerve. Studies in the dog found that only bipolar needle needles, with the two poles inside the needle rather than concentric was the only needle accurate for sampling the thyroarytenoid without interference from the cricothyroid (Dedo, 1970). Unfortunately bipolar needle electrodes that are not concentric are difficult to obtain in the United States and single fiber EMG electrodes may be too selective to record from motor units.
To study most laryngeal reflexes, hooked wire electrodes are inserted into the thyroarytenoid muscles which comprise the vocal folds and are adductor muscles. Pairs of hooked wires that are annealed, reduce movement artifacts as no motion occurs between the wires except inside the muscle (made by California Wire Co.). After threading the wires into 27 gauge 1.5 inch epidermic needles, one end of the annealed pairs of wire are made into hooks by quickly passing a flame past the ends burning off the casing for 1 mm and separating the two poles. The poles are bent backwards on either side of the needle so that the two poles will not connect shorting the circuit. The two poles are close together but not touching both for unit analysis and EMG pattern. The other ends are bared for 1–2 cm for attaching to separate poles into a differential amplifier.
Placement of the hooked wires is difficult to assure that the target muscle is being recorded. In our practice we first used a concentric needle electrode to identify the location using verification gestures for each muscle (Table 2). Otolaryngologists who have the knowledge of laryngeal anatomy and an experienced electromyographer are the best team for making accurate recordings of the laryngeal muscles. However, the motor units in the laryngeal muscles are much smaller and have a shorter duration than limb muscles and the motor units may sound like fibrillation potentials to an electromyographer who is experienced in doing limb EMG studies. Normative data on laryngeal muscle motor unit characteristics using a concentric needle were provided for 40 healthy volunteers by Koivu and associates (Koivu, Jaaskelainen and Falck, 2002) and are provided in Table 3.
Table 3.
Normative data on motor unit amplitude and duration for the laryngeal muscles from (Koivu, Jaaskelainen and Falck, 2002) CT is Cricothyroid, TA is thyroarytenoid.
| Measures | CT amp μV | TA Amp μV | CT dur ms | TA dur ms |
|---|---|---|---|---|
| Men Mean/S.D. | 331.3/ 86 | 395.8/84.97 | 4.5/ 0.56 | 4.4/ 0.47 |
| Women Mean/S.D. | 232.8/ 51.7 | 307.4/ 97.3 | 4.7/ 0.8 | 4.7/ 0.7 |
| All Mean/S.D. | 282.0/ 86.1 | 349.4/ 100.6 | 4.6/ 0.7 | 4.5/ 0.6 |
Training on Laryngeal EMG
The importance of having clinically valid and reliable neurophysiological methods for assessing sensory and motor functioning of the larynx is of great importance to the field. In 2004, an evidence based review found that laryngeal EMG was only accurate for injecting botulinum toxin into the thyroarytenoid muscle (Sataloff, Mandel, Mann, et al., 2003). Later the Neurolaryngology Study Group of the American Academy of Otolaryngology –Head and neck Surgery conducted a further review and found that laryngeal EMG could only be used for qualitative EMG (Blitzer, Crumley, Dailey, et al., 2009). The European Laryngological Society provided a description of the methods and techniques currently available for laryngeal electromyography and recommended workshops be provided to provide training for larynoglogists on its use in clinical practice for diagnosis of superior laryngeal nerve injury (cricothyroid muscle) and recurrent laryngeal nerve injury (the thyroarytenoid muscle)(Volk, Hagen, Pototschnig, et al., 2012). As follow-up the use of a training workshop for allowing practitioners to develop skills in the practice and interpretation of laryngeal EMG showed encouraging results indicating improved reliability with training (Volk, Pototschnig, Mueller, et al., 2015). A website is now available for training otolaryngologists on how to use these techniques at www.lemg.org.
Sensory Stimulation for eliciting the Laryngeal Adductor Response
1. Electrical stimulation of the internal branch of the superior laryngeal nerve
Most of the laryngeal reflexes require stimulation of the internal branch of the superior laryngeal nerve which branches between the external efferent branch to the cricothyroid and the internal afferent branch. Branching occurs lateral to the upper lateral corner of the thyroid cartilage. The internal branch enters the larynx between the upper edge of the thyroid cartilage and the inferior surface of the hyoid bone and descends through the thyrohyoid membrane. The only access to this nerve for electrical stimulation is to use bipolar needle electrodes or hooked wires inserted along the inferior edge of the hyoid and the superior edge of the thyroid cartilage (see Figure 1 in Yasmashita et al., (Yamashita, Nash, Tanaka, et al., 1997)). This is a difficult procedure and is accurate for stimulating the nerve about two-thirds of the time by experienced otolaryngologists. It should only be done unilaterally as bilateral stimulation could produce bilateral laryngeal closure, interfering with respiration. At low levels of stimulation, only the early ipsilateral laryngeal adductor response, R1, occurs at around 16 ms. The later R2 bilateral response occurs around 65 ms at higher levels of stimulation. As the amplitude of R2 is unrelated to the amplitude of R1 these two components likely involve different pathways (Yamashita, Nash, Tanaka, et al., 1997).
The internal branch of the superior laryngeal nerve innervates the mechanoreceptors in the mucosa in the laryngeal vestibule and the vocal fold. Pressure on the intrinsic mucosa covering the vocal folds in the anesthetized cat elicits the R1 and R2 responses similar to the awake human; when the mucosa is removed from the vocal fold and pressure is applied to the muscle body in the vocal fold no response occurs demonstrating that this response is to mechanoreceptors in the mucosa (Andreatta, Mann, Poletto, et al., 2002). Air puffs to the mucosa over the arytenoid in the awake human elicits the laryngeal closure response (Aviv, Martin, Kim, et al., 1999) which occurs around 100 ms bilaterally in the thyroarytenoid muscle (Bhabu, Poletto, Mann, et al., 2003). The bilateral response in the thyroarytenoid muscles which briefly closes the larynx with air puff stimulation of the mucosa over the arytenoid on one side, is likely related to the R2 response elicited when stimulating the internal branch of the superior laryngeal nerve electrically (Figure 2).
2. Air Puff Stimulation of the Laryngeal Mucosa
Clinically, it is feasible to stimulate the mucosa over the arytenoid with air puffs through the working channel of a nasolaryngoscope. A device was developed that measured the pressure of the air puff emitted from the tip of the scope and could be applied for determining the threshold for sensation by the patient or the elicitation of the laryngeal adductor response. Normally threshold responses occur between 2 and 4 mmHg. Moderate sensory impairment thresholds are between 4 and 6 mmHg. Thresholds or no response at > 6 mmHg are used as criteria for identifying sensory deficits in a patient (Aviv, Kim, Sacco, et al., 1998; Aviv, Martin, Kim, et al., 1999). Difficulties with this procedure are as follows: the Pentax instrument used by Aviv and his colleagues is no longer available commercially. In the Pentax instrument the airpuff presentation could clearly be heard. This could interfere with accurate reports of sensation by the patient although the occurrence of the laryngeal adductor reflex is not likely to be produced by the sound. The distance of the endoscope from the mucosa can vary making the response unreliable. Laboratory setups can be developed which overcome some of these difficulties and are used in research (Hammer, 2009; Kearney, Poletto, Mann, et al., 2004) but are not feasible for use in a clinical setting.
3. Electrical Stimulation of the Laryngeal Mucosa
More recently, a unipolar wire stimulating electrode passed through the working channel of a nasoendoscope was used to stimulate either the mucosa overlying the arytenoid, on the aryepiglottic fold or the ventricular fold unilaterally (Carey, Sulica, Wu, et al., 2013; Sulica, Carey and Branski, 2013). This elicited the laryngeal adductor response with an R1 around 16 ms similar to previous studies using an electrical stimulus to the internal branch to the superior laryngeal nerve. In addition, a bilateral R2 was elicited which had a latency of 49 to 50 ms (Carey, Sulica, Wu, et al., 2013; Sulica, Carey and Branski, 2013). It is unclear why the latency of the bilateral response is earlier than the bilateral response elicited by stimulation to the internal superior laryngeal nerve (Yamashita, Nash, Tanaka, et al., 1997). If this technique is found reliable it could be used by others as a clinically viable approach to assessing laryngeal sensory and motor integrity.
CLINICAL IMPACT OF LARYNGEAL REFLEXES
Needs for Research
Several challenges must be overcome before laryngeal reflexes are ready for implementation in laryngology. First as mentioned above, is the need for clinical training on methods for use of laryngeal EMG in laryngology training (Volk, Pototschnig, Mueller, et al., 2015; Volk, Hagen, Pototschnig, et al., 2012). Accurate procedures are needed to record from the laryngeal muscles during reflex testing. Second are the techniques for sensory stimulation. As described above, electrical stimulation of the internal branch of the superior laryngeal nerve is a difficult technique not useful in clinical practice. Further, air puff stimulation of the mucosa over the arytenoid depends upon a calibrated pressure source which is no longer commercially available. The recent alternative to stimulate the mucosa using an electrical wire through the working channel of a nasoendoscope which is described as an “irritative” stimulus has been studied in 17 healthy controls (Carey, Sulica, Wu, et al., 2013) and three patients (Sulica, Carey and Branski, 2013). Studies of the sensitivity and specificity of this technique for application in a clinical setting for differentiating between patients with sensory (internal branch of the superior laryngeal nerve) and motor (recurrent laryngeal nerve) deficits are needed.
Rapid presentations of stimuli to elicit the laryngeal adductor response have been used to examine conditioning effects for the laryngeal adductor response in laryngeal motor control disorders such as spasmodic dysphonia (Deleyiannis, Gillespie, Bielamowicz, et al., 1999; Ludlow, Schulz, Yamashita, et al., 1995). A lack of suppression of the conditioning responses suggests a similar disinhibition to that found using conditioning studies of the blink reflex in spasmodic dysphonia (Cohen, Ludlow, Warden, et al., 1989). Similar conditioning effects were also found in healthy adults using the air puff method for eliciting the laryngeal adductor response (Kearney, Poletto, Mann, et al., 2005). As the air puff or electrical stimulation of the mucosa are clinically feasible techniques, these could be applied to examine central disinhibition of laryngeal reflexes in laryngeal control disorders.
A major need in the field of laryngology and voice disorders is to understand the mechanisms involved in the large numbers of patients affected by laryngeal control disorders considered to be sensory hyper-sensitivity disorders. These include problems such as paradoxical vocal fold movement disorder (Vertigan, Theodoros, Gibson, et al., 2006b; Vertigan, Gibson, Theodoros, et al., 2007; Vertigan, Theodoros, Gibson, et al., 2007), chronic cough (Murry, Tabaee and Aviv, 2004) and muscular tension dysphonia (Morrison, Rammage and Emami, 1999; Morrison, Rammage, Belisle, et al., 1983). The laryngeal adductor reflex has not been studied quantitatively in such patients and could provide a diagnostic technique for assessing whether these patients have a sensory hypersensitivity.
Sensory feedback from the larynx is also involved in swallowing as this feedback is important to maintaining airway protection during the pharyngeal phase of swallowing (Jafari, Prince, Kim, et al., 2003). Development of clinical feasible methods for quantifying the laryngeal adductor reflex in response to electrical stimulation of the laryngeal mucosa could be applicable to determining if loss of laryngeal sensation could be contributing to aspiration in dysphagia following stroke or following treatment of head and neck cancer. Study of the pharyngoglottal reflex closing the vocal folds in response to water in the pharynx (Shaker, Ren, Bardan, et al., 2003) could also be of importance to examining the role of pharyngeal sensation in swallowing disorders with different etiologies.
CONCLUSIONS
This review has examined the current level of knowledge and techniques available for the study of laryngeal reflexes. Overall, the larynx is under constant control of several systems (such as respiration, swallowing and cough) as well as sensory-motor reflex responses. Techniques for the clinical assessment of these reflexes are emerging and need to be examined for sensitivity and specificity for identifying laryngeal sensory disorders. Quantitative assessment methods are needed for the diagnosis of sensory reductions as well as abnormalities in sensory –motor reflexes which may interfere with volitional control of the larynx to suppress chronic cough, reduce hyper-sensitivity and allow for precise control for speech.
Acknowledgments
Support for this work included U54 NS065701.
References
- Abu-Shaweesh JM, Dreshaj IA, Haxhiu MA, Martin RJ. Central GABAergic mechanisms are involved in apnea induced by SLN stimulation in piglets. J Appl Physiol. 2001;90:1570–1576. doi: 10.1152/jappl.2001.90.4.1570. [DOI] [PubMed] [Google Scholar]
- Addington WR, Stephens RE, Gilliland KA. Assessing the laryngeal cough reflex and the risk of developing pneumonia after stroke: an interhospital comparison. Stroke. 1999;30:1203–1207. doi: 10.1161/01.str.30.6.1203. [DOI] [PubMed] [Google Scholar]
- Addington WR, Stephens RE, Widdicombe JG, Rekab K. Effect of stroke location on the laryngeal cough reflex and pneumonia risk. Cough. 2005;1:4. doi: 10.1186/1745-9974-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, Tanaka Y, Selbie WS, Ludlow CL. Neuronal activation in the medulla oblongata during selective elicitation of the laryngeal adductor response. J Neurophysiol. 2004;92:2920–2932. doi: 10.1152/jn.00064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambalavanar R, Purcell L, Miranda M, Evans F, Ludlow CL. Selective suppression of late laryngeal adductor responses by N-Methyl-D-Asparate receptor blockade in the cat. J Neurophysiol. 2002;87:1252–1262. doi: 10.1152/jn.00595.2001. [DOI] [PubMed] [Google Scholar]
- Amis TC, Brancatisano A, Tully A. Thyroid cartilage movements during breathing. J Appl Physiol. 1995;78:441–448. doi: 10.1152/jappl.1995.78.2.441. [DOI] [PubMed] [Google Scholar]
- Andreatta RD, Mann EA, Poletto CJ, Ludlow CL. Mucosal afferents mediate laryngeal adductor responses in the cat. J Appl Physiol. 2002;93:1622–1629. doi: 10.1152/japplphysiol.00417.2002. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Martin JH, Sacco RL, et al. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105:92–97. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Kim T, Sacco RL, et al. FEESST: a new bedside endoscopic test of the motor and sensory components of swallowing. Ann Otol Rhinol Laryngol. 1998;107:378–387. doi: 10.1177/000348949810700503. [DOI] [PubMed] [Google Scholar]
- Aviv JE, Martin JH, Kim T, et al. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol. 1999;108:725–730. doi: 10.1177/000348949910800802. [DOI] [PubMed] [Google Scholar]
- Barkmeier JM, Bielamowicz S, Takeda N, Ludlow CL. Modulation of laryngeal responses to superior laryngeal nerve stimulation by volitional swallowing in awake humans. J Neurophysiol. 2000;83:1264–1272. doi: 10.1152/jn.2000.83.3.1264. [DOI] [PubMed] [Google Scholar]
- Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112:834–840. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- Blitzer A, Crumley RL, Dailey SH, et al. Recommendations of the Neurolaryngology Study Group on laryngeal electromyography. Otolaryngol Head Neck Surg. 2009;140:782–793. doi: 10.1016/j.otohns.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bongianni F, Corda M, Fontana G, Pantaleo T. Influences of superior laryngeal afferent stimulation on expiratory activity in cats. J Appl Physiol. 1988;65:385–392. doi: 10.1152/jappl.1988.65.1.385. [DOI] [PubMed] [Google Scholar]
- Carey B, Sulica L, Wu A, Branski R. A novel electrodiagnostic assessment of the laryngeal closure reflex. Muscle Nerve. 2013;47:432–436. doi: 10.1002/mus.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanaud CM, Ludlow CL. Single motor unit activity of human intrinsic laryngeal muscles during respiration. Ann Otol Rhinol Laryngol. 1992;101:832–840. doi: 10.1177/000348949210101006. [DOI] [PubMed] [Google Scholar]
- Chi-Fishman G, Capra NF, Mccall GN. Thermomechanical facilitation of swallowing evoked by electrical nerve stimulation in cats. Dysphagia. 1994;9:149–155. doi: 10.1007/BF00341258. [DOI] [PubMed] [Google Scholar]
- Cohen LG, Ludlow CL, Warden M, et al. Blink reflex curves in patients with spasmodic dysphonia. Neurology. 1989;39:572–577. doi: 10.1212/wnl.39.4.572. [DOI] [PubMed] [Google Scholar]
- Dedo HH. The paralyzed larynx: an electromyographic study in dogs and humans. Laryngoscope. 1970;80:1445–1517. doi: 10.1288/00005537-197010000-00001. [DOI] [PubMed] [Google Scholar]
- Deleyiannis FW, Gillespie M, Bielamowicz S, Yamashita T, Ludlow CL. Laryngeal long latency response conditioning in abductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108:612–619. doi: 10.1177/000348949910800615. [DOI] [PubMed] [Google Scholar]
- Ekberg O. Closure of the laryngeal vestibule during deglutition. Acta Otolaryngol. 1982;93:123–129. doi: 10.3109/00016488209130862. [DOI] [PubMed] [Google Scholar]
- Fung ML, Tomori Z, St John WM. Medullary neuronal activities in gasping induced by pharyngeal stimulation and hypoxia. Respir Physiol. 1995;100:195–202. doi: 10.1016/0034-5687(94)00141-l. [DOI] [PubMed] [Google Scholar]
- Gestreau C, Bianchi AL, Grelot L. Differential brainstem fos-like immunoreactivity after laryngeal-induced coughing and its reduction by codeine. J Neurosc. 1997;17:9340–9352. doi: 10.1523/JNEUROSCI.17-23-09340.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ. Design of a new somatosensory stimulus delivery device for measuring laryngeal mechanosensory detection thresholds in humans. IEEE Trans Biomed Eng. 2009;56:1154–1159. doi: 10.1109/TBME.2008.2007968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L, Wollmer P, Dahlback M, Karlsson JA. Regional sensitivity of human airways to capsaicin-induced cough. Am RevRespir Dis. 1992;145:1191–1195. doi: 10.1164/ajrccm/145.5.1191. [DOI] [PubMed] [Google Scholar]
- Hegland KW, Pitts T, Bolser DC, Davenport PW. Urge to cough with voluntary suppression following mechanical pharyngeal stimulation. Bratislavske lekarske listy. 2011;112:109–114. [PMC free article] [PubMed] [Google Scholar]
- Henriquez VM, Schulz GM, Bielamowicz S, Ludlow CL. Laryngeal reflex responses are not modulated during human voice and respiratory tasks. J Physiol. 2007;585:779–789. doi: 10.1113/jphysiol.2007.143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillel AD. The study of laryngeal muscle activity in normal human subjects and in patients with laryngeal dystonia using multiple fine-wire electromyography. Laryngoscope. 2001;111:1–47. doi: 10.1097/00005537-200104001-00001. [DOI] [PubMed] [Google Scholar]
- Hirano M, Ohala J. Use of hooked-wire electrodes for electromyography of the intrinsic laryngeal muscles. J Speech Hear Res. 1969;12:362–373. doi: 10.1044/jshr.1202.362. [DOI] [PubMed] [Google Scholar]
- Hirano M, Bless DM. Videostroboscopic Examination of the Larynx. San Diego: Singular Publishing; 1993. [Google Scholar]
- Honda K, Hirai H, Masaki S, Shimada Y. Role of vertical larynx movement and cervical lordosis in F0 control. Lang Speech. 1999;42 ( Pt 4):401–411. doi: 10.1177/00238309990420040301. [DOI] [PubMed] [Google Scholar]
- Ikari T, Sasaki CT. Glottic closure reflex: Control mechanisms. Ann Otol. 1980;89:220–224. doi: 10.1177/000348948008900305. [DOI] [PubMed] [Google Scholar]
- Insalaco G, Kuna ST, Cibella F, Villeponteaux RD. Thyroarytenoid muscle activity during hypoxia, hypercapnia, and voluntary hyperventilation in humans. J Appl Physiol. 1990;69:268–273. doi: 10.1152/jappl.1990.69.1.268. [DOI] [PubMed] [Google Scholar]
- Jafari S, Prince RA, Kim DY, Paydarfar D. Sensory regulation of swallowing and airway protection: a role for the internal superior laryngeal nerve in humans. J Physiol. 2003;550:287–304. doi: 10.1113/jphysiol.2003.039966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajii Y, Shingai T, Kitagawa J, et al. Sour taste stimulation facilitates reflex swallowing from the pharynx and larynx in the rat. Physiol Behav. 2002;77:321–325. doi: 10.1016/s0031-9384(02)00854-5. [DOI] [PubMed] [Google Scholar]
- Kawakita S, Aibara R, Kawamura Y, Yumoto E, Desaki J. Motor innervation of the guinea pig interarytenoid muscle: reinnervation process following unilateral denervation. Laryngoscope. 1998;108:398–402. doi: 10.1097/00005537-199803000-00016. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Fukuda H, Shiotani A, Kanzaki J. Study of movements of individual structures of the larynx during swallowing. Auris Nasus Larynx. 2001;28:75–84. doi: 10.1016/s0385-8146(00)00087-0. [DOI] [PubMed] [Google Scholar]
- Kearney P, Poletto C, Mann EA, Ludlow CL. Suppression of thyroarytenoid muscle responses during repeated air pressure stimulation of the laryngeal mucosa in awake humans. Annals of Otology Rhinology and Laryngology. 2004 doi: 10.1177/000348940511400403. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney PR, Poletto CJ, Mann EA, Ludlow CL. Suppression of thyroarytenoid muscle responses during repeated air pressure stimulation of the laryngeal mucosa in awake humans. Ann Otol Rhinol Laryngol. 2005;114:264–270. doi: 10.1177/000348940511400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kianicka I, Diaz V, Renolleau S, Canet E, Praud JP. Laryngeal and abdominal muscle electrical activity during periodic breathing in nonsedated lambs. J Appl Physiol. 1998;84:669–675. doi: 10.1152/jappl.1998.84.2.669. [DOI] [PubMed] [Google Scholar]
- Kitagawa J, Nakagawa K, Hasegawa M, et al. Facilitation of reflex swallowing from the pharynx and larynx. J Oral Sci. 2009;51:167–171. doi: 10.2334/josnusd.51.167. [DOI] [PubMed] [Google Scholar]
- Koivu MK, Jaaskelainen SK, Falck BB. Multi-MUP analysis of laryngeal muscles. Clin Neurophysiol. 2002;113:1077–1081. doi: 10.1016/s1388-2457(02)00127-x. [DOI] [PubMed] [Google Scholar]
- Korpas J, Jakus J. The expiration reflex from the vocal folds. Acta physiologica Hungarica. 2000;87:201–215. doi: 10.1556/APhysiol.87.2000.3.1. [DOI] [PubMed] [Google Scholar]
- Korpas J, Misik A, Kalocsayova G. The expiration reflex in man. Physiologia Bohemoslovaca. 1975;24:249–252. [PubMed] [Google Scholar]
- Kuna ST, Vanoye CR. Laryngeal response during forced vital capacity maneuvers in normal adult humans. Am J Respir Crit Care Med. 1994;150:729–734. doi: 10.1164/ajrccm.150.3.8087344. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol. 1988;65:1332–1339. doi: 10.1152/jappl.1988.65.3.1332. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Insalaco G, Villeponteaux RD. Arytenoideus muscle activity in normal adult humans during wakefulness and sleep. J Appl Physiol. 1991;70:1655–1664. doi: 10.1152/jappl.1991.70.4.1655. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Vanoye CR, Griffin JR, Updegrove JD. Effect of hypercapnia on laryngeal airway resistance in normal adult humans. J Appl Physiol. 1994;77:2797–2803. doi: 10.1152/jappl.1994.77.6.2797. [DOI] [PubMed] [Google Scholar]
- Kuna ST, Insalaco G, Villeponteaux DR, Vanoye CR, Smickley JS. Effect of hypercapnia and hypoxia on arytenoideus muscle activity in normal adult humans. J Appl Physiol. 1993;75:1781–1789. doi: 10.1152/jappl.1993.75.4.1781. [DOI] [PubMed] [Google Scholar]
- Leow LP, Huckabee ML, Sharma S, Tooley TP. The influence of taste on swallowing apnea, oral preparation time, and duration and amplitude of submental muscle contraction. Chem Senses. 2007;32:119–128. doi: 10.1093/chemse/bjl037. [DOI] [PubMed] [Google Scholar]
- Logemann JA. Factors affecting ability to resume oral nutrition in the oropharyngeal dysphagic individual. Dysphagia. 1990;4:202–208. doi: 10.1007/BF02407266. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Pauloski BR, Colangelo L, Lazarus C, Fujiu M, Kahrilas PJ. Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res. 1995;38:556–563. doi: 10.1044/jshr.3803.556. [DOI] [PubMed] [Google Scholar]
- Ludlow CL. Central nervous system control of the laryngeal muscles in humans. Respir Physiol Neurobiol. 2005;147:205–222. doi: 10.1016/j.resp.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow CL, Vanpelt F, Koda J. Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol. 1992;101:127–134. doi: 10.1177/000348949210100204. [DOI] [PubMed] [Google Scholar]
- Ludlow CL, Schulz GM, Yamashita T, Deleyiannis FW. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104:928–935. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- Lundy DS, Smith C, Colangelo L, et al. Aspiration: cause and implications. Otolaryngol Head Neck Surg. 1999;120:474–478. doi: 10.1053/hn.1999.v120.a91765. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Loizzi RF. Anatomical and functional differentiation of superior laryngeal nerve fibers affecting swallowing and respiration. Exp Neurol. 1974;42:369–387. doi: 10.1016/0014-4886(74)90033-8. [DOI] [PubMed] [Google Scholar]
- Morrison M, Rammage L, Emami AJ. The irritable larynx syndrome. J Voice. 1999;13:447–455. doi: 10.1016/s0892-1997(99)80049-6. [DOI] [PubMed] [Google Scholar]
- Morrison MD, Rammage LA, Belisle GM, Pullan CB, Nichol H. Muscular tension dysphonia. J Otolaryngol. 1983;12:302–306. [PubMed] [Google Scholar]
- Murry T, Tabaee A, Aviv JE. Respiratory retraining of refractory cough and laryngopharyngeal reflux in patients with paradoxical vocal fold movement disorder. Laryngoscope. 2004;114:1341–1345. doi: 10.1097/00005537-200408000-00005. [DOI] [PubMed] [Google Scholar]
- Odom JL. Airway emergencies in the post anesthesia care unit. Nurs Clin North Am. 1993;28:483–491. [PubMed] [Google Scholar]
- Paterson WG. Alteration of swallowing and oesophageal peristalsis by different initiators of deglutition. Neurogastroenterol Motil. 1999;11:63–67. doi: 10.1046/j.1365-2982.1999.00131.x. [DOI] [PubMed] [Google Scholar]
- Paydarfar D, Gilbert RJ, Poppel CS, Nassab PF. Respiratory phase resetting and airflow changes induced by swallowing in humans. J Physiol. 1995;483 ( Pt 1):273–288. doi: 10.1113/jphysiol.1995.sp020584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson WG, Jr, Hindson DF, Langmore SE, Zumwalt AC. Evaluating swallowing muscles essential for hyolaryngeal elevation by using muscle functional magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2013;85:735–740. doi: 10.1016/j.ijrobp.2012.07.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poletto CJ, Verdun LP, Strominger R, Ludlow CL. Correspondence between laryngeal vocal fold movement and muscle activity during speech and nonspeech gestures. J Appl Physiol. 2004;97:858–866. doi: 10.1152/japplphysiol.00087.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullerits T, Ternesten-Hasseus E, Johansson EL, Millqvist E. Capsaicin cough threshold test in diagnostics. Respiratory medicine. 2014;108:1371–1376. doi: 10.1016/j.rmed.2014.07.012. [DOI] [PubMed] [Google Scholar]
- Robbins J, Coyle J, Rosenbek J, Roecker E, Wood J. Differentiation of normal and abnormal airway protection during swallowing using the penetration-aspiration scale. Dysphagia. 1999;14:228–232. doi: 10.1007/PL00009610. [DOI] [PubMed] [Google Scholar]
- Rosenbek JC, Robbins JA, Roecker EB, Coyle JL, Wood JL. A penetration-aspiration scale. Dysphagia. 1996;11:93–98. doi: 10.1007/BF00417897. [DOI] [PubMed] [Google Scholar]
- Sanes JN, Ison JR. Habituation and sensitization of components of the human eyeblink reflex. Behavioral Neuroscience. 1983;97:833–836. doi: 10.1037//0735-7044.97.5.833. [DOI] [PubMed] [Google Scholar]
- Sasaki CT, Suzuki M. Laryngeal reflexes in cat, dog and man. Arch Otolaryngol. 1976;102:400–402. doi: 10.1001/archotol.1976.00780120048004. [DOI] [PubMed] [Google Scholar]
- Sasaki CT, Jassin B, Kim YH, Hundal J, Rosenblatt W, Ross DA. Central facilitation of the glottic closure reflex in humans. Ann Otol Rhinol Laryngol. 2003;112:293–297. doi: 10.1177/000348940311200401. [DOI] [PubMed] [Google Scholar]
- Sataloff RT, Mandel S, Mann E, Ludlow CL. Laryngeal electromyography: an evidence-based review. Muscle Nerve. 2003;28:767–772. doi: 10.1002/mus.10503. [DOI] [PubMed] [Google Scholar]
- Shaker R, Medda BK, Ren J, Jaradeh S, Xie P, Lang IM. Pharyngoglottal closure reflex: identification and characterization in a feline model. Am J Physiol. 1998;275:G521–525. doi: 10.1152/ajpgi.1998.275.3.G521. [DOI] [PubMed] [Google Scholar]
- Shaker R, Ren J, Bardan E, et al. Pharyngoglottal closure reflex: characterization in healthy young, elderly and dysphagic patients with predeglutitive aspiration. Gerontology. 2003;49:12–20. doi: 10.1159/000066504. [DOI] [PubMed] [Google Scholar]
- Strohl KP, Hensley MJ, Hallett M, Saunders NA, Ingram RHJ. Activation of upper airway muscles before onset of inspiration in normal humans. The American Physiological Society. 1980:638–642. doi: 10.1152/jappl.1980.49.4.638. [DOI] [PubMed] [Google Scholar]
- Sulica L, Carey B, Branski RC. A novel technique for clinical assessment of laryngeal nerve conduction: normal and abnormal results. Laryngoscope. 2013;123:2202–2208. doi: 10.1002/lary.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Sasaki CT. Laryngeal spasm: A neurophysiologic redefinition. Ann Otol Rhinol Laryngol. 1977;86:150–158. doi: 10.1177/000348947708600203. [DOI] [PubMed] [Google Scholar]
- Tatar M, Hanacek J, Widdicombe J. The expiration reflex from the trachea and bronchi. The European respiratory journal. 2008;31:385–390. doi: 10.1183/09031936.00063507. [DOI] [PubMed] [Google Scholar]
- Theurer JA, Bihari F, Barr AM, Martin RE. Oropharyngeal stimulation with air-pulse trains increases swallowing frequency in healthy adults. Dysphagia. 2005;20:254–260. doi: 10.1007/s00455-005-0021-1. [DOI] [PubMed] [Google Scholar]
- Titze IR, Luschei ES, Hirano M. Role of the thyroarytenoid muscle in regulation of fundamental frequency. Journal of Voice. 1989;3(3):213–224. [Google Scholar]
- Tomori Z, Stransky A. Electroneurographic and pneumotachographic analysis of the expiration reflex. Physiologia Bohemoslovaca. 1973;22:589–601. [PubMed] [Google Scholar]
- Tomori Z, Benacka R, Donic V. Mechanisms and clinicophysiological implications of the sniff- and gasp-like aspiration reflex. Respir Physiol. 1998;114:83–98. doi: 10.1016/s0034-5687(98)00077-2. [DOI] [PubMed] [Google Scholar]
- Tomori Z, Donic V, Benacka R, Jakus J, Gresova S. Resuscitation and auto resuscitation by airway reflexes in animals. Cough. 2013;9:21. doi: 10.1186/1745-9974-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully A, Brancatisano A, Loring SH, Engel LA. Influence of posterior cricoarytenoid muscle activity on pressure-flow relationship of the larynx. J Appl Physiol. 1991;70:2252–2258. doi: 10.1152/jappl.1991.70.5.2252. [DOI] [PubMed] [Google Scholar]
- Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax. 2006a;61:1065–1069. doi: 10.1136/thx.2006.064337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. The relationship between chronic cough and paradoxical vocal fold movement: a review of the literature. J Voice. 2006b;20:466–480. doi: 10.1016/j.jvoice.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Vertigan AE, Gibson PG, Theodoros DG, Winkworth AL. A review of voice and upper airway function in chronic cough and paradoxical vocal cord movement. Current opinion in allergy and clinical immunology. 2007;7:37–42. doi: 10.1097/ACI.0b013e328012c587. [DOI] [PubMed] [Google Scholar]
- Vertigan AE, Theodoros DG, Gibson PG, Winkworth AL. Voice and upper airway symptoms in people with chronic cough and paradoxical vocal fold movement. J Voice. 2007;21:361–383. doi: 10.1016/j.jvoice.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Volk GF, Pototschnig C, Mueller A, et al. Teaching laryngeal electromyography. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2015 Feb 25; doi: 10.1007/s00405-015-3568-y. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Volk GF, Hagen R, Pototschnig C, et al. Laryngeal electromyography: a proposal for guidelines of the European Laryngological Society. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2012;269:2227–2245. doi: 10.1007/s00405-012-2036-1. [DOI] [PubMed] [Google Scholar]
- Widdicombe J, Addington R. Modified endoscopic swallowing test for improved diagnosis and prevention of aspiration. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies. 2006;263:1057–1058. doi: 10.1007/s00405-006-0130-y. author reply 1059. [DOI] [PubMed] [Google Scholar]
- Woodson GE, Sant'ambrogio F, Mathew O, Sant'ambrogio G. Effects of cricothyroid muscle contraction on laryngeal resistance and glottic area. Ann Otol Rhinol Laryngol. 1989;98:119–124. doi: 10.1177/000348948909800207. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Nash EA, Tanaka Y, Ludlow CL. Effects of stimulus intensity on laryngeal long latency responses in awake humans. Otolaryngol Head Neck Surg. 1997;117:521–529. doi: 10.1016/S0194-59989770025-1. [DOI] [PubMed] [Google Scholar]
- Zealear DL, Billante CR, Courey MS, et al. Reanimation of the paralyzed human larynx with an implantable electrical stimulation device. Laryngoscope. 2003;113:1149–1156. doi: 10.1097/00005537-200307000-00010. [DOI] [PubMed] [Google Scholar]