Abstract
Background
We previously reported initial results of the first multi-center randomized, double blind, placebo controlled clinical trial of peanut sublingual immunotherapy (SLIT), observing a favorable safety profile associated with modest clinical and immunologic effects in the first year.
Objective
To provide long-term (3-year) clinical and immunologic outcomes for our peanut SLIT trial. Key endpoints: (1) percentage of responders at 2 years (could consume 5g of peanut powder or a 10-fold increase from baseline), 2) percentage reaching desensitization at 3 years, (3) percentage attaining sustained unresponsiveness after 3 years, (4) immunologic endpoints and (5) assessment of safety parameters.
Methods
Response to treatment was evaluated in 40 subjects aged 12-40 years by performing a 10g peanut powder oral food challenge (OFC) following 2 and 3 years of daily peanut SLIT therapy. At 3 years, SLIT was discontinued for 8 weeks followed by another 10g OFC, and an open feeding of peanut butter to assess sustained unresponsiveness.
Results
Approximately 98% of the 18,165 doses were tolerated without adverse reactions beyond the oropharynx, with no severe symptoms or uses of epinephrine. A high rate (>50%) discontinued therapy. By study end, 4/37 (10.8%) of SLIT treated participants were fully desensitized to 10g of peanut powder, and all 4 achieved sustained unresponsiveness. Responders at 2 years showed a significant decrease in peanut-specific basophil activation and skin prick test titration compared to non-responders.
Conclusions
Peanut SLIT induced a modest level of desensitization, decreased immunologic activity over 3 years in responders, and had an excellent long-term safety profile. However, most patients discontinued therapy by the end of year 3, and only 10.8% of subjects achieved sustained unresponsiveness.
Keywords: Peanut allergy, sublingual immunotherapy, desensitization, food allergy, follow-up
Peanut allergy is a leading cause of fatal food-induced anaphylaxis, affects approximately 1.4% of children and 0.6% of adults, and adversely affects quality of life.1–3 Standard clinical care for peanut allergy involves strict dietary avoidance and ready access to emergency medications.4 The onset of peanut allergy generally occurs in childhood, persists to adulthood in the vast majority of individuals, and requires life-long dietary avoidance to prevent severe allergic reactions.3,5 Although the need is great, there are presently no treatments for peanut allergy ready for broad implementation in mainstream clinical care. The risk of potentially fatal reactions coupled with the need for life-long and life-altering dietary and lifestyle modifications places significant burdens on affected individuals and their families. The development of a safe and efficacious active therapy targeting peanut allergy is a critical unmet need to mitigate the adverse medical, psychosocial and economic effects of this increasingly prevalent disorder.3
Traditional subcutaneous immunotherapy (SCIT) has proven unsafe for peanut allergy;6,7 however, mucosally targeted immunotherapeutic approaches such as oral immunotherapy (OIT) and sublingual immunotherapy (SLIT) have shown promise in Phase I and early Phase II trials.8–13 Collectively, this work has established that mucosal immunotherapy can induce desensitization (reduced reactivity while on therapy) in subsets of subjects, characterized by increases in the threshold dose required to elicit symptoms during peanut challenge and associated with changes in antigen-specific immune responses.
Although peanut OIT has shown potential as a treatment, it has been limited by heterogeneous clinical responses, high rates of adverse reactions, and potential for loss of protection with cessation of therapy.14 Attempts to balance enhanced therapeutic efficacy with reduced allergic side effects have generated increased interest in the application of potentially safer and more convenient immunotherapeutic approaches. SLIT is an appealing alternative to OIT, with some studies reporting a better safety profile and demonstrated efficacy in treatment of food allergy to multiple foods including kiwi, hazelnut, peach, milk and peanut.10,11,15–18 We previously reported initial results of the first multi-center randomized, double blind, placebo controlled clinical trial of peanut SLIT,11 observing that peanut SLIT had a favorable safety profile associated with modest clinical and immunologic effects in the first year of therapy. After 44 weeks of SLIT, 70% of treated subjects (14/20) were defined as responders (those who could consume either a cumulative dose of 5 grams of peanut powder or a 10-fold increase in the amount of peanut powder compared with baseline oral food challenge), compared with 15% (3/20) of placebo treated subjects. After 68 weeks of SLIT, the median successfully consumed dose was significantly increased compared to week 44, suggesting the possibility that longer treatment duration conferred additional benefit to treated subjects.11 However, longer-term safety and efficacy outcomes of peanut SLIT have not been reported, and these data are crucial for understanding the therapeutic potential of this approach.19 The goal of the current report is to provide long-term (3-year) clinical and immunologic outcomes for subjects undergoing a peanut SLIT trial.
METHODS
Study design
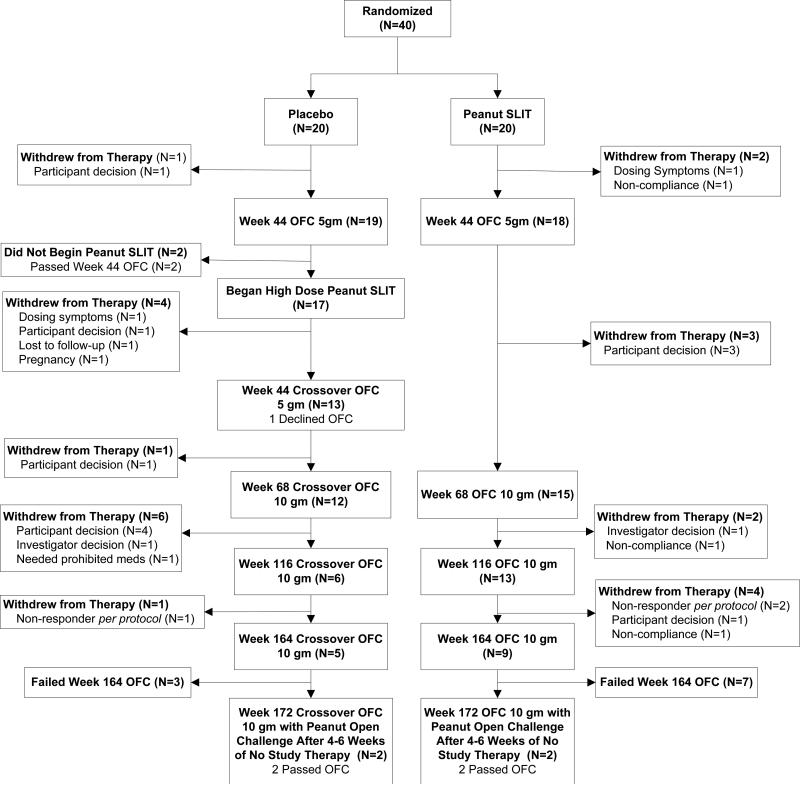
The first phase of this randomized, double-blind, placebo-controlled peanut SLIT trial was reported previously.11 In the first phase, 40 subjects were randomized 1:1 to active versus placebo SLIT with 20 subjects randomized to each group. The initial active SLIT subjects were treated through week 44 with up to 1386 μg of peanut protein SLIT daily. At week 44 the Peanut SLIT and Placebo subjects completed a 5g oral food challenge (OFC) and were unblinded, while placebo crossover subjects were escalated after unblinding at week 44 to higher dose peanut SLIT up to 3696 μg daily (designated as High Dose Crossover Group; the original Peanut SLIT group maintained a maximum dose of 1386 μg of peanut protein daily). The second, open-label phase of this study is reported here; both groups were to receive up to a total of 164 weeks (3 years) of active peanut SLIT (Figure 1).
Figure 1.
Subject Disposition
Response to treatment was evaluated by performing a 10g peanut powder (~5g peanut protein; for reference, 1 peanut has 250-280 mg of peanut protein, thus 5 grams is equivalent to 16-18 peanuts). The intent was to escalate to a dose of 1386 mcg in all subjects, but some subjects tolerated only a much smaller dose. OFC at years 2 and 3 (week 116 and 164) while on peanut SLIT daily maintenance therapy. OFCs were performed per standard protocol as previously reported and included an initial OFC while on SLIT to assess clinical desensitization.11 Subjects who passed the full 10g OFC at 3 years were discontinued from SLIT dosing for 8 weeks. The sustained unresponsiveness OFC after the 8 weeks was a combination of a 10g OFC followed by an open feeding of 2 tablespoons of peanut butter 1 hour later. Treatment responders were defined as the following: 1) Two-year Responder: subjects who either successfully consumed a cumulative dose of 5g of peanut powder (~2.5g peanut protein) while on peanut SLIT dosing or experienced at least a 10-fold increase in the amount of peanut powder compared to baseline OFC without dose-limiting symptoms; 2) Three-year Desensitization Responder: subjects who successfully consumed a cumulative dose of 10g of peanut powder (~5g peanut protein) without dose-limiting symptoms while on peanut SLIT dosing; and 3) Three-year Sustained Unresponsiveness Responder: subjects who successfully consumed a cumulative dose of 10g peanut powder (~5g peanut protein) plus an open feeding of peanut protein without dose-limiting symptoms (8 weeks after discontinuation of peanut SLIT dosing). If a desensitization response was not attained by year 2, as defined above, dosing was discontinued. Subjects were scheduled for a three-year evaluation whether on SLIT dosing or not, with those discontinuing from dosing followed for mechanistic studies only. Key endpoints for SLIT treatment and for comparison between standard SLIT and higher dose SLIT included the following: (1) the percentage of subjects who were responders at year 2 (could consume 5g of peanut powder or at least a 10-fold increase from baseline during an oral food challenge), (2) the percentage of subjects reaching desensitization at each time point, (3) the percentage of subjects attaining sustained unresponsiveness by year 3, (4) immunologic endpoints, including changes in peanut IgE, IgG4, endpoint titration skin prick test (SPT) and basophil activation, and (5) assessment of safety parameters including adverse events, serious adverse events in response to peanut SLIT and long-term tolerability.
Study population
Subject recruitment, including inclusion and exclusion criteria, was previously described and included 40 subjects, ages 12-40 years, from 5 US sites (New York, NY, Baltimore, MD, Little Rock, AR, Denver, CO, Durham, NC; the North Carolina subjects moved with the investigative team from Duke to the University of North Carolina-Chapel Hill in March 2012).11 The study was conducted with investigational new drug approval from the US Food and Drug Administration. The National Institute of Allergy and Infectious Diseases Allergy and Asthma Data and Safety Monitoring Board and local Institutional Review Boards approved study procedures, and written informed consents were obtained.
Study protocol
Subjects were instructed to remain on a peanut-free diet throughout the entire study and were required to carry an epinephrine auto-injector. Solicited dosing symptoms were recorded on a daily basis using a home diary. Other unsolicited adverse events were separately recorded. Study drug was administered sublingually, held for 2 minutes, and then swallowed.
Maintenance SLIT dosing
A standard peanut SLIT solution was manufactured and administered to all subjects (see Online Repository), as previously described.11 For subjects initially treated with active peanut SLIT, maintenance dosing continued at a minimum dose of 165 μg and a maximum maintenance dose of 1386 μg of peanut protein through the end of study. For placebo crossover subjects on active peanut SLIT, maintenance dosing continued at a minimum of 165 μg and a maximum maintenance dose of 3696 μg through the end of study.
Oral food challenge
A 10g OFC with peanut powder (~5g peanut protein) was conducted at years 2 and 3 of maintenance peanut SLIT dosing per protocol (see Online Repository for full OFC methods). Subjects who passed the year 3 OFC assessing desensitization discontinued peanut SLIT therapy for 8 weeks and completed a sustained responsiveness OFC with a combination of a 10g OFC and an open feeding of 2 tablespoons peanut butter. Subjects who passed the final year 3 OFC assessing sustained unresponsiveness were instructed to add peanut to their diet, while those that failed either the year 3 OFC assessing desensitization or the year 3 OFC assessing sustained unresponsiveness were provided dietary guidance based on their OFC outcome, with most participants resuming strict avoidance but others introducing peanut to the diet in amounts specified by the site investigator.
Adverse events
Mild, moderate and severe adverse events were defined using standard adverse event reporting criteria. Severity of adverse events was determined via site reporting with serious adverse events reviewed by a SACCC medical monitor. For dosing and OFC symptoms, the site reported a severity associated with the symptoms. Severity was determined based on type of reaction e.g. a mild skin reaction could be 1-2 hives, a moderate reaction could be a few hives, and a severe reaction could be extensive hives and swelling. Sites were provided with guidance on how to assess severity based on standard CoFAR case report forms and a Manual of Procedures.
Endpoint titration skin prick testing
Endpoint titration SPTs were performed with serial ten-fold dilutions of peanut extract at baseline and annually, as previously reported.11
Immunologic studies
Basophil activation
Basophil activation as measured by CD63 up-regulation was evaluated by flow cytometry at baseline, week 29, week 44 and annually at the time of OFC, as previously described.11
Immunoglobulins
Total IgE was measured by immunoassay, and peanut-specific-IgE (PN-IgE) and peanut-specific IgG4 (PN-IgG4) were measured using the ImmunoCAP 100 (Thermo Fisher Scientific, Waltham, MA) at baseline and at weeks 29, 44, 68 and annually at the time of OFC, as previously reported.11
Statistical analysis
High Dose Crossover and Peanut SLIT groups as well as responder versus non-responder were compared using Fisher's Exact test for categorical variables and the Wilcoxon Rank Sum test for continuous variables. Repeated measures models were fit using unstructured covariance to evaluate basophil activation, immunoglobulin levels, and peanut endpoint titration area under the curve. As subjects who were non-responders were intentionally discontinued from dosing at year 2 per protocol, models were limited to data through year 2. Covariates included study visit, baseline values, and year 2 response. Interactions were evaluated and included if statistically significant. All analyses were performed with the use of SAS software, version 9.2 (SAS Institute).
RESULTS
As reported in the manuscript by Fleischer et al.,11 a total of 40 subjects were initially randomized with 20 receiving low dose peanut SLIT and 20 receiving placebo. Among the 20 subjects who received Peanut SLIT, 14 (70%) were defined as a responder by week 44, compared to only 3 (15%) on the placebo arm (P = 0.001). From the placebo group, 17 subjects crossed over to the High Dose Crossover arm and 7/16 (44%) were categorized as responders at the week 44 crossover OFC (one subject declined the week 44 crossover OFC, and 4 discontinued dosing before the OFC and were counted as non-responders per the protocol). There were no statistical differences in baseline characteristics between treatment groups (Table 1).
Table 1.
Baseline Characteristics
| Treatment | ||
|---|---|---|
| High Dose Crossover (n = 17 [%]) | Peanut SLIT (n = 20 [%]) | |
| Male sex | 64.7 | 65.0 |
| Additional food allergy | 64.7 | 85.0 |
| Physician's diagnosis of asthma | 58.8 | 55.0 |
| Allergic rhinitis | 70.6 | 70.0 |
| Median (Q1–Q3) | Median (Q1–Q3) | |
|---|---|---|
| Age (y) | 16.0 (14.0–18.0) | 14.0 (13.0–18.0) |
| Baseline SPT peanut score (mm) | 12.0 (10.0–14.8) | 13.3 (9.5–17.5) |
| Baseline peanut IgE (kUA/L) | 30.4 (7.1–91.1) | 31.3 (3.2–42.4) |
| Baseline OFC dose at first symptom (mg) | 6.0 (1.0–71.0) | 6.0 (1.0–46.0) |
| Baseline OFC successfully consumed dose (mg) | 71.0 (6.0–146.0) | 21.0 (1.0–146.0) |
Subject disposition over the course of the study is represented in Figure 1, including the reasons for subject withdrawals and the final subject status at the final OFC at year 3. In the High Dose Crossover group, 12/17 withdrew prior to the year 3 OFC, 2/5 passed the year 3 OFC, and both of those subjects passed the year 3 sustained unresponsiveness OFC after being off treatment for 8 weeks. In the initial active Peanut SLIT group, 11/20 withdrew prior to the year 3 OFC, and 2/9 passed the year 3 OFC, both of whom passed the year 3 sustained unresponsiveness OFC. Using the definitions provided above, 4/17 (23.5%) in the High Dose Crossover group versus 11/20 (55%) in the Peanut SLIT group were categorized as responders at year 2 (P = 0.09), while 2/17 (11.8%) in the High Dose Crossover and 2/20 (10%) in the Peanut SLIT groups were categorized both as desensitized at year 3 and having sustained unresponsiveness at year 3.
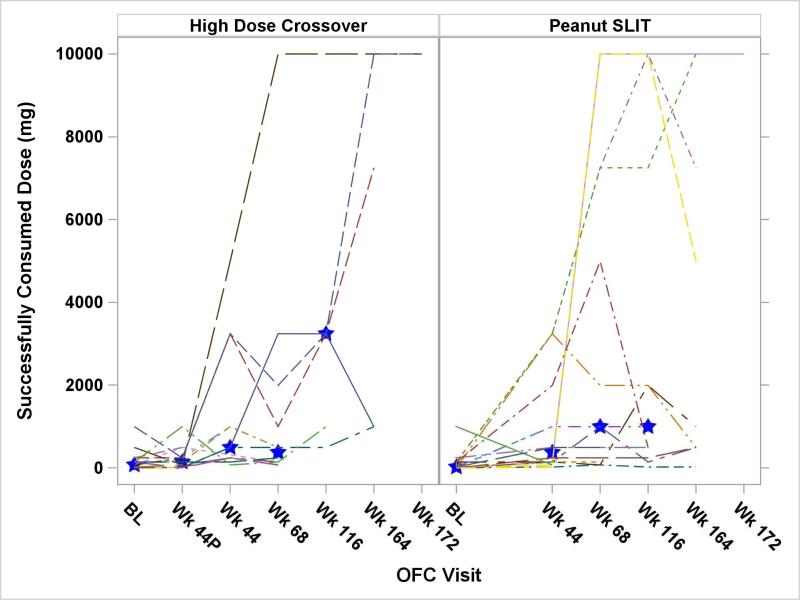
A comparison of OFC results between the High Dose Crossover and Peanut SLIT groups is presented in Figure 2. This figure shows the median successfully consumed dose between these groups only to the year 2 OFC, because non-responders at year 2 were subsequently withdrawn from dosing, per protocol. There were no significant differences in successfully consumed dose at any challenge time point between the 2 treatment groups. The impact of dosing beyond year 2 could not be determined because of subject withdrawal and per-protocol discontinuation of dosing for those not responding by year 2. Table 2 displays the details of the OFCs, divided by the treatment group, as well as the year 2 response. The median time on dosing through year 2 was 771 days for the High Dose Crossover subjects and 825 days for the Peanut SLIT subjects. Of note, there are 3 fewer year 2 responders in both treatment groups compared to the 44 week OFC because these subjects withdrew prior to the year 2 OFC.
Figure 2.
Oral Food Challenge Results
Note: Subjects were discontinued from treatment per protocol if they did not meet specific criteria at the year 2 OFC (OFC threshold at least 5000 mg or 10 times baseline). Therefore, the median values at year 3 are not presented. Blue stars indicate the group median at each time point. There was no statistically significant difference in the medians between the treatment groups.
Table 2.
Successfully Consumed Dose by Year 2 Response and Treatment Group
| Treatment Group | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Placebo/High Dose Crossover | Peanut SLIT | ||||||||||||||
| n | Mean | Min | Q1 | Median | Q3 | Max | n | Mean | Min | Q1 | Median | Q3 | Max | ||
| OFC Type | 2 Year Responder | 4 | 186 | 1.0 | 1.0 | 123.5 | 371.0 | 496 | 11 | 44.1 | 0.0 | 1.0 | 1.0 | 146 | 146 |
| Baseline (2g) | Yes | ||||||||||||||
| No | 13 | 159.1 | 1.0 | 21.0 | 71.0 | 146.0 | 996 | 9 | 297.6 | 0.0 | 21.0 | 146 | 246 | 996 | |
| All | 17 | 165.4 | 1.0 | 6.0 | 71.0 | 146.0 | 996 | 20 | 158.2 | 0.0 | 1.0 | 21.0 | 146 | 996 | |
| Initial Week 44 (5g) | 2 Year Responder | 4 | 82.3 | 6.0 | 6.0 | 38.5 | 158.5 | 246 | 11 | 1091.5 | 21.0 | 146 | 246 | 3246 | 3246 |
| Yes | |||||||||||||||
| No | 13 | 240.2 | 1.0 | 21.0 | 146 | 246 | 996 | 7 | 603.1 | 21.0 | 71 | 496 | 996 | 1996 | |
| All | 17 | 203.1 | 1.0 | 6.0 | 146 | 246 | 996 | 18 | 901.6 | 21.0 | 146 | 371 | 996 | 3246 | |
| Crossover Week 44 (5g) | 2 Year Responder | 4 | 2308.5 | 496 | 496 | 1871 | 4121 | 4996 | . | . | . | . | . | . | . |
| Yes | |||||||||||||||
| No | 8 | 774.1 | 71 | 196 | 246 | 996 | 3246 | . | . | . | . | . | . | . | |
| All | 12 | 1285.6 | 71 | 246 | 496 | 2121 | 4996 | . | . | . | . | . | . | . | |
| Crossover Week 68/Week 68 (10g) | 2 Year Responder | 4 | 3683.5 | 496 | 746 | 2121.0 | 6621 | 10000 | 11 | 3578.5 | 71 | 246 | 996 | 7246 | 10000 |
| Yes | |||||||||||||||
| No | 8 | 414.8 | 71 | 108.5 | 146.0 | 371 | 1996 | 4 | 1533.5 | 146 | 321 | 496 | 2746 | 4996 | |
| All | 12 | 1504.3 | 71 | 146 | 371.0 | 1496 | 10000 | 15 | 3033.2 | 71 | 246 | 996 | 7246 | 10000 | |
| Crossover Year 2/Year 2 (10g) | 2 Year Responder | 4 | 4246 | 496 | 1871 | 3246 | 6621 | 10000 | 11 | 3922.1 | 21.0 | 246 | 1996 | 10000 | 10000 |
| Yes | |||||||||||||||
| No | 2 | 2121 | 996 | 996 | 2121 | 3246 | 3246 | 2 | 496 | 496 | 496 | 496 | 496 | 496 | |
| All | 6 | 3537.7 | 496 | 996 | 3246 | 3246 | 10000 | 13 | 3395 | 21.0 | 496 | 996 | 7246 | 10000 | |
| Crossover Year 3/Year 3 (10g) | 2 Year Responder | 4 | 4808.5 | 996 | 996 | 4121 | 8621 | 10000 | 9 | 3860.3 | 21.0 | 496 | 996 | 7246 | 10000 |
| Yes | |||||||||||||||
| No | 1 | 10000 | 10000 | 10000 | 10000 | 10000 | 10000 | . | . | . | . | . | . | . | |
| All | 5 | 5846.8 | 996 | 996 | 7246 | 10000 | 10000 | 9 | 3860.3 | 21.0 | 496 | 996 | 7246 | 10000 | |
| Crossover Year 3/Year 3 (10g) | 2 Year Responder | 1 | 10000 | 10000 | 10000 | 10000 | 10000 | 10000 | 2 | 10000 | 10000 | 10000 | 10000 | 10000 | 10000 |
| Yes | |||||||||||||||
| No | 1 | 10000. | 10000 | 10000 | 10000 | 10000 | 10000 | . | . | . | . | . | . | . | |
| All | 2 | 10000 | 10000 | 10000 | 10000 | 10000 | 10000 | 2 | 10000 | 10000 | 10000 | 10000 | 10000 | 10000 | |
As noted, there was a high rate of subject withdrawal from this protocol. One subject withdrew while on placebo and, of the 17 subjects who crossed over to the high dose group, one withdrew due to dosing symptoms, 6 withdrew due to participant decision, one was withdrawn per protocol as a non-responder, and the remaining 4 withdrew for other miscellaneous reasons (lost to follow-up, investigator decision, need for a prohibited medication, pregnancy). From the original Peanut SLIT group, 4 withdrew due to participant decision, 3 due to non-compliance, 2 were withdrawn as non-responders, 1 withdrew due to dosing symptoms, and 1 was withdrawn due to investigator decision. With regard to the participants who chose to withdraw for reasons other than dosing symptoms or non-compliance, most felt that the daily dosing was too difficult to maintain.
Dose-related adverse reactions after the OFC at 44 weeks on active therapy for High Dose Crossover subjects and after the week 44 OFC for Peanut SLIT subjects are summarized in Table 3. Overall, dose-related symptoms were reported in 18.3% of doses in the High Dose Crossover subjects following 44 weeks of active therapy and 18.1% doses received by Peanut SLIT subjects following 44 weeks of active therapy. The vast majority of reactions were isolated oropharyngeal symptoms; 1 Peanut SLIT subject had a moderate dosing symptom of throat tightness without hoarseness. No subjects had severe dosing related symptoms and no dosing related reaction required treatment with epinephrine. Adverse events were reported separately from dosing reactions. In the period following 44 weeks of active therapy, there were 112 adverse events from 12 High Dose Crossover subjects reported; 6 were of moderate severity, none were severe, and all were unrelated to study product. During this same period, there were 83 adverse events from 13 Peanut SLIT subjects reported; 14 were of moderate severity, 1 was a life-threatening anaphylactic reaction to the year 3 OFC. The only adverse event definitely related to study product was a mild contact reaction to the study product.
Table 3.
Post-Week 44 Crossover OFC/Week 44 OFC Dosing Symptom Summary by Dose
| High Dose Crossover Subjects | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Symptom | Any Symptom Excluding Oral Phary | Oral Phary Symptoms | Skin | Resp. | GI | Other | Treated | Treated with Epi. | Mild | Moderate | Severe | ||||||||||||||
| Visit Type | # Doses | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| All | 7385 | 1352 | 18.3 | 301 | 4.1 | 1326 | 18.0 | 17 | 0.2 | 262 | 3.5 | 16 | 0.2 | 13 | 0.2 | 26 | 0.4 | 0 | 0.0 | 301 | 4.1 | 0 | 0.0 | 0 | 0.0 |
| Clinic | 12 | 9 | 75.0 | 3 | 25.0 | 9 | 75.0 | 0 | 0.0 | 3 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 25.0 | 0 | 0.0 | 0 | 0.0 |
| Home | 7373 | 1343 | 18.2 | 298 | 4.0 | 1317 | 17.9 | 17 | 0.2 | 259 | 3.5 | 16 | 0.2 | 13 | 0.2 | 26 | 0.4 | 0 | 0.0 | 298 | 4.0 | 0 | 0.0 | 0 | 0.0 |
| Peanut SLIT Subjects | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Any Symptom | Any Symptom Excluding Oral Phary | Oral Phary Symptoms | Skin | Resp. | GI | Other | Treated | Treated with Epi. | Mild | Moderate | Severe | ||||||||||||||
| Visit Type | # Doses | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % |
| All | 10780 | 1950 | 18.1 | 75 | 0.7 | 1912 | 17.7 | 8 | 0.1 | 60 | 0.6 | 7 | 0.1 | 2 | 0.0 | 5 | 0.0 | 0 | 0.0 | 74 | 0.7 | 1 | 0.0 | 0 | 0.0 |
| Escalation | 5 | 5 | 100.0 | 4 | 80.0 | 4 | 80.0 | 0 | 0.0 | 4 | 80.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 4 | 80.0 | 0 | 0.0 | 0 | 0.0 |
| Clinic | 55 | 26 | 47.3 | 1 | 1.8 | 25 | 45.5 | 0 | 0.0 | 1 | 1.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.8 | 0 | 0.0 | 0 | 0.0 |
| Home | 10720 | 1919 | 17.9 | 70 | 0.7 | 1883 | 17.6 | 8 | 0.1 | 55 | 0.5 | 7 | 0.1 | 2 | 0.0 | 5 | 0.0 | 0 | 0.0 | 69 | 0.6 | 1 | 0.0 | 0 | 0.0 |
Mechanistic Results
Immunologic changes
In the repeated measures analysis described in the methods, there was no significant difference between treatment groups over time in immunoglobulin levels, basophil activation, or peanut titrated SPTs. We focused our analysis on differences between subjects who were responders and those who were non-responders at 2 years. For the subjects who were treated with placebo during the first year and then given a high dose of peanut SLIT (High Dose Crossover group), immunoglobulin and basophil baseline values for the analyses were from the time point just prior to crossing over.
Immunoglobulins
Total IgE, PN-IgE levels, and PN-IgG4 were not statistically different for those categorized as year 2 responders versus those who were not. However, median PN-IgG4 levels were observed to be slightly higher for year 2 responders (Online Repository, Figure E1).
Basophil activation
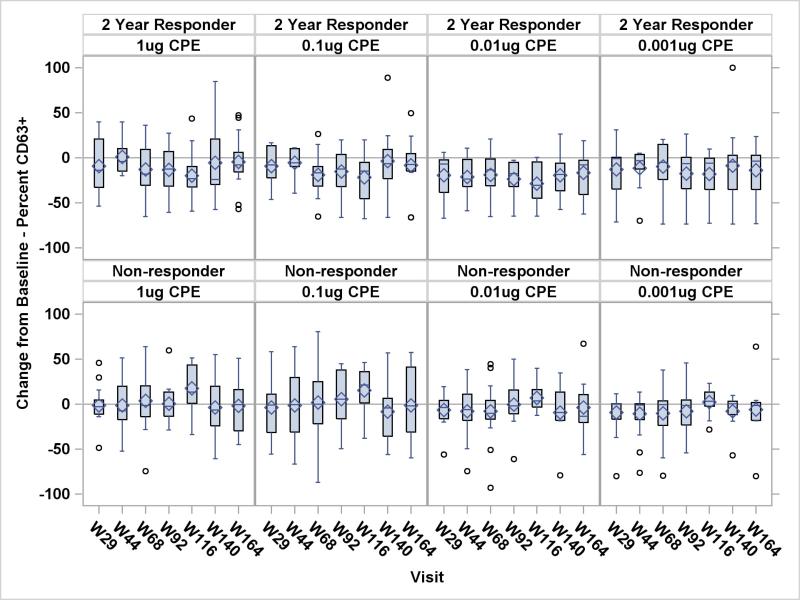
Based on results from the repeated measures analyses of percent CD63 positivity (CD63+) basophils for the 4 different peanut stimulant levels, the 2 year responders had significantly lower percent CD63+ basophils than non-responders for the 0.1 μg (P = 0.02), 0.01 μg (P = 0.002), and 0.001 μg (P = 0.03) peanut stimulant levels. There was a significant interaction between study visit and 2 year responder status at the 0.01 μg (P = 0.009), and 0.001 μg (P = 0.03) peanut stimulant levels, indicating that the magnitude of the effect is not constant over time. The change from baseline of percent CD63+ was observed to be lower for the 2 year responders at all peanut stimulant levels at almost every visit (Figure 3). Note in Figure 3 that the median percent CD63+ basophil is predominantly below zero for the 2 year responders but not for the non-responders.
Figure 3.
Change from Baseline in Percent CD63+ Basophils by 2-Year Response – All Subjects
Peanut Skin Prick Test Endpoint Titration
Peanut SPT endpoint titration was performed at baseline and at around 1, 2 and 3 years of therapy. In a repeated measures analysis of the peanut endpoint titration area under the curve through year 2, there was a significantly greater decrease over time in area under the curve in 2 year responders versus non-responders (P = 0.003; Online Repository, Figure E2).
DISCUSSION
This study presents unique data on long term open-label follow-up from the first multi-center, randomized, placebo-controlled trial of peanut SLIT.11 Briefly, our previous study showed that peanut SLIT was generally safe and induced a modest level of desensitization in the majority of treated subjects compared to placebo. The results presented here extend these observations beyond our previous report of week 68 data from the lower dose (1386 μg peanut protein/day) Peanut SLIT treated arm of the randomized study and week 44 results from the High Dose Crossover (3696 μg peanut protein/day) participants. The current study includes up to 3 years of therapy with a daily maintenance dose of 1386 μg peanut protein in persons originally randomized to active treatment and a dose of 3696 μg peanut protein in the group crossing over to active treatment from the placebo group. This longer-term study includes assessment of sustained unresponsiveness (after 8 weeks off of peanut SLIT) at year 3 for participants showing desensitization to 10g peanut powder (~5g peanut protein). By study end, 4 participants were fully desensitized to 10g of peanut powder, one of whom was not considered a responder at year 2 and all 4 showed sustained unresponsiveness. Overall, we report here 4 important new findings in the novel context of long term treatment with peanut SLIT: 1) Differences in outcomes using 1386 or 3696 μg of daily peanut protein were not observed but conclusions are limited due to the high drop-out rate; 2) Peanut SLIT induced a modest level of desensitization, but only a few achieved sustained unresponsiveness; 3) A high rate of participants discontinued therapy; and 4) Peanut SLIT has a favorable long-term safety profile. Additionally, we observed immunologic responses to therapy correlating with clinical outcomes.
Of the above findings, two key observations were the low rate of significant adverse reactions to dosing and, despite this, a high rate of participant withdrawal. Regarding safety, the previously reported first 44 weeks of treatment included 10,855 doses where 95.2% were symptom free-excluding oropharyngeal symptoms. During the initial 44 weeks of therapy, one participant had experienced a dose-related serious adverse event. This follow-up after 44 weeks of therapy included 18,165 additional doses with > 97.9% of doses without reactions beyond the oropharynx, and no severe symptoms or use of epinephrine. Despite this safety profile,20,21 participant withdrawal was high, and evenly distributed between the 2 phases of the study. In the first phase of the study through 44 weeks of active therapy, 2 participants withdrew because of dosing symptoms, and during long-term follow-up none did. Therefore, dosing side effects after a year of therapy do not appear to be a cause for withdrawal. However, withdrawal was common for “participant decision” (n = 11) or non-adherence (n = 3). Although motivation for discontinuation was not formally assessed, the difficulty of maintaining daily therapies, mild oral discomfort (17.8% of doses), and a lack of robust responses as measured during OFCs are likely causes (i.e., subjects still reacting at follow-up OFCs may have been discouraged by the absence of more significant protection). Additionally, the participants were adolescents and adults where lifestyle issues may be a concern, in contrast to longer-term studies of food immunotherapy with young children where parental oversight may maintain adherence.22 The high rate of discontinuation in this study was still not as high as that seen in clinical treatment for environmental allergies. In a review of 6486 patients starting SLIT or subcutaneous immunotherapy (SCIT) for environmental allergens in the Netherlands, only 18% of users reached the minimally required duration of treatment of 3 years (SCIT, 23%; SLIT, 7%); for those on SLIT, 62% discontinued by 1 year and 93% by 3 years.23 Clearly, more studies will be needed to evaluate the practical application of SLIT and other proposed daily immunotherapies to address safety and adherence.
We previously noted improved desensitization with longer duration of therapy from 44 to 68 weeks of treatment with peanut SLIT.11 Unfortunately in this follow-up study, it is not possible to conclude whether longer treatment, beyond 68 weeks, resulted in improved desensitization due to participant drop out and elimination from dosing per protocol when participants did not meet the definition of a responder at year 2. However, there were sufficient participant data to address 2-year outcomes, comparing responders to non-responders for mechanistic studies. Among the antibody tests, only median peanut-specific IgG4 levels were observed to be slightly higher among the year 2 responders, but the repeated measures analysis did not find a statistically significant difference. This marker of successful desensitization has been noted in prior immunotherapy studies,9,22,24 with a more robust response observed in OIT compared to SLIT.12 Similarly, our year 2 responders showed a stronger reduction in basophil activation than nonresponders, an effect that is an extension of our initial observation where basophil activation was suppressed in treated compared to untreated participants.11 The change from baseline for the area under the SPT end point titration curve was improved in responders compared to non-responders to year 2, an extension of our prior observation on this difference from week 44. These markers confirm the immune activity associated with clinical outcomes for long term SLIT.
It is notable that treatment with very low doses of antigen, on the order of 1-4 μg peanut protein compared to the gram quantities used in OIT, is associated with median increases in desensitization of >1 gm at year 2, and with evidence of immune changes. The magnitude of desensitization in this study is similar to that reported in a similar study in younger children.10 While OIT may induce far greater degrees of desensitization, it still may be reasonable to pursue interventions with low rates of risk that provide some measure of protection from accidental exposure. Thus, the results here underscore the notion that low dose peanut SLIT, requiring approximately 1386 μg, and with a favorable safety profile, could result in useful rates of desensitization, and, for a few individuals, large improvements with sustained unresponsiveness. In addition, SLIT may also represent a safe means to progress toward OIT in highly sensitive patients, and/or may be a particularly advantageous approach to combining a type of oral mucosal immunotherapy with adjuvants.
The limitations of the current study include the definition of a responder, which might have over represented relative success, exclusion of patients with a past history of life-threatening peanut allergic reactions who may benefit from such therapies and respond differently to them, the high rate of dropouts in the study, and the lack of a placebo control for final endpoint assessments due to the crossover design.
Overall, these results suggest that SLIT is safe and can result in modest desensitization at low doses. However, the response is overall less robust than with OIT, and there may be a high likelihood of patient discontinuation. Future studies may focus on understanding patient motivation, addressing patient expectations of this therapy, and investigating alternative schedules to improve adherence, using SLIT as a gateway toward transitioning to additional therapies such as OIT,25 or attempting to augment responses by the use of adjuvants.26
Supplementary Material
Key Messages.
In our multicenter trial, Peanut SLIT had an excellent long-term safety profile
Peanut SLIT induced a modest level of desensitization, with <15% achieving sustained unresponsiveness. Responders at 2 years showed a significant decrease in peanut-specific basophil activation and skin prick test titration compared to non-responders.
A high rate of participants discontinued therapy, a finding similar to other SLIT trials. Many had difficulty maintaining daily dosing as the primary reason.
Capsule Summary.
Long-term follow-up (3-year) of the first multi-center randomized, double blind, placebo controlled clinical trial of peanut sublingual immunotherapy (SLIT) demonstrated a favorable safety profile associated with modest clinical and immunologic effects.
Acknowledgments
Supported by National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) grant U19AI066738 and U01AI066560. The project was also supported by grant no. UL1 RR025780 from the National Center for Research Resources (NCRR)/National Institutes of Health (NIH) and grant nos. UL1 TR000154 from the NIH/National Center for Advancing Translational Sciences (National Jewish) and grant nos. UL1 TR000067 (Mount Sinai), UL1 TR000039 (Arkansas), UL 1 RR024128 (North Carolina), and UL1 RR 025005 (Johns Hopkins) from the NCRR. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
Abbreviations used
- %CD63
Percentage of CD63 positivity
- DBPCFC
Double-blind, placebo-controlled food challenge
- OFC
Oral food challenge
- OIT
Oral immunotherapy
- PN-IgE
Peanut-specific IgE
- PN-IgG4
Peanut-specific IgG4
- SCD
Successfully consumed dose
- SLIT
Sublingual immunotherapy
- SPT
Skin prick test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of potential conflict of interest: A.W. Burks has board memberships with the American Academy of Allergy, Asthma & Immunology, the NIH Hypersensitivity, Autoimmune, and Immune-mediated Diseases study section, the US Food and Drug Administration, and the Journal of Allergy and Clinical Immunology; is on advisory boards for the Food Allergy & Anaphylaxis Network, ActoGeniX, and Exploramed Development; has consultant arrangements with Merck, Novartis Pharma AG, the Dannon Company, McNeill Nutritionals, and Schering-Plough; is employed by UNC Children's Hospital and Duke University; has received grants from the NIH; has grants pending from the Department of Defense and the Wallace Research Foundation; receives payment for lectures from Myland Specialty; receives royalties from UpToDate; receives payment for development of educational presentations from Current Views; has stock/stock options with Allertein, Mastcell Pharmaceuticals, and Dow AgroSciences; and has received travel expenses from the European Academy of Allergy and Clinical Immunology. D. M. Fleischer has received grants from the National Institutes of Health (NIH)/National Institutes of Allergy and Infectious Diseases (NIAID), has consultant arrangements with LabCorp, has received payment for lectures from Nestle Nutrition Institute, is employed by University of Colorado and Children's Hospital Colorado, is on the Medical Advisory Board for the Food Allergy & Anaphylaxis Connection Team, and receives royalties from UpToDate. A. M. Scurlock has received grants from the NIH. R. A. Wood has consultant arrangements with the Asthma and Allergy Foundation of America, is employed by Johns Hopkins University, has provided expert testimony for the NIH, and received royalties from UpToDate. S. M. Jones has received grants from the NIH, Food Allergy Educational and Research, and the National Peanut Board; has received clinical trials funding from DBV Technologies, Astra Zeneca, Inc., Kedrion, Inc., Nutricia, Inc., has board memberships with the Food Allergy Research and Education organization and the Journal of Allergy and Clinical Immunology; has received payment for educational lectures from the Greater Kentucky Allergy Society, Mercy Children's Hospital, Southwestern Medical School, and travel funds from the European Academy of Allergy and Clinical Immunology; and serves on the National Institute of Allergy and Infectious Disease Safety Monitoring Committee, and the Arkansas Medicaid Drug Review Committee. S. H. Sicherer has received grants from the NIH/NIAID, has consultant arrangements with the Food Allergy Initiative, and receives royalties from UpToDate. B. P. Vickery has received grants and travel support from the NIH/NIAID and has received grants from the American Lung Association, Cephalon, the Foundation of the American College of Allergy, Asthma&Immunology, and the Thrasher Research Fund. A. K. Henning has received grants from the NIH. R. Lindblad has received grants from the NIH/NIAID. H. A. Sampson has received grants and travel support from the NIAID, has received grants from the NIH, is on the Danone Scientific Advisory Board, has consultant arrangements with Allertein Therapeutics and the Food Allergy Initiative, is employed by Mount Sinai Medical School, and has received royalties from Elsevier-Wiley and UpToDate. The rest of the authors declare that they have no relevant conflicts of interest.
Trial registration: This study is registered with ClinicalTrials.gov with ID NCT00580606.
REFERENCES
- 1.Bock SA, Muñoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–3. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 2.Bock SA, Muñoz-Furlong A, Sampson HA. Further fatalities caused by anaphylactic reactions to food, 2001-2006. J Allergy Clin Immunol. 2007;119:1016–8. doi: 10.1016/j.jaci.2006.12.622. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Muñoz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, and sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–6. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 4.Boyce JA, Assa'a A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. Nutrition. 2011;27:253–67. doi: 10.1016/j.nut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Fleischer DM, Conover-Walker MK, Christie L, Burks AW, Wood RA. The natural progression of peanut allergy: Resolution and the possibility of recurrence. J Allergy Clin Immunol. 2003;112:183–9. doi: 10.1067/mai.2003.1517. [DOI] [PubMed] [Google Scholar]
- 6.Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. J Allergy Clin Immunol. 1997;99:744–51. doi: 10.1016/s0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 7.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. J Allergy Clin Immunol. 1992;90:256–62. doi: 10.1016/0091-6749(92)90080-l. [DOI] [PubMed] [Google Scholar]
- 8.Jones SM, Pons L, Roberts JL, Scurlock AM, Perry TT, Kulis M, et al. Clinical efficacy and immune regulation with peanut oral immunotherapy. J Allergy Clin Immunol. 2009;124:292–300. 300, e1–97. doi: 10.1016/j.jaci.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varshney P, Jones SM, Scurlock AM, Perry TT, Kemper A, Steele P, et al. A randomized controlled study of peanut oral immunotherapy: clinical desensitization and modulation of the allergic response. J Allergy Clin Immunol. 2011;127:654–60. doi: 10.1016/j.jaci.2010.12.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim EH, Bird JA, Kulis M, Laubach S, Pons L, Shreffler W, et al. Sublingual immunotherapy for peanut allergy: clinical and immunologic evidence of desensitization. J Allergy Clin Immunol. 2011;127:640–646. e1. doi: 10.1016/j.jaci.2010.12.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleischer DM, Burks AW, Vickery BP, Scurlock AM, Wood RA, Jones SM, et al. Sublingual immunotherapy for peanut allergy: a randomized, double-blind, placebo-controlled multicenter trial. J Allergy Clin Immunol. 2013;131:119–127. e1–7. doi: 10.1016/j.jaci.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chin SJ, Vickery BP, Kulis MD, Kim EH, Varshney P, Steele P, et al. Sublingual versus oral immunotherapy for peanut-allergic children: a retrospective comparison. J Allergy Clin Immunol. 2013;132:476–478. e2. doi: 10.1016/j.jaci.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anagnostou K, Islam S, King Y, Foley L, Pasea L, Bond S, et al. Assessing the efficacy of oral immunotherapy for the desensitisation of peanut allergy in children (STOP II): a phase 2 randomised controlled trial. Lancet. 2014;383:1297–304. doi: 10.1016/S0140-6736(13)62301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sampson HA. Peanut oral immunotherapy: is it ready for clinical practice? J Allergy Clin Immunol Pract. 2013;1:15–21. doi: 10.1016/j.jaip.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Kerzl R, Simonowa A, Ring J, Ollert M, Mempel M. Life-threatening anaphylaxis to kiwi fruit: protective sublingual allergen immunotherapy effect persists even after discontinuation. J Allergy Clin Immunol. 2007;119:507–8. doi: 10.1016/j.jaci.2006.09.041. [DOI] [PubMed] [Google Scholar]
- 16.Mempel M, Rakoski J, Ring J, Ollert M. Severe anaphylaxis to kiwi fruit: Immunologic changes related to successful sublingual allergen immunotherapy. J Allergy Clin Immunol. 2003;111:1406–9. doi: 10.1067/mai.2003.1497. [DOI] [PubMed] [Google Scholar]
- 17.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–455. 455, e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Enrique E, Pineda F, Malek T, Bartra J, Basagaña M, Tella R, et al. Sublingual immunotherapy for hazelnut food allergy: a randomized, double-blind, placebo-controlled study with a standardized hazelnut extract. J Allergy Clin Immunol. 2005;116:1073–9. doi: 10.1016/j.jaci.2005.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Greenhawt MJ. Oral and sublingual peanut immunotherapy is not ready for general use. Allergy Asthma Proc. 2013;34:197–204. doi: 10.2500/aap.2013.34.3661. [DOI] [PubMed] [Google Scholar]
- 20.Pleskovic N, Bartholow A, Skoner DP. Sublingual immunotherapy in children: the recent experiences. Curr Opin Allergy Clin Immunol. 2014 doi: 10.1097/ACI.0000000000000112. [DOI] [PubMed] [Google Scholar]
- 21.Sun J, Hui X, Ying W, Liu D, Wang X. Efficacy of allergen-specific immunotherapy for peanut allergy: a meta-analysis of randomized controlled trials. Allergy Asthma Proc. 2014;35:171–7. doi: 10.2500/aap.2014.35.3730. [DOI] [PubMed] [Google Scholar]
- 22.Vickery BP, Scurlock AM, Kulis M, Steele PH, Kamilaris J, Berglund JP, et al. Sustained unresponsiveness to peanut in subjects who have completed peanut oral immunotherapy. J Allergy Clin Immunol. 2014;133:468–75. doi: 10.1016/j.jaci.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiel MA, Röder E, Gerth van Wijk R, Al MJ, Hop WCJ, Rutten-van Mölken MPMH. Real-life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353–360. e2. doi: 10.1016/j.jaci.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Blumchen K, Ulbricht H, Staden U, Dobberstein K, Beschorner J, de Oliveira LCL, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J Allergy Clin Immunol. 2010;126:83–91. e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 25.Keet CA, Wood RA. Emerging therapies for food allergy. J Clin Invest. 2014;124:1880–6. doi: 10.1172/JCI72061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones SM, Burks AW, Dupont C. State of the art on food allergen immunotherapy: oral, sublingual, and epicutaneous. J Allergy Clin Immunol. 2014;133:318–23. doi: 10.1016/j.jaci.2013.12.1040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.