Abstract
Background
Accumulating but inconsistent data about the role of rs13266634 variant of SLC30A8 in type 2 diabetes have been reported, partly due to small sample sizes and non-identical ethnicity.
Material/Methods
We searched PubMed and Cochrane Library to identify eligible studies and extract data of baseline characteristics, genotype count, odds ratio (OR), and 95% confidence interval (CI). Both adjusted OR with 95% CI and genotype counts were employed to assess the association. Genotype data were further pooled to provide estimates under different genetic models and the most appropriate model was determined. Sensitivity and cumulative analysis were conducted to assure the strength of results.
Results
Fifty-five datasets of 39 studies (including 38 of 24 with genotype count) were included. Significant associations were found in allelic contrasts using adjusted ORs and raw genotype count, respectively, overall in Asian and European populations (overall: OR=1.147/1.157, 95% CI 1.114–1.181/1.135–1.180; Asian: OR=1.186/1.165, 95% CI 1.150–1.222/1.132–1.198; European: OR=1.100/1.151, 95% CI 1.049–1.153/1.120–1.183; All p=0.00), but not in African populations (African: OR=1.255/1.111, 95% CI 0.964–1.634/0.908–1.360, p=0.091/0.305). Further analysis with genotype count under different genetic models all showed that individuals with CC genotype had 33.0% and 16.5% higher risk of type 2 diabetes than those carrying TT and CT genotypes, respectively, under the most likely codominant model. Cumulative analysis indicated gradually improved precision of estimation after studies accumulated.
Conclusions
Our results suggest that rs13266634 may be an important genetic factor of type 2 diabetes risk among Asian and European but not African populations.
MeSH Keywords: Diabetes Mellitus, Type 2; Meta-Analysis; Polymorphism, Genetic
Background
Diabetes is a group of worldwide prevalent, metabolic diseases characterized by hyperglycemia resulting from defects in insulin secretion, insulin action, or both. Two major etiopathogenetic groups of diabetes mellitus have been defined: type 1 and type 2. Type 1 diabetes is an autoimmune disease. Diabetes type 2 is much more prevalent and has a more complicated etiology involving a combination of genetic to environmental factors. Diabetes mellitus affects approximately 347 million people worldwide[1], 90% of which have type 2 diabetes, and accounts for an estimated 3.4 million death in 2004 [2]. The economic costs and burden of diabetes are considerable, given the substantial morbidity and vital multi-organ complications [3].
In recent years many genes have been identified to be associated with type 2 diabetes susceptibility [4,5]. SLC30A8, as a coding gene of zinc transporter 8 (ZnT8), was reported to contribute to type 2 diabetes susceptibility [6–9]. ZnT8, a novel member of ZnT family, is predominately expressed in pancreatic islet beta cells and is responsible for cellular efflux of zinc from cytoplasm into intracellular vesicles. It localizes into insulin secretory granules and is indispensable for insulin crystallization, storage, and secretion [10–12]. Antibodies against ZnT8 have been identified as novel biomarkers for autoimmune diabetes [13–19]. The association of SLC30A8 variants with type 2 diabetes has also received much attention, with an important polymorphism, rs13266634, being the most studied [19–29]. This non-synonymous variant (rs13266634) is a C-to-T variant (arginine to tryptophan at position 325, R325W) and had been confirmed to be associated with higher risk of type 2 diabetes in various ethnic populations [6–9,30–33].
Although a number of studies, including some meta-analyses, have been conducted to investigate the association between rs13266634, the most common variant of SLC30A8, and the risk of type 2 diabetes in diverse populations, the results were mixed and inconclusive, possibly due to the relatively small sample size in the included studies. Meta-analysis is a cost-effective method to increase sample size by combining data from different independent studies, and is recognized as a ideal tool for summarizing inconsistent results from multiple studies. There has recently been an increase in the number of these studies, but there is no updated meta-analysis investigating the association between them. To provide the most comprehensive assessment of the relationship between rs13266634 variant and type 2 diabetes risk, we performed an updated meta-analysis of all available studies.
Material and Methods
The meta-analysis was conducted according to the PRISMA statement (Preferred reporting items for systematic reviews and meta-analyses, checklist S1) [34] and meta-analysis on genetic association studies (checklist S2).
Search strategy
We searched PubMed and Cochrane Library from 2007, the year when the association of SLC30A8 and diabetes was first reported [6–9], to identify all relevant papers on humans published in English. The following terms were used in searching: (rs13266634 or zinc transporter protein member 8 or ZnT8 or ZnT-8 or SLC30A8) and (polymorphism or variant or loci) and diabetes. All eligible studies were retrieved and their references were checked for other relevant publications in English. The titles and abstracts were scanned to exclude studies that were clearly irrelevant to the current topic. The full text of the remaining articles was read to determine whether they contained information of interest. Two independent reviewers (Cheng and Zhang) performed a systematic search in PubMed and Cochrane databases, with the last search updated on June 25, 2014.
Inclusion and exclusion criteria
Studies included in the meta-analysis were required to meet the following criteria: (1) case-control study or prospective study that evaluated the association between rs13266634 and type 2 diabetes in humans; and (2) had an odds ratio (OR) with 95% confidence interval (CI) or detailed genotype data for estimating OR (95% CI). Exclusion criteria are: (1) duplication of previous publications; (2) comment, review, or editorial; and (3) family-based studies of pedigrees.
Data extraction
Two investigators (Cheng and Zhang) independently extracted information from all eligible publications. The results were compared and disagreements were discussed until a consensus was reached. Data extracted from each study included the following characteristics: the first author’s name, the year of publication, ethnicity of participants, age, age at diagnosis, body mass index (BMI), fasting plasma glucose (FPG), total number, genotype numbers and frequency, and the most completely adjusted estimate (OR and 95% CI, respectively) of the rs13266634 polymorphism in the cases and controls. If dissent still existed, the third investigator (Zhou) would be involved to adjudicate the disagreements. For those included articles without all desired wanted data, we emailed the authors, providing contact information, to ask for details.
Quality score assessment
The quality of selected studies was assessed by 3 investigators (Cheng, Zhang, and Zhou) independently scoring studies according to a set of predetermined criteria adjusted from previous reports [35,36]. Quality scores ranged from 0 to 10 and studies were scored as “good” if the score was 8–10, “fair” if the score was 5–7, and “poor” if the score was <4. Discrepancies were resolved by discussion.
Statistical analyses
All statistical tests were conducted with STATA software version 11.0 (STATA Corp, College Station, Texas).
To investigate the association strength between SLC30A8 rs13266634 polymorphism and the susceptibility of type 2 diabetes, we used adjusted ORs and corresponding 95% CIs from all studies, and calculated ORs from studies with genotype distribution information to pool ORs and corresponding 95% CIs. For those studies with raw genotype count, we then obtained pooled ORs and corresponding 95% CIs from combination of single studies by homozygote and heterozygote comparison (CC vs. TT, OR1; CT vs. TT, OR2; CC vs. CT, OR3), overdominant (CC+CT vs. TT) an dominant and recessive models (CC vs. CT+TT and CC+CT vs. TT), respectively. We also performed subgroup analysis on ethnicity, Hardy-Weinberg equilibrium (HWE), genotyping methods, sample size (large sample ≥1000, small sample <1000), study design (population-based case-control study, hospital-based case-control study, and prospective study), and quality of studies in these comparisons. Environmental effects associated with diabetes, such as diet and exercise, were not analyzed due to limited details. Z-test was used for assessing the significance of the pooled ORs, with p<0.05 considered statistically significant. A biological justification for the choice of the genetic model was estimated according the relationships of OR1, OR2 and OR3 [37]. Cumulative meta-analysis by publication year was conducted to investigate time-based fluctuation and robustness of results.
Heterogeneity among the studies was evaluated as notable by the chi-square-based Q-test (significance level of p<0.10) and/or I2 index (greater than 50% as evidence of significant inconsistency) [38] and the random-effects model (DerSimonian and Laird method); otherwise, the fixed-effects model (Mantel and Haenszel method) was employed. The significance of pooled ORs was determined by Z-test and p<0.05 was considered as statistically significant. For the controls of each study with detailed genotype count, HWE was evaluated using the goodness-of-fit chi-square test and a p<0.05 was considered to deviate from HWE. To identify the effect of any individual study, especially studies deviating from HWE, we pooled results and assessed the stability of the results. Sensitivity analysis was also carried out by deleting a single study each time to examine the influence of individual data sets on the pooled ORs. Publication bias of literature was examined using Begg’s funnel plots and Egger’s test [39]. An asymmetrical plot suggests possible publication bias and the p value of Egger’s test less than 0.05 was considered the presence of potential publication bias [40].
Results
Flow of included studies and characteristics of studies
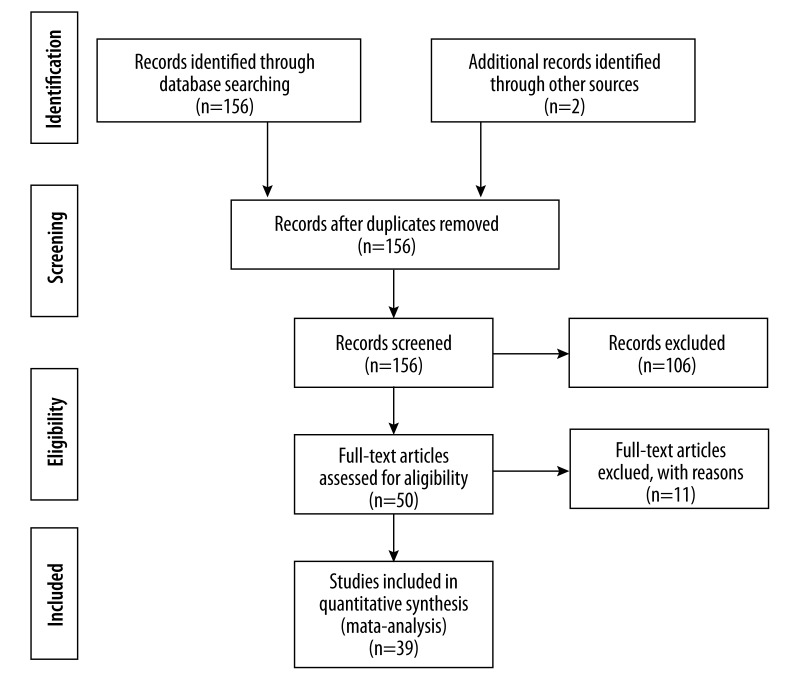
Figure 1 is a flow diagram illustrating the strategy used to identify and select studies for inclusion in the meta-analysis. According to the PubMed and Cochrane database with associated key words, a total of 156 articles were retrieved and 2 studies from references were included. After excluding 2 duplicates, 156 articles were screened by titles and abstracts and 106 of them were excluded as review articles or irrelevant studies. Fifty studies appeared to satisfy our criteria and their full texts were evaluated. In further examination, we excluded 11 studies deviating from inclusion criteria. Finally, 39 studies, including 36 case-control studies, 2 prospective studies, and 1 study including both case-control and prospective groups, were eligible and included in our meta-analysis[6–9,28,30,31,33,41–71].
Figure 1.
Flow diagram of study selection.
A summary of characteristics of each selected study, including first author, year of publication, ethnicity, genotype distribution, HWE, score of studies, and adjusted estimate (OR and 95% CI).
Overall meta-analysis and stratified analysis
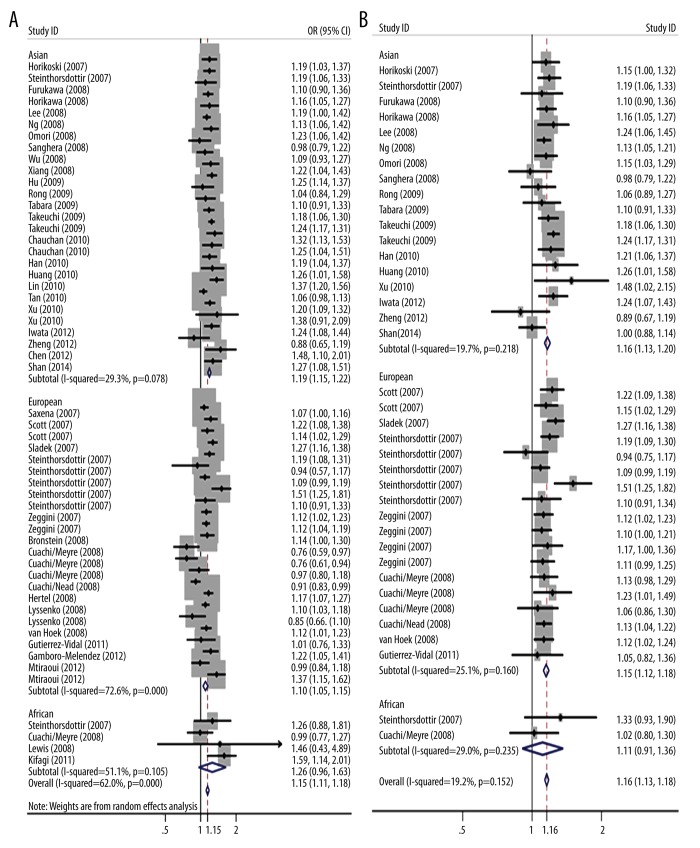
We pooled overall and ethnic-specific ORs for allelic contrast with adjusted or calculated ORs, respectively, from the included studies to assess the association between rs13266634 variants and susceptibility to type 2 diabetes (Table 1 and Figure 2). A significant association was identified with adjusted ORs from all eligible studies in the overall population, Asian and European populations, but not in African population (Overall population: OR=1.147, 95% CI 1.114–1.181, p=0.000; Asian: OR=1.186, 95% CI 1.150–1.222, p=0.000; European: OR=1.100, 95% CI 1.049–1.153, p=0.000; African: OR=1.255, 95% CI 0.964–1.634, p=0.091) using the random-effects model from 55 data sets of 39 independent studies with 65 767 cases and 100 182 controls. Similar results were acquired using calculated ORs from 38 datasets of 24 studies with detailed genotype distribution under the fixed-effects model (overall population: OR=1.157, 95% CI 1.135–1.180, p=0.000; Asian: OR=1.165, 95% CI 1.132–1.198, p=0.000; European: OR=1.151, 95% CI 1.120–1.183, p=0.000; African: OR=1.111, 95% CI 0.908–1.360, p=0.305).
Table 1.
Meta-analysis of the association between rs13266634 variant and susceptibility to type 2 diabetes (all conducted under fixed-effects model except those marked with an asterisk).
| Genetic contrasts | Ethnic group | Data sets (n) | OR (95% CI) | P | Heterogeneity test | |
|---|---|---|---|---|---|---|
| I2 (%) | P (Q) | |||||
| C vs. T(ajusted ORs)* | Overall | 55 | 1.147 (1.114–1.181) | 0.000 | 62.0 | 0.000 |
| Asian | 27 | 1.186 (1.150–1.222) | 0.000 | 29.3 | 0.078 | |
| European | 24 | 1.100 (1.049–1.153) | 0.000 | 72.6 | 0.000 | |
| African | 4 | 1.255 (0.964–1.634) | 0.091 | 51.1 | 0.105 | |
| C vs. T (calculated ORs) | Overall | 38 | 1.157 (1.135–1.180) | 0.000 | 19.2 | 0.152 |
| Asian | 18 | 1.165 (1.132–1.198) | 0.000 | 19.7 | 0.218 | |
| European | 18 | 1.151 (1.120–1.183) | 0.000 | 25.1 | 0.160 | |
| African | 2 | 1.111 (0.908–1.360) | 0.305 | 29.0 | 0.235 | |
| CC + CT vs. TT | Overall | 38 | 1.216 (1.123–1.318) | 0.000 | 17.1 | 0.182 |
| Asian | 18 | 1.221 (1.160–1.286) | 0.000 | 47.5 | 0.014 | |
| European | 18 | 1.221 (1.149–1.298) | 0.000 | 0.0 | 0.793 | |
| African | 2 | 1.207 (0.575–2.533) | 0.618 | 0.0 | 0.730 | |
| CC vs. CT + TT | Overall | 38 | 1.197 (1.165–1.229) | 0.000 | 21.7 | 0.120 |
| Asian | 18 | 1.215 (1.167–1.266) | 0.000 | 9.9 | 0.336 | |
| European | 18 | 1.185 (1.143–1.228) | 0.000 | 34.2 | 0.078 | |
| African | 2 | 1.118 (0.895–1.397) | 0.326 | 23.1 | 0.254 | |
| CC + TT vs. CT* | Overall | 38 | 1.086 (1.050–1.123) | 0.000 | 29.4 | 0.048 |
| Asian | 18 | 1.056 (1.000–1.116) | 0.050 | 38.3 | 0.050 | |
| European | 18 | 1.108 (1.062–1.156) | 0.000 | 21.7 | 0.196 | |
| African | 2 | 1.112 (0.855–1.446) | 0.428 | 21.4 | 0.259 | |
| CC vs. TT(OR1) | Overall | 38 | 1.330 (1.274–1.388) | 0.000 | 5.7 | 0.369 |
| Asian | 18 | 1.346 (1.270–1.426) | 0.000 | 31.0 | 0.103 | |
| European | 18 | 1.312 (1.230–1.398) | 0.000 | 0.0 | 0.665 | |
| African | 2 | 1.213 (0.577–2.552) | 0.611 | 0.0 | 0.718 | |
| CT vs. TT(OR2) | Overall | 38 | 1.136 (1.089–1.184) | 0.000 | 21.2 | 0.126 |
| Asian | 18 | 1.141 (1.080–1.206) | 0.004 | 51.5 | 0.005 | |
| European | 18 | 1.128 (1.058–1.203) | 0.000 | 0.0 | 0.853 | |
| African | 2 | 1.144 (0.532–2.459) | 0.731 | 0.0 | 0.938 | |
| CC vs. CT(OR3) | Overall | 38 | 1.165 (1.132–1.198) | 0.000 | 21.6 | 0.122 |
| Asian | 18 | 1.176 (1.126–1.228) | 0.000 | 17.7 | 0.242 | |
| European | 18 | 1.155 (1.115–1.202) | 0.000 | 31.4 | 0.100 | |
| African | 2 | 1.109 (0.882–1.394) | 0.377 | 19.5 | 0.265 | |
Figure 2.
Stratified analysis based on ethnicity for the association between SLC30A8 polymorphism rs13266634 and type 2 diabetes risk under allelic model. (A) Using individual adjusted ORs from all original articles, (B) using individual calculated ORs from studies with detailed genotype distribution. Each study is shown by the point estimate of the odds ratio, and a horizontal line denotes the 95% confidence interval. The pooled odds ratio is represented by a diamond. The area of the grey squares reflects the weight of the study in the meta-analysis.
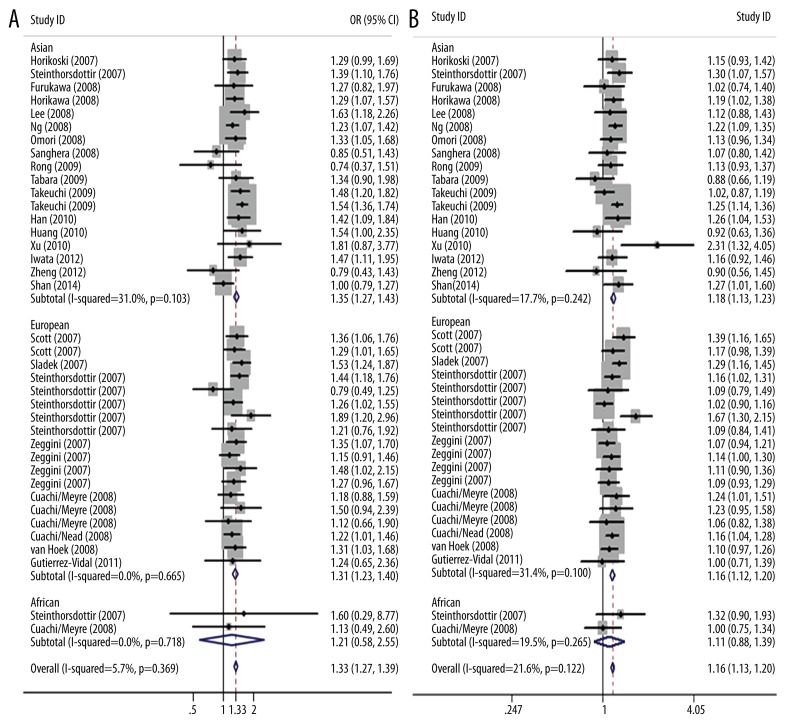
Then 38 data sets from 24 studies with detailed genotype distribution were analyzed employing different genetic models. The fixed-effects model was conducted in all genetic models except for overdominant model analysis, which showed significant heterogeneity (p<0.1) The pooled analysis of all genetic models yielded significant overall association (CC+CT vs. TT: OR=1.216, 95% CI: 1.123–1.318, p=0.000; CC vs. CT+TT: OR=1.197, 95% CI: 1.165–1.229, p=0.000; CC+TT vs. CT: OR=1.086, 95% CI: 1.050–1.123, p=0.000; CC vs. TT: OR1=1.330, 95% CI: 1.274–1.388, p=0.000; CT vs. TT: OR2=1.136, 95% CI: 1.089–1.184, p=0.000; CC vs. CT: OR3=1.165, 95% CI: 1.132–1.198, p=0.000). When stratifying the data by ethnicity, we observed an increased risk among Asians in all except the overdominant model (CC+TT vs. CT, p=0.050). Significant associations were also found among Europeans. Not surprisingly, this polymorphism did not appear to influence risk of African population and type 2 diabetes in all genetic models (Table 1 and Figure 3).
Figure 3.
Stratified analysis based on ethnicity for the association between SLC30A8 polymorphism rs13266634 and type 2 diabetes risk under codominant genetic model. (A) CC vs. TT, (B) CC vs. CT. Each study is shown by the point estimate of the odds ratio, and a horizontal line denotes the 95% confidence interval. The pooled odds ratio is represented by a diamond. The area of the grey squares reflects the weight of the study in the meta-analysis.
According to the recommendation of Thakkinstian et al. [37], pairwise differences of OR1, OR2, and OR3 were used to indicate the most appropriate genetic model (OR1=OR3≠1,OR2=1, recessive model; OR1=OR2≠1, OR3=1, dominant model;OR2=1,OR3≠1, OR1=1, overdominant model; OR1>OR2>1,OR1>OR3<1 or OR1<OR2<1, OR1<OR3<1, codominant model). The pooled OR1 for CC versus TT, OR2 for CT versus TT, OR3 for CC versus CT(OR1=1.330, OR2=1.136, OR3=1.165) suggested that a codominant effect was most likely and that individuals with CC genotype had 33.0% and 16.5% higher risk of type 2 diabetes than those carrying TT and CT genotypes, respectively. We further carried out subgroup analysis under this genetic model based on HWE, genotyping methods, sample size, study design and quality of studies (conducted by fixed-effects model). We observed increased risk of type 2 diabetes regardless of HWE, genotyping methods, and study design. In subgroup analysis stratified by ethnicity, we found that this polymorphism was associated with type 2 diabetes risk both in Asians and Europeans but not in Africans. In subgroup analysis stratified by sample size, we found increased risk in large sample sizes but not small sample sizes. In subgroup analysis stratified by quality of studies, however, fair quality subgroup in CC vs. CT comparison, unlike that in CC vs. TT comparison, indicated no significant association.
Test of heterogeneity and sensitivity analysis
In allelic comparison using adjusted ORs, there was heterogeneity across studies (P=0.000 and I2=62.0%); thus, a random-effects model was finally employed to obtain summary OR. The source of heterogeneity was further explored by subgroup analysis based on ethnicity, which indicated that studies of Europeans were mainly responsible for the overall heterogeneity; in allelic comparison using calculated ORs from raw data, there was no heterogeneity across the study (p=0.152 and I2=19.2%); thus, a fixed-effects model was finally employed to obtain summary OR. For comparisons using studies with raw genotype count, the fixed-effects model was conducted in all contrasts except overdominant model (CC+CT vs. TT: p=0.182, I2=17.1%; CC vs. CT+TT: p=0.120, I2=21.7%; CC vs. TT: p=0.369, I2=5.7%; CT vs. TT: p=0.126, I2=21.2%; CC vs. CT: p=0.122, I2=21.6%; CC+TT vs. CT: p=0.048, I2=29.4%) due to heterogeneity (Table 1).
To evaluate the effect of each individual study, especially studies deviating from HWE, on the pooled estimate, we performed a sensitivity analysis by deleting 1 study each time in turn. It showed that all the results hardly changed after sequential removal of each study from the total analysis, indicating the robustness of these results (data not shown). We also observed that the significance of the overall data for the different genetic models was not statistically altered under either random- or fixed-effects models.
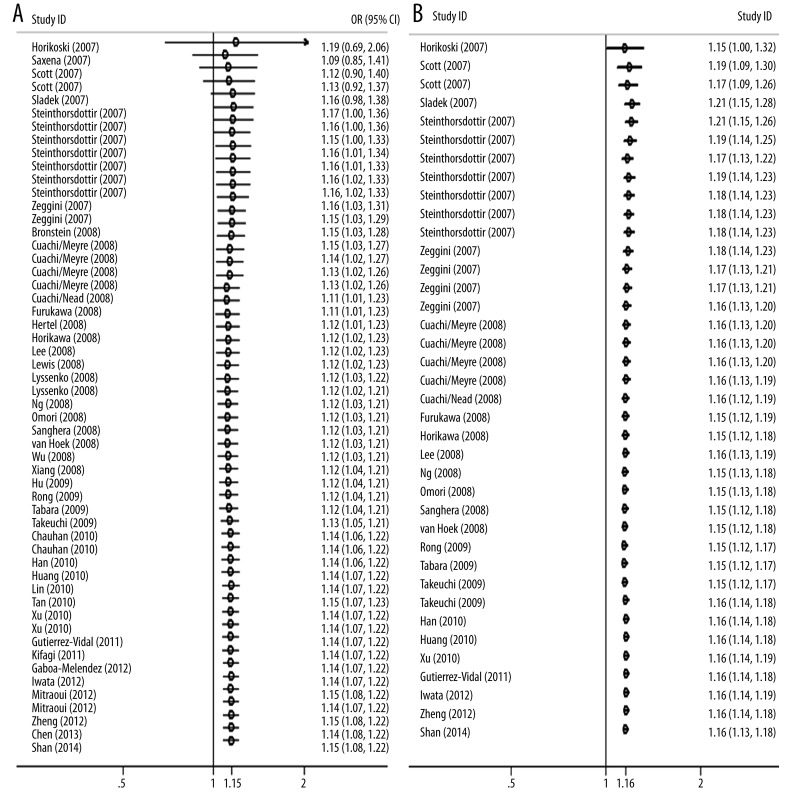
Cumulative meta-analysis
Cumulative meta-analysis was also performed in allelic contrast with adjusted and calculated ORs by gradually including each additional study according to published year. The pooled genetic risk effect of both adjusted and calculated allelic contrasts remained significant in the entire period studied and the 95% CI for pooled ORs became progressively narrower after adding 1 more study, indicating that the precision of our estimation was gradually improved after adding more studies (Figure 4).
Figure 4.
Cumulative meta-analysis of associations between the rs13266634 variant of SLC30A8 with type 2 diabetes risk in allelic contrasts sorted by publication year. The horizontal line shows the accumulation of estimates as each study was added, and is not the estimate of a single study. (A) Using individual adjusted ORs from all original articles, (B) using individual calculated ORs from studies with detailed genotype distribution.
Publication bias
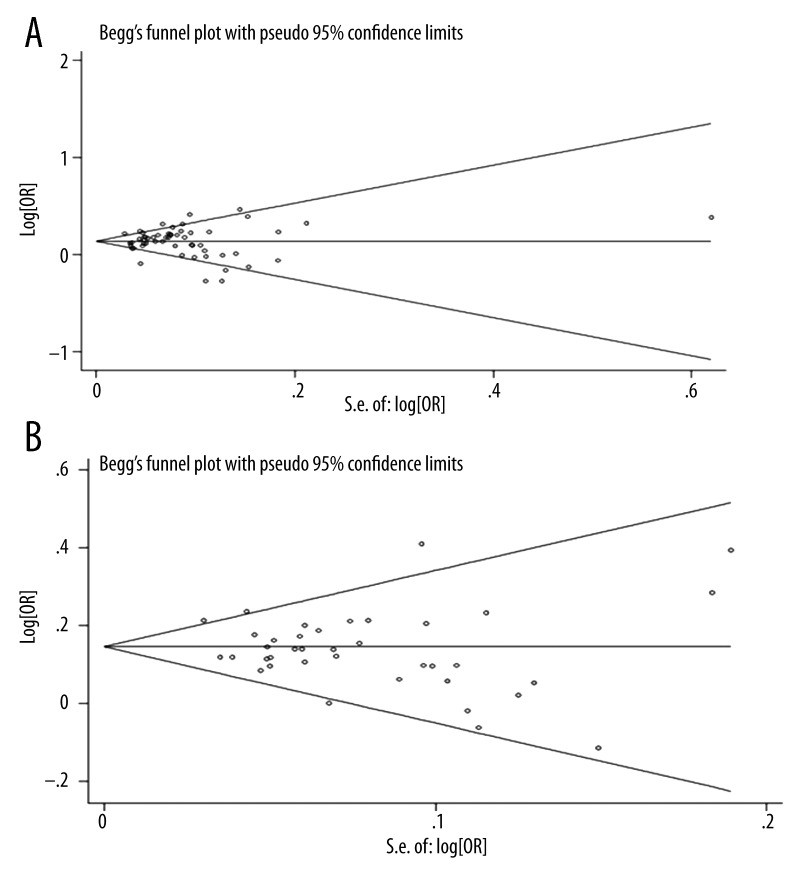
Funnel plot and Egger’s test were performed to assess the publication bias of included studies. As shown in Figure 5, the shapes of the Begg’s funnel plot did not reveal any evidence of obvious asymmetry in both stages. Then, Egger’s test was used to provide statistical evidence of funnel plot symmetry. The results still did not show any evidence of publication bias (p=0.496/0.249, respectively).
Figure 5.
Begg’s publication bias funnel plot of the ORs for SLC30A8 mutation rs13266634 and the standard error of natural logarithm of the ORs for the included studies. Circles represent individual studies and a dash line indicates 95% confidence interval. (A) Using individual adjusted ORs from all original articles, (B) using individual calculated ORs from studies with detailed genotype distribution.
Discussion
So far, 3 previous meta-analysis have been conducted to explore the function of SLC30A8 gene polymorphisms in type 2 diabetes [72–74]. One of them pooled allelic ORs but provided no details about selecting criteria, another also conducted allelic contrast with adjusted individual OR, and the last one carried out contrasts under different genetic models with calculated individual OR. Although all 3 articles indicated increased risk of type 2 diabetes with polymorphism rs13266634, they employed either adjusted or calculated individual OR only rather than both, and did not conclude which was the appropriate genetic model. Additionally, considering several new original studies on this variant, we therefore decided to perform this updated meta-analysis, with more samples (including 38 studies, involving 65 767 type 2 diabetes patients and 100 182 controls) and more comprehensive comparisons (using both adjusted and calculated individual OR, under most likely and overall genetic models), to elucidate the relationship between rs13266634 variant and type 2 diabetes. The summary results from adjusted ORs demonstrated that C allele carriers of rs13266634 are strongly associated with type 2 diabetes risk in the total population. Subgroup analysis by ethnicity showed the same result in Asian and European populations but not in African populations. Furthermore, meta-analysis with calculated raw ORs generated similar results in overall and ethnic populations under different s models. Finally, we decided on the most likely genetic model, the codominant model, and performed subgroup analysis based on HWE, genotyping methods, sample size, study design, and quality of studies, and observed increased risk of type 2 diabetes in all populations except Africans, small sample size subgroups, and fair subgroup in CC vs. CT comparison. Hence, the result of subgroup analysis also confirmed the association between polymorphism rs13266634 and risk of type 2 diabetes. This differences in ethnicity may be due to the different genetic backgrounds of and limited articles on the African population. The difference in sample size subgroup is reasonable and indicates the importance of sample size in research. The difference in study quality subgroup between CC vs. TT and CC vs. CT contrasts may result from gene additive effect besides quality itself. Cumulative meta-analysis indicated a consistent and more precise estimation as evidence from published studies accumulated according to year published.
To date, 5 genome-wide association studies (GWAS) involving type 2 diabetes and SLC30A8 polymorphism rs13266634 have been published [6–9, 64], which were all included in this meta-analysis. Four of them reported that this common variant is also associated with the risk of type 2 diabetes in Europeans (combined OR=1.12 and 95% CI=1.07–1.16), although data from 1 study were less compelling (p=0.90 in the genome scan and p=0.01 in replication samples) [9]. One replication in Asians also showed similar results as in Europeans (OR=1.16 and 95% CI=1.05–1.27). Therefore, results from GWAS were consistent with that of our meta-analysis, which indicated that SLC30A8 polymorphism rs13266634 confers risk of type 2 diabetes.
Potential mechanisms whereby rs13266634 variant of ZnT8 may modulate insulin biophysiological activities and plasma glucose metabolism have been extensively studied. There are several possible explanations for the association between type 2 diabetes risk and rs13266634. Firstly, given the significant role of ZnT8 in insulin activities, this mutation may affect the basal function of ZnT8 and subsequent insulin synthesis. Zinc is essential for the correct processing, storage, secretion, and action of insulin in pancreatic β cells [75–77]. The W325 variant displayed higher zinc transport activity than R325 ZnT8 in a murine β-cell line[78] and protective effect against cyclosporin A-induced suppression of insulin secretion [79]. Similarly, studies also demonstrated that this polymorphism is strongly associated with abnormal insulin behaviors in humans, including decreased insulin secretion response to glucose stimulations, lower fasting insulin levels, reduced disposition index, and decreased proinsulin-to-insulin conversion [56,80–82]. Controversially, the risk C-allele does not affect ex-vivo insulin secretion and SLC30A8 expression in isolated human islets [74]. Secondly, this mutation may regulate plasma insulin concentration via decreasing hepatic insulin clearance. A recent study revealed that humans carrying rs13266634 exhibited increased insulin clearance, as assessed by c-peptide/insulin ratio, and mice with beta cell-specific SLC30A8 knockout demonstrated the similar results; thus, we speculate that the rs13266634 variant may result in the same consequence [83]. The studies cited above suggest that rs13266634 variant can modulate plasma glucose homeostasis via full-scale insulin activities from synthesis to clearance [56,80–82].
Several strengths of this meta-analysis could be listed. First of all, we followed a rigorous protocol of meta-analysis [34] and meta-analysis of genetic association studies [37]. We performed subgroup analysis, HWE test, sensitivity analysis, and funnel plots to explore the source of heterogeneity, indicating the reliability of our study. Next, we included more original studies and more samples than previous studies to enhance the statistical power of the study. Moreover, we used adjusted and calculated ORs to summarize estimate and raw genotype count, respectively, analyzed under different genetic models with the codominant model deemed to be most likely. All previous meta-analyses used either adjusted or calculated ORs and did not specify a most likely genetic model. Lastly, we conducted cumulative meta-analysis to investigate the dynamic change trend of the research results and the potential impact of small samples on estimate effect size. The small sample sizes without cumulative analyses may have influenced the strength of results of previous studies.
Unavoidable limitations of this meta-analysis should also be pointed out. The first limitation comes from multiple and diverse methods for SNP detection in different studies. The second limitation is that type 2 diabetes is a complex and multifactorial disorder and potential interactions among gene-gene and gene-environment should be considered; however, insufficient information, including nutrient, lifestyle behavior, and demographic details, hinder us from performing further adjusted analysis. The third limitation is that although we tried our best to contact the corresponding authors of published articles, we could not get information on gain genotypes data of all studies. The final limitation is that studies of Africans and prospective research were limited; thus, the interpretation of results should be cautious.
Conclusions
The present meta-analysis suggests that SLC30A8 gene polymorphism rs13266634 may be an important genetic factor in the risk for developing type 2 diabetes among Asian and European populations but not African populations. Mechanism studies are needed to explain and support this finding in various ethnic groups.
Footnotes
Disclosure
No competing financial interests exist.
Source of support: This study was partly supported by the Scientific and Technological plans of Chongqing (cstc2014yykfA110004)
References
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 2.WHO. Mortality and burden of disease attributable to selected major risks. Geneva: 2009. Global health risks. [Google Scholar]
- 3.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014:S81–90. doi: 10.2337/dc14-S081. [DOI] [PubMed] [Google Scholar]
- 4.Williams AL, Jacobs SB, Moreno-Macias H, et al. Sequence variants in SLC16A11 are a common risk factor for type 2 diabetes in Mexico. Nature. 2014;506:97–101. doi: 10.1038/nature12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinthorsdottir V, Thorleifsson G, Sulem P, et al. Identification of low-frequency and rare sequence variants associated with elevated or reduced risk of type 2 diabetes. Nat Genet. 2014;46:294–98. doi: 10.1038/ng.2882. [DOI] [PubMed] [Google Scholar]
- 6.Zeggini E, Weedon MN, Lindgren CM, et al. Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science. 2007;316:1336–41. doi: 10.1126/science.1142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sladek R, Rocheleau G, Rung J, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–85. doi: 10.1038/nature05616. [DOI] [PubMed] [Google Scholar]
- 8.Scott LJ, Mohlke KL, Bonnycastle LL, et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316:1341–45. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saxena R, Voight BF, Lyssenko V, et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316:1331–36. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 10.Chimienti F, Devergnas S, Pattou F, et al. In vivo expression and functional characterization of the zinc transporter ZnT8 in glucose-induced insulin secretion. J Cell Sci. 2006;119:4199–206. doi: 10.1242/jcs.03164. [DOI] [PubMed] [Google Scholar]
- 11.Chimienti F, Devergnas S, Favier A, Seve M. Identification and cloning of a beta-cell-specific zinc transporter, ZnT-8, localized into insulin secretory granules. Diabetes. 2004;53:2330–37. doi: 10.2337/diabetes.53.9.2330. [DOI] [PubMed] [Google Scholar]
- 12.Lemaire K, Ravier MA, Schraenen A, et al. Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA. 2009;106:14872–77. doi: 10.1073/pnas.0906587106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skarstrand H, Lernmark A, Vaziri-Sani F. Antigenicity and epitope specificity of ZnT8 autoantibodies in type 1 diabetes. Scand J Immunol. 2013;77:21–29. doi: 10.1111/sji.12008. [DOI] [PubMed] [Google Scholar]
- 14.Long AE, Gillespie KM, Aitken RJ, et al. Humoral responses to islet antigen-2 and zinc transporter 8 are attenuated in patients carrying HLA-A*24 alleles at the onset of type 1 diabetes. Diabetes. 2013;62(6):2067–71. doi: 10.2337/db12-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingemansson S, Vaziri-Sani F, Lindblad U, et al. Long-term sustained autoimmune response to beta cell specific zinc transporter (ZnT8, W, R, Q) in young adult patients with preserved beta cell function at diagnosis of diabetes. Autoimmunity. 2013;46:50–61. doi: 10.3109/08916934.2012.730585. [DOI] [PubMed] [Google Scholar]
- 16.Andersson C, Vaziri-Sani F, Delli A, et al. Triple specificity of ZnT8 autoantibodies in relation to HLA and other islet autoantibodies in childhood and adolescent type 1 diabetes. Pediatr Diabetes. 2013;14:97–105. doi: 10.1111/j.1399-5448.2012.00916.x. [DOI] [PubMed] [Google Scholar]
- 17.Andersen MK, Harkonen T, Forsblom C, et al. Zinc transporter type 8 autoantibodies (ZnT8A): prevalence and phenotypic associations in latent autoimmune diabetes patients and patients with adult onset type 1 diabetes. Autoimmunity. 2013;46:251–58. doi: 10.3109/08916934.2012.741155. [DOI] [PubMed] [Google Scholar]
- 18.Trabucchi A, Faccinetti NI, Guerra LL, et al. Detection and characterization of ZnT8 autoantibodies could help to screen latent autoimmune diabetes in adult-onset patients with type 2 phenotype. Autoimmunity. 2012;45:137–42. doi: 10.3109/08916934.2011.604658. [DOI] [PubMed] [Google Scholar]
- 19.Sorgjerd EP, Skorpen F, Kvaloy K, et al. Prevalence of ZnT8 antibody in relation to phenotype and SLC30A8 polymorphism in adult autoimmune diabetes: results from the HUNT study, Norway. Autoimmunity. 2013;46:74–79. doi: 10.3109/08916934.2012.732132. [DOI] [PubMed] [Google Scholar]
- 20.Yakala GK. Protein truncating variants of SLC30A8 reduce type 2 diabetes mellitus risk in humans. Clin Genet. 2014;86(2):121–22. doi: 10.1111/cge.12412. [DOI] [PubMed] [Google Scholar]
- 21.Turki A, Al-Zaben GS, Khirallah M, et al. Gender-dependent associations of CDKN2A/2B, KCNJ11, POLI, SLC30A8, and TCF7L2 variants with type 2 diabetes in (North African) Tunisian Arabs. Diabetes Res Clin Pract. 2014;103:e40–43. doi: 10.1016/j.diabres.2013.12.040. [DOI] [PubMed] [Google Scholar]
- 22.Sprouse C, Gordish-Dressman H, Orkunoglu-Suer EF, et al. SLC30A8 nonsynonymous variant is associated with recovery following exercise and skeletal muscle size and strength. Diabetes. 2014;63:363–68. doi: 10.2337/db13-1150. [DOI] [PubMed] [Google Scholar]
- 23.Salem SD, Saif-Ali R, Ismail IS, et al. Contribution of SLC30A8 variants to the risk of type 2 diabetes in a multi-ethnic population: a case control study. BMC Endocr Disord. 2014;14:2. doi: 10.1186/1472-6823-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pineda-Tenor D, Micheloud D, Berenguer J, et al. SLC30A8 rs13266634 polymorphism is related to a favorable cardiometabolic lipid profile in HIV/hepatitis C virus-coinfected patients. AIDS. 2014;28(9):1325–32. doi: 10.1097/QAD.0000000000000215. [DOI] [PubMed] [Google Scholar]
- 25.Faghih H, Khatami SR, Azarpira N, Foroughmand AM. SLC30A8 gene polymorphism (rs13266634 C/T) and type 2 diabetes mellitus in south Iranian population. Mol Biol Rep. 2014;41:2709–15. doi: 10.1007/s11033-014-3158-x. [DOI] [PubMed] [Google Scholar]
- 26.Billings LK, Jablonski KA, Ackerman RJ, et al. The Influence of Rare Genetic Variation in SLC30A8 on Diabetes Incidence and beta-Cell Function. J Clin Endocrinol Metab. 2014;99:E926–30. doi: 10.1210/jc.2013-2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaki M, Fujitani Y, Hara A, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123:4513–24. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zheng X, Ren W, Zhang S, et al. Association of type 2 diabetes susceptibility genes (TCF7L2, SLC30A8, PCSK1 and PCSK2) and proinsulin conversion in a Chinese population. Mol Biol Rep. 2012;39:17–23. doi: 10.1007/s11033-011-0705-6. [DOI] [PubMed] [Google Scholar]
- 29.Xu J, Wang J, Chen B. SLC30A8 (ZnT8) variations and type 2 diabetes in the Chinese Han population. Genet Mol Res. 2012;11:1592–98. doi: 10.4238/2012.May.24.1. [DOI] [PubMed] [Google Scholar]
- 30.Tan JT, Ng DP, Nurbaya S, et al. Polymorphisms identified through genome-wide association studies and their associations with type 2 diabetes in Chinese, Malays, and Asian-Indians in Singapore. J Clin Endocrinol Metab. 2010;95:390–97. doi: 10.1210/jc.2009-0688. [DOI] [PubMed] [Google Scholar]
- 31.Wu Y, Li H, Loos RJ, et al. Common variants in CDKAL1, CDKN2A/B, IGF2BP2, SLC30A8, and HHEX/IDE genes are associated with type 2 diabetes and impaired fasting glucose in a Chinese Han population. Diabetes. 2008;57:2834–42. doi: 10.2337/db08-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waters KM, Stram DO, Hassanein MT, et al. Consistent association of type 2 diabetes risk variants found in europeans in diverse racial and ethnic groups. PLoS Genet. 2010;6:1001078. doi: 10.1371/journal.pgen.1001078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kifagi C, Makni K, Boudawara M, et al. Association of genetic variations in TCF7L2, SLC30A8, HHEX, LOC387761, and EXT2 with Type 2 diabetes mellitus in Tunisia. Genet Test Mol Biomarkers. 2011;15:399–405. doi: 10.1089/gtmb.2010.0199. [DOI] [PubMed] [Google Scholar]
- 34.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo J, Jin M, Zhang M, Chen K. A genetic variant in miR-196a2 increased digestive system cancer risks: a meta-analysis of 15 case-control studies. PLoS One. 2012;7:e30585. doi: 10.1371/journal.pone.0030585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thakkinstian A, McEvoy M, Minelli C, et al. Systematic review and meta-analysis of the association between β2-adrenoceptor polymorphisms and asthma: A HuGE review. Am J Epidemiol. 2005;162:201–11. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 37.Thakkinstian A, McElduff P, D’Este C, et al. A method for meta-analysis of molecular association studies. Stat Med. 2005;24:1291–306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA. 2006;295:676–80. doi: 10.1001/jama.295.6.676. [DOI] [PubMed] [Google Scholar]
- 40.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shan Z, Bao W, Zhang Y, et al. Interactions between zinc transporter-8 gene (SLC30A8) and plasma zinc concentrations for impaired glucose regulation and type 2 diabetes. Diabetes. 2014;63:1796–803. doi: 10.2337/db13-0606. [DOI] [PubMed] [Google Scholar]
- 42.Chen G, Xu Y, Lin Y, et al. Association study of genetic variants of 17 diabetes-related genes/loci and cardiovascular risk and diabetic nephropathy in the Chinese She population. J Diabetes. 2013;5:136–45. doi: 10.1111/1753-0407.12025. [DOI] [PubMed] [Google Scholar]
- 43.Mtiraoui N, Turki A, Nemr R, et al. Contribution of common variants of ENPP1, IGF2BP2, KCNJ11, MLXIPL, PPARgamma, SLC30A8 and TCF7L2 to the risk of type 2 diabetes in Lebanese and Tunisian Arabs. Diabetes Metab. 2012;38:444–49. doi: 10.1016/j.diabet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 44.Iwata M, Maeda S, Kamura Y, et al. Genetic risk score constructed using 14 susceptibility alleles for type 2 diabetes is associated with the early onset of diabetes and may predict the future requirement of insulin injections among Japanese individuals. Diabetes Care. 2012;35:1763–70. doi: 10.2337/dc11-2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gamboa-Melendez MA, Huerta-Chagoya A, Moreno-Macias H, et al. Contribution of common genetic variation to the risk of type 2 diabetes in the Mexican Mestizo population. Diabetes. 2012;61:3314–21. doi: 10.2337/db11-0550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutierrez-Vidal R, Rodriguez-Trejo A, Canizales-Quinteros S, et al. LOC387761 polymorphism is associated with type 2 diabetes in the Mexican population. Genet Test Mol Biomarkers. 2011;15:79–83. doi: 10.1089/gtmb.2010.0107. [DOI] [PubMed] [Google Scholar]
- 47.Xu M, Bi Y, Xu Y, et al. Combined effects of 19 common variations on type 2 diabetes in Chinese: results from two community-based studies. PLoS One. 2010;5:e14022. doi: 10.1371/journal.pone.0014022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin Y, Li P, Cai L, et al. Association study of genetic variants in eight genes/loci with type 2 diabetes in a Han Chinese population. BMC Med Genet. 2010;11:97. doi: 10.1186/1471-2350-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang Q, Yin JY, Dai XP, et al. Association analysis of SLC30A8 rs13266634 and rs16889462 polymorphisms with type 2 diabetes mellitus and repaglinide response in Chinese patients. Eur J Clin Pharmacol. 2010;66:1207–15. doi: 10.1007/s00228-010-0882-6. [DOI] [PubMed] [Google Scholar]
- 50.Han X, Luo Y, Ren Q, et al. Implication of genetic variants near SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, FTO, TCF2, KCNQ1, and WFS1 in type 2 diabetes in a Chinese population. BMC Med Genet. 2010;11:81. doi: 10.1186/1471-2350-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chauhan G, Spurgeon CJ, Tabassum R, et al. Impact of common variants of PPARG, KCNJ11, TCF7L2, SLC30A8, HHEX, CDKN2A, IGF2BP2, and CDKAL1 on the risk of type 2 diabetes in 5,164 Indians. Diabetes. 2010;59:2068–74. doi: 10.2337/db09-1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takeuchi F, Serizawa M, Yamamoto K, et al. Confirmation of multiple risk loci and genetic impacts by a genome-wide association study of type 2 diabetes in the Japanese population. Diabetes. 2009;58:1690–99. doi: 10.2337/db08-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tabara Y, Osawa H, Kawamoto R, et al. Replication study of candidate genes associated with type 2 diabetes based on genome-wide screening. Diabetes. 2009;58:493–98. doi: 10.2337/db07-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rong R, Hanson RL, Ortiz D, et al. Association analysis of variation in/near FTO, CDKAL1, SLC30A8, HHEX, EXT2, IGF2BP2, LOC387761, and CDKN2B with type 2 diabetes and related quantitative traits in Pima Indians. Diabetes. 2009;58:478–88. doi: 10.2337/db08-0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hu C, Zhang R, Wang C, et al. PPARG, KCNJ11, CDKAL1, CDKN2A-CDKN2B, IDE-KIF11-HHEX, IGF2BP2 and SLC30A8 are associated with type 2 diabetes in a Chinese population. PLoS One. 2009;4:e7643. doi: 10.1371/journal.pone.0007643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiang J, Li XY, Xu M, et al. Zinc transporter-8 gene (SLC30A8) is associated with type 2 diabetes in Chinese. J Clin Endocrinol Metab. 2008;93:4107–12. doi: 10.1210/jc.2008-0161. [DOI] [PubMed] [Google Scholar]
- 57.van Hoek M, Dehghan A, Witteman JC, et al. Predicting type 2 diabetes based on polymorphisms from genome-wide association studies: a population-based study. Diabetes. 2008;57:3122–28. doi: 10.2337/db08-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanghera DK, Ortega L, Han S, et al. Impact of nine common type 2 diabetes risk polymorphisms in Asian Indian Sikhs: PPARG2 (Pro12Ala), IGF2BP2, TCF7L2 and FTO variants confer a significant risk. BMC Med Genet. 2008;9:59. doi: 10.1186/1471-2350-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Omori S, Tanaka Y, Takahashi A, et al. Association of CDKAL1, IGF2BP2, CDKN2A/B, HHEX, SLC30A8, and KCNJ11 with susceptibility to type 2 diabetes in a Japanese population. Diabetes. 2008;57:791–95. doi: 10.2337/db07-0979. [DOI] [PubMed] [Google Scholar]
- 60.Ng MC, Park KS, Oh B, et al. Implication of genetic variants near TCF7L2, SLC30A8, HHEX, CDKAL1, CDKN2A/B, IGF2BP2, and FTO in type 2 diabetes and obesity in 6,719 Asians. Diabetes. 2008;57:2226–33. doi: 10.2337/db07-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyssenko V, Jonsson A, Almgren P, et al. Clinical risk factors, DNA variants, and the development of type 2 diabetes. N Engl J Med. 2008;359:2220–32. doi: 10.1056/NEJMoa0801869. [DOI] [PubMed] [Google Scholar]
- 62.Lewis JP, Palmer ND, Hicks PJ, et al. Association analysis in african americans of European-derived type 2 diabetes single nucleotide polymorphisms from whole-genome association studies. Diabetes. 2008;57:2220–25. doi: 10.2337/db07-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee YH, Kang ES, Kim SH, et al. Association between polymorphisms in SLC30A8, HHEX, CDKN2A/B, IGF2BP2, FTO, WFS1, CDKAL1, KCNQ1 and type 2 diabetes in the Korean population. J Hum Genet. 2008;53:991–98. doi: 10.1007/s10038-008-0341-8. [DOI] [PubMed] [Google Scholar]
- 64.Horikawa Y, Miyake K, Yasuda K, et al. Replication of genome-wide association studies of type 2 diabetes susceptibility in Japan. J Clin Endocrinol Metab. 2008;93:3136–41. doi: 10.1210/jc.2008-0452. [DOI] [PubMed] [Google Scholar]
- 65.Hertel JK, Johansson S, Raeder H, et al. Genetic analysis of recently identified type 2 diabetes loci in 1,638 unselected patients with type 2 diabetes and 1,858 control participants from a Norwegian population-based cohort (the HUNT study) Diabetologia. 2008;51:971–77. doi: 10.1007/s00125-008-0982-3. [DOI] [PubMed] [Google Scholar]
- 66.Furukawa Y, Shimada T, Furuta H, et al. Polymorphisms in the IDE-KIF11-HHEX gene locus are reproducibly associated with type 2 diabetes in a Japanese population. J Clin Endocrinol Metab. 2008;93:310–14. doi: 10.1210/jc.2007-1029. [DOI] [PubMed] [Google Scholar]
- 67.Cauchi S, Nead KT, Choquet H, et al. The genetic susceptibility to type 2 diabetes may be modulated by obesity status: implications for association studies. BMC Med Genet. 2008;9:45. doi: 10.1186/1471-2350-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cauchi S, Meyre D, Durand E, et al. Post genome-wide association studies of novel genes associated with type 2 diabetes show gene-gene interaction and high predictive value. PLoS One. 2008;3:e2031. doi: 10.1371/journal.pone.0002031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bronstein M, Pisante A, Yakir B, Darvasi A. Type 2 diabetes susceptibility loci in the Ashkenazi Jewish population. Hum Genet. 2008;124:101–4. doi: 10.1007/s00439-008-0520-x. [DOI] [PubMed] [Google Scholar]
- 70.Steinthorsdottir V, Thorleifsson G, Reynisdottir I, et al. A variant in CDKAL1 influences insulin response and risk of type 2 diabetes. Nat Genet. 2007;39:770–75. doi: 10.1038/ng2043. [DOI] [PubMed] [Google Scholar]
- 71.Horikoshi M, Hara K, Ito C, et al. Variations in the HHEX gene are associated with increased risk of type 2 diabetes in the Japanese population. Diabetologia. 2007;50:2461–66. doi: 10.1007/s00125-007-0827-5. [DOI] [PubMed] [Google Scholar]
- 72.Jing YL, Sun QM, Bi Y, et al. SLC30A8 polymorphism and type 2 diabetes risk: evidence from 27 study groups. Nutr Metab Cardiovasc Dis. 2011;21:398–405. doi: 10.1016/j.numecd.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 73.Xu K, Zha M, Wu X, et al. Association between rs13266634 C/T polymorphisms of solute carrier family 30 member 8 (SLC30A8) and type 2 diabetes, impaired glucose tolerance, type 1 diabetes – a meta-analysis. Diabetes Res Clin Pract. 2011;91:195–202. doi: 10.1016/j.diabres.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 74.Cauchi S, Del Guerra S, Choquet H, et al. Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab. 2010;100:77–82. doi: 10.1016/j.ymgme.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 75.Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine. 2014;45(2):178–89. doi: 10.1007/s12020-013-0032-x. [DOI] [PubMed] [Google Scholar]
- 76.Cousins RJ, Liuzzi JP, Lichten LA. Mammalian zinc transport, trafficking, and signals. J Biol Chem. 2006;281:24085–89. doi: 10.1074/jbc.R600011200. [DOI] [PubMed] [Google Scholar]
- 77.Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M. Overview of mammalian zinc transporters. Cell Mol Life Sci. 2004;61:49–68. doi: 10.1007/s00018-003-3148-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nicolson TJ, Bellomo EA, Wijesekara N, et al. Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes. 2009;58:2070–83. doi: 10.2337/db09-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim I, Kang ES, Yim YS, et al. A low-risk ZnT-8 allele (W325) for post-transplantation diabetes mellitus is protective against cyclosporin A-induced impairment of insulin secretion. Pharmacogenomics J. 2011;11:191–98. doi: 10.1038/tpj.2010.22. [DOI] [PubMed] [Google Scholar]
- 80.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42:105–16. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Staiger H, Machicao F, Stefan N, et al. Polymorphisms within novel risk loci for type 2 diabetes determine beta-cell function. PLoS One. 2007;2:e832. doi: 10.1371/journal.pone.0000832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kirchhoff K, Machicao F, Haupt A, et al. Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia. 2008;51:597–601. doi: 10.1007/s00125-008-0926-y. [DOI] [PubMed] [Google Scholar]
- 83.Tamaki M, Fujitani Y, Hara A, et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 2013;123:4513–24. doi: 10.1172/JCI68807. [DOI] [PMC free article] [PubMed] [Google Scholar]