Abstract
Tet (ten-eleven translocation) methylcytosine dioxygenases, which belong to the iron and 2-oxoglutarate (2OG)-dependent dioxygenase superfamily, convert 5-methylcytosine (5mC) to 5-hydroxymethylcytosine (5hmC) in DNA. We recently reported that ascorbate (vitamin C) induces Tet-mediated generation of 5hmC. To initially delineate the role of ascorbate on 5hmC generation, we analyzed whether the effect of ascorbate is dependent upon the conditions of other components involved in the hydroxylation of 5mC catalyzed by Tet. We found that removing iron from the culture medium did not affect the induction of 5hmC by ascorbate (10 µM) in mouse embryonic fibroblasts (MEFs). The effect of ascorbate did not involve an increased expression of Tet1-3 or isocitrate dehydrogenases (IDH1-2), the enzymes responsible for producing 2OG. Interestingly, MEFs cultured with different concentrations of glucose, a major precursor of 2OG, exhibited nearly identical responses to ascorbate treatment. Further, blocking the uptake of the reduced form of vitamin C, ascorbic acid, through the sodium-dependent vitamin C transporters (SVCTs) inhibited the effect of ascorbate on 5hmC. However, inhibition of the facilitative glucose transporters (GLUTs), which mediate the incorporation of the oxidized form of vitamin C, dehydroascorbic acid (DHA), did not modify the ability of ascorbate to induce 5hmC generation. These results indicate that the effect of ascorbate on 5hmC is not dependent upon iron uptake, the expression of Tet and IDH, or the production of 2OG, suggesting that ascorbate may directly participate in the generation of 5hmC, most likely as a cofactor of Tet.
Keywords: Ascorbate, 5-hydroxymethylcytosine, Tet methylcytosine dioxygenase, iron, 2-oxoglutarate, glucose, isocitrate dehydrogenase, sodium-dependent vitamin C transporter
1. Introduction
Vitamin C (L-ascorbic acid) exists predominantly as the ascorbate anion under physiological pH conditions. In addition to functioning as a general antioxidant, ascorbate is responsible for maintaining the catalytic activity of a group of iron and 2-oxoglutarate (2OG)-dependent dioxygenases [1]. To catalyze the hydroxylation of a diverse variety of substrates, the iron and 2OG-dependent dioxygenases utilize Fe2+ as a cofactor, 2OG as a co-substrate, and some of them also require ascorbate as an additional cofactor in order to reach full catalytic activity. One classic member of this family is collagen prolyl 4-hydroxylase (P4H), which is well known for its involvement in scurvy. In the absence of ascorbate, the initial hydroxylation catalyzed by collagen P4H can proceed at a maximal rate. However, the catalytically inactive oxidized iron species accumulates and quickly causes the inactivation of collagen P4H, leading to an incomplete hydroxylation of residues in collagen. Ultimately, this leads to the characteristic symptoms of scurvy [2]. However, when available, ascorbate has the capacity to reduce oxidized iron species to catalytically active Fe2+, thus reconstituting the catalytic activity of these dioxygenases.
We recently reported that ascorbate induces the generation of 5-hydroxymethylcytosine (5hmC) in cultured cells [3]. 5hmC is generated from the hydroxylation of 5-methylcytosine (5mC), which is catalyzed by a group of enzymes termed Tet methylcytosine dioxygenases (also known as Tet proteins), members of the iron and 2OG-dependent dioxygenase superfamily [4], [5] and [6]. Tet can further oxidize 5hmC to 5-formylcytosine and 5-carboxylcytosine, which could eventually be replaced by unmodified cytosine, constituting the most consistent mechanism underlying the active demethylation of DNA [7], [8] and [9]. In our previous study, we discovered that the content of 5hmC was extremely low in cells cultured in ascorbate-free medium. When ascorbate was added to the cell culture medium, the generation of 5hmC was induced in a dose- and time-dependent manner while other reducing agents such as glutathione (GSH) had no effect on 5hmC. Furthermore, blocking ascorbate entry into cells and knocking down Tet expression inhibited the effect of ascorbate on the levels of 5hmC [3]. These results suggest that ascorbate enhances 5hmC generation, possibly by acting as a cofactor to maintain the activity of Tet as it does for a variety of other iron and 2OG-dependent dioxygenases. To further solidify these results, the effect of ascorbate on the conversion of 5mC to 5hmC was confirmed by another group using embryonic stem cells [10]. However, a detailed mechanism that explains ascorbate effectively modulates the catalytic activity of Tet has yet to be elucidated. To delineate how ascorbate induces 5hmC generation, we analyzed the effect of varying iron, 2OG, and the activity of the vitamin C transporters on this reaction.
2. Methods
2.1. Cell culture and treatments
Mouse embryonic fibroblasts (MEFs), derived from a wild type C57BL/6 mouse, were maintained in DMEM (Invitrogen), which did not contain any ascorbate in its formula. MEFs were cultured in 6-well plates with or without cover slips. After seeding for 24 hr, cells were treated with L-ascorbic acid (Sigma) at 10 µM for varying durations. Each treatment group consisted of three wells for every experiment. Each experiment was repeated at least three times. To test whether the effect of ascorbate is independent upon the presence of iron in the medium, MEFs were cultured in DMEM (Invitrogen) that did not contain any iron in its formula, but was supplemented with dialyzed FBS. Fe(NO3)3·9H2O (Sigma) was dissolved in PBS, sterilized by filtration and added to the medium when indicated. The final concentrations of iron in the media were 0, 0.25 and 2.5 µM. In commercially available cell culture media, 0.25 µM of iron is the most typical concentration. To test whether the effect of ascorbate is independent on glucose in the medium, DMEM of high glucose (25mM), DMEM of low glucose (5.56 mM), and DMEM containing no glucose (0 mM) were prepared. The only difference among the three media was the concentration of glucose in their formulas. MEFs were usually maintained in DMEM of low glucose. After the cells had been seeded for 24 hr, the medium was changed to DMEM containing the different concentrations of glucose.
2.2. Dot-blot assay
Genomic DNA was extracted from MEFs using QIAamp DNA mini kits according to the manufacturer’s instructions (Qiagen). A Qubit Fluorometer (Life Technology) was used to quantify the concentration of DNA. The dot-blot procedure followed the published methods as conducted in our previous studies [3] and [11]. Briefly, DNA samples were diluted with 2N NaOH and 10 mM Tris·Cl, pH 8.5, then loaded on a Hybond N+ nylon membrane (GE Health) using a 96-well dot-blot apparatus (Bio-Rad). After baking at 80°C for 30 min and being blocked by 5% non-fat milk for 1 hr at room temperature, the membrane was incubated in a polyclonal anti-5hmC antibody (Active Motif #39769, 1:10,000) at 4°C overnight. 5hmC was visualized by chemiluminescence. The densities of the dots were captured by AlphaImager. To ensure equal loading, the membrane was stained with methylene blue post-immunoblotting. Statistical significance of differences in 5hmC content between different treatments were assessed by t test, at α = 0.05.
2.3. Immunostaining
Immunostaining of 5hmC was performed using the published method [11]. Briefly after treatment, MEFs were fixed with 4% paraformaldehyde and then incubated with 1N HCl at 37°C for 30min. After washing with PBS and blocking with 3% serum in PBS for 1 hr, the cells were incubated with anti-5hmC antibody (Active Motif #39769, 1:10,000) at 4°C overnight. Cy3-conjugated donkey anti-rabbit IgG (1:400; Jackson ImmunoResearch) was used as an immunofluorescent secondary antibody. Cells were then counterstained with DAPI. Double fluorescence images were acquired with a Zeiss LSM710 confocal microscope.
2.4. RT-PCR
RNA was extracted from MEFs using the RNeasy kit (Qiagen). A nanodrop 8000 photospectrometer was used to measure the yield of RNA extraction. The SuperScript III First-Strand Synthesis System (Invitrogen) was used for reverse transcription (RT) according to the manufacturer’s instructions. The PCR was applied by the following 3 pairs of primers, SVCT1 forward: 5′-CAGCAGGGACTTCCACCA-3′; SVCT1 reverse: 5′-CCACACAGGTGAAGATG GTA-3′. SVCT2 forward: 5′-CTCCTGGTGAAC AAAGTCAGAATGG-3′; SVCT2 reverse: 5′-GAGGCCAATGACCACTTCTATC-3′. GAPDH-forward: 5′-TTAGCACCCCTGGCCAAGG-3′; GAPDH-reverse: 5′-CTTACTCCTTGGAGG CCATG-3′. The PCR products were separated in 2% agarose gel. The gel image was captured using the AlphaImager gel documentation system.
2.5. Quantitative PCR
Quantitative PCR was performed using an ABI HP7900. Within the plate, samples were plated in duplicate in adjacent wells within the column; one for amplification of each Tet (Tet1, Tet2 and Tet3) or IDH (IDH1, IDH2), the other one for the housekeeper gene GAPDH as the internal control. Each sample was used three times at different locations in the 384-well plate. After the PCR run was complete, quantitative gene expression data were acquired and analyzed using the ABI Prism 7900HT Sequence Detection System (RQ manager). Statistical significance of differences in expression levels between different treatments were assessed by t test, at α = 0.05.
3. Results
3.1. The generation of 5hmC induced by ascorbate is independent on the cellular uptake of iron
Iron is a known cofactor for Tet proteins to hydroxylate 5mC to 5hmC. In the absence of iron, recombinant Tet proteins are not functional [5], [6] and [12]. The presence of ascorbate at physiological concentration has been shown to enhance the uptake of iron by cells, raising the possibility that the effect of ascorbate on 5hmC is indirect and mediated by an ascorbate-induced increase in the cellular uptake of iron [13], [14] and [15]. To test this possibility, we examined whether ascorbate could induce the generation of 5hmC in an iron-deprived medium, and whether addition of iron could change the effect of ascorbate.
First, we cultured MEFs in DMEM that contained different concentrations of Fe3+, which could be reduced to Fe2+ within the cell. We observed that treatments of ascorbate (10 µM) did indeed cause an elevated generation of 5hmC in MEFs, even when cultured in iron-free medium (no iron in the formula of the medium and supplemented with dialyzed FBS). The level of induction was similar in conditions of iron deprivation and normal (0.25 uM) or elevated (2.5 uM) iron supplementation. This was determined by immunostaining (Fig. 1A) and semiquantitative dot-blot (Fig. 1B and 1C). These results suggest that the effect of ascorbate on 5hmC is independent of cellular uptake of iron.
Fig. 1.
The generation of 5hmC induced by ascorbate is independent on the iron uptake by MEFs. (A) Immunostaining shows that the 5hmC signal induced by ascorbate (10 µM) treatments for 24 hr is at a similar level in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (B) A representative dot-blot shows that ascorbate (10 µM) increases the content of 5hmC in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (C) Semiquantitative analysis of the dot-blot indicates that ascorbate (10 µM) increases 5hmC at a similar level (~4 fold of the basal level) in MEFs cultured with different concentrations (0, 0.25, 2.5µM) of iron. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
3.2. The presence of glucose does not change the effect of ascorbate on the generation of 5hmC
It has been shown that Tet proteins cannot convert 5mC to 5hmC in the absence of 2OG, which is an important co-substrate for Tet proteins [5], [6] and [16]. It should be noted that it is quite typical for 2OG, one of the intermediate metabolites in the Krebs cycle, to be highly abundant within eukaryotic cells. Although it seems unlikely that the effect of ascorbate on the generation of 5hmC is mediated by an influence of ascorbate on the level of 2OG, we tested whether glucose, which is one major precursor of 2OG, modifies the effect of ascorbate on 5hmC generation. The results showed that the basal level of 5hmC was extremely low in MEFs cultured in media containing any level of glucose – i.e. no (0 mM), low (5.56 mM) or high (25 mM) glucose (Fig. 3). Furthermore, the generation of 5hmC, induced by ascorbate (10 µM), is similar in MEFs cultured with different concentrations of glucose, as shown by immunostaining (Fig. 2A) and semiquantitative dot-blot (Fig. 2B and 2C). These results suggest that the generation of 5hmC, induced by ascorbate, is independent of presence of glucose in the cell culture the glucose in the medium.
Fig. 3.
Ascorbate treatment does not change the expression of Tet and IDH genes. (A) Quantitative PCR shows that ascorbate (0 – 1,000 µM) does not significantly change the expression of Tet genes in MEFs. (P > 0.05 assessed by t test. Date are represented as mean ± SEM). (B) Quantitative PCR shows that ascorbate (0 – 1,000 µM) does not significantly change the expression of IDH genes in MEFs. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
Fig. 2.
Glucose in the medium does not change the generation of 5hmC induced by ascorbate treatment. (A) Immunostaining shows that glucose (0, 5.56, 25 mM) does not change 5hmC signal induced by ascorbate (10 µM) treatment for 24 hr. (B) A representative dot-blot shows that ascorbate (10 µM) increases the content of 5hmC in MEFs cultured with different concentrations (0, 5.56, 25 mM) of glucose. (C) Semiquantitative analysis of the dot-blot indicates that ascorbate (10 µM) increases 5hmC at a similar level (~4 fold of the basal level) in MEFs cultured with different concentrations (0, 5.56, 25 mM) of glucose. (P > 0.05 assessed by t test. Date are represented as mean ± SEM).
3.3. Ascorbate treatment does not change the expression of Tet and IDH genes
The production of 2OG relies on a family of enzymes that are termed isocitrate dehydrogenases (IDH). Changes in the expression of IDH have been shown to affect 5hmC generation presumably by controlling the production of 2OG [17]. To test whether the effect of ascorbate on 5hmC generation is mediated by an increase in the expression of IDH proteins, we analyzed the level of IDH1-2 mRNA in cells treated with ascorbate. Through quantitative PCR we determined that ascorbate (0 – 1,000 µM) treatments for 24 hr did not alter the mRNA level of IDH1-2 (P > 0.05; Fig. 3A). We further proved that ascorbate treatment did not affect the expression of Tet1-3, as primarily observed (Fig. 3B) [3]. These findings suggest that the effect of ascorbate on the generation of 5hmc is not due to alterations of the expression of Tet or IDH.
3.4. Blocking SVCTs, but not GLUTs, inhibits the effect of ascorbate on 5hmC
Although being critical in the maintenance of a group of iron and 2OG-dependent dioxygenases, ascorbate is rarely included in standard cell culture media [18]. However, when present in the growth medium, ascorbate can effectively enter and accumulate within cells – mainly via the assistance of sodium-dependent vitamin C transporters (SVCTs). In contrast, the oxidized form of vitamin C (dehydroascorbic acid, DHA) enters via facilitative glucose transporters (GLUTs) [19].
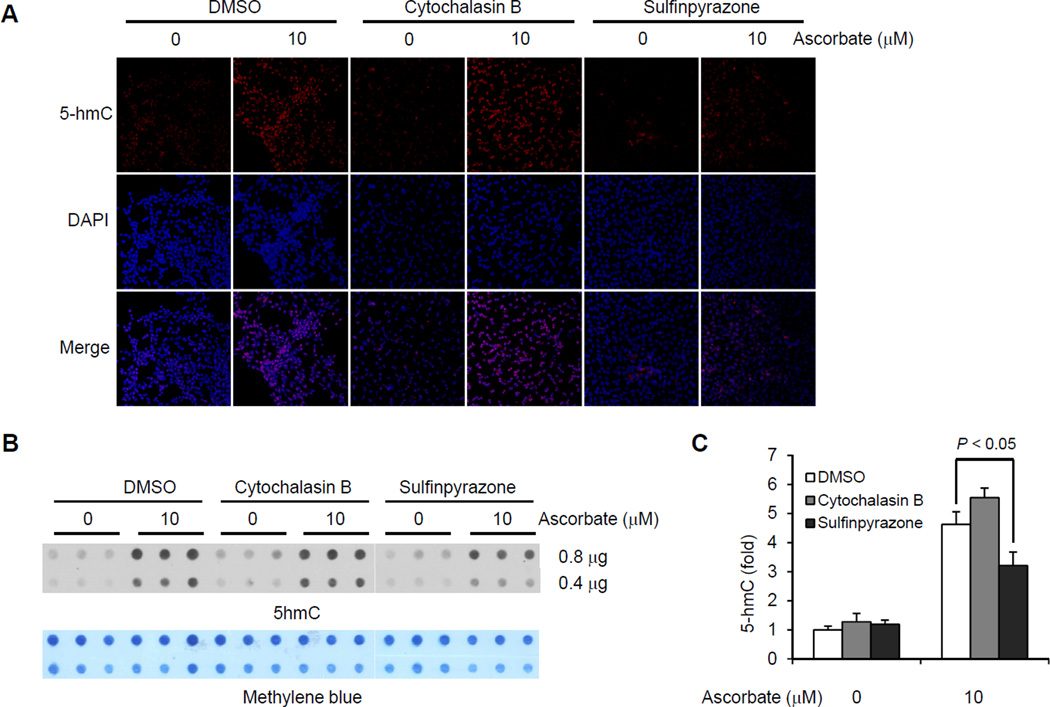
Due to the fact that the large majority of physiologic vitamin C exists in the form of ascorbate, and DHA is not detectable in the plasma of healthy subjects [20], this study focused on ascorbate rather than DHA. Upon entering the cell, ascorbate can easily penetrate the pores of the nuclear membrane to reach the 5mC hydroxylation complex that includes Tet proteins bound with iron and 2OG. By RT-PCR, we verified that MEFs express SVCT2, not SVCT1 (data not shown). It is known that GLUTs are ubiquitously expressed. We previously reported that the generation of 5hmC, induced by ascorbate treatment, was reduced by phloretin, which inhibits the uptake of ascorbate -- possibly via acting upon SVCTs as well as GLUTs [3] and [21]. To further verify that the entry of ascorbate into cells is essential towards inducing the generation of 5hmC, we pre-treated some MEFs with sulfinpyrazone (2 mM, a preferential inhibitor of SVCTs) and others with cytochalasin B (20µM, a typical inhibitor of GLUTs). The concentration of these inhibitors was designated based on previous reports [22] and [23]. The addition of the inhibitors to the medium did not alter the 5hmC content in ascorbate-free MEFs. However, only additions of sulfinpyrazone, but not cytochalasin B, reduced the effect of ascorbate on 5hmC from approximately 4.5-fold to less than 3-fold compared to basal levels, as shown by immunostaining (Fig. 4A) and semiquantitative dot blot (P < 0.05; Fig. 4B and 4C). These results suggest that the generation of 5hmC in MEFs indeed requires the entry of ascorbate, more likely through SVCTs, rather than GLUTs, into the cells.
Fig. 4.
The effect of ascorbate on 5hmC is partially reduced by sulfinpyrazone. (A) Immunostaining shows that both sulfinpyrazone (2 mM) and cytochalasin B (20 µM) do not change 5hmC signal in ascorbate-free MEFs. Only sulfinpyrazone blocks the induction of 5hmC by ascorbate (10 µM) treatment for 24 hr. (B) A representative dot-blot shows that sulfinpyrazone (2 mM), but not cytochalasin B (20 µM), inhibits the effects of ascorbate (10 µM) on 5hmC content. (C) Semiquantitative analysis of the dot-blot indicates that sulfinpyrazone (2 mM), but not cytochalasin B (20 µM), inhibits the induction of 5hmC by ascorbate (* P < 0.05 assessed by t test. Date are represented as mean ± SEM).
4. Discussion
We recently reported that ascorbate induces the Tet-mediated generation of 5hmC in cultured cells [3]. This effect also has been verified by another team [10]. However, a detailed mechanism to explain how ascorbate affects the catalytic activity of Tet has yet to be elucidated. Experimental evidence is required to determine whether ascorbate reconstitutes Tet by reducing the inactive Fe3+ state to the active Fe2+ state (as is the case with P4H mediated hydroxylation) after Tet is inactivated by coupled or uncoupled 2OG turnover [2]. As the first step of the endeavor to delineate the mechanism of ascorbate on 5hmC generation, we investigated whether the effect of ascorbate is dependent upon other components in the hydroxylation of 5mC.
Ascorbate has been shown to enhance the uptake of iron by cells [13], [14] and [15]. This may cause one to infer that the effect of ascorbate on 5hmC is indirect and mediated by an increased uptake of iron. Our experiments showed that the ascorbate treatment induced the generation of 5hmC in MEFs without iron present in the medium. Addition of different concentrations of iron did not change the effect of ascorbate on 5hmC. This suggests that the effect of ascorbate on the generation of 5hmC is independent of the cellular uptake of iron. MEFs used in our study have been maintained in medium containing iron for a long time and it is known that cells tend to accumulate iron intracellularly [24] and [25]. Thus, it is of a high likelihood that these MEFs have accumulated enough iron to allow full catalytic activity of Tet proteins and are fully independent of the extracellular concentration of iron.
The expression of Tet and IDH genes is critical for the hydroxylation of 5mC to 5hmC. Mutations and dysregulated expression of these genes have been associated with abnormal levels of 5hmC and a number of potentially fatal diseases [17] and [26]. Previously, we demonstrated that the effect of ascorbate on 5hmC is mediated by Tet. In this study, we found that the expression of Tet1-3 and IDH1-2 was maintained at a similar level after treatment with different concentrations of ascorbate. This suggests that the effect of ascorbate on 5hmC is not driven by alterations in Tet or IDH expression. These results are congruent with our previous observation of the rapid effect of ascorbate on 5hmC, which suggests that no protein synthesis is required, but that intracellular accumulation of ascorbate is sufficient to enhance the activity of available Tet [3]. Furthermore, we showed that differing concentrations of glucose, the major precursor of 2OG, had no effect on the level of 5hmC induced by ascorbate treatment.
Blocking the intracellular accumulation of ascorbate using phloretin inhibited the effect of ascorbate on 5hmC, as shown in our previous report [3]. It is necessary to mention that phloretin has been suggested to block the activity of both SVCTs and GLUTs [27] and [28]. Ascorbate enters cells mainly through SVCTs while the oxidized DHA does so through GLUTs. To further verify that SVCTs mediate the cellular uptake of ascorbate that ultimately induces the generation of 5hmC, SVCTs and GLUTs specific inhibitors were added to the culture medium. The results showed that the preferential SVCTs inhibitor (sulfinpyrazone) partially reduced the effect of ascorbate on 5hmC generation. In contrast, the GLUTs inhibitor (cytochalasin B) had no effect on the generation of 5hmC induced by ascorbate. This suggests that the accumulation of ascorbate via SVCTs is critical in the induction of the Tet-mediated generation of 5hmC in MEFs.
Thus, we have discovered that the effect of ascorbate on 5hmC is fully independent of the cellular uptake of iron, enhanced expression of Tet or IDH, or changes in the production of 2OG. The accumulation of ascorbate through SVCTs is critical for inducing the generation of 5hmC. It is possible that ascorbate reconstitutes Tet by reducing the inactive Fe3+ state to the active Fe2+ state (as is the case with P4H mediated hydroxylation) after Tet is inactivated by 2OG turnover.
Highlights for review.
Ascorbate-induced generation of 5hmC is independent on the cellular uptake of iron.
The presence of glucose does not change the effect of ascorbate on 5hmC.
Ascorbate does not change the expression of Tet and IDH genes.
Blocking SVCTs, but not GLUTs, inhibits the effect of ascorbate on 5hmC.
Acknowledgment
We thank Brenda Court for technical support. This research was supported by a James and Esther King biomedical research award (3KN08) to G.W. and a NIH grant (R01NS075764) to S.Z.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
G.W., J.I.Y. and S.Z. designed the experiments. K.M.D. and C.B.G performed cell culture, immunostaining and dot-blot experiments. G.W., J.I.Y. and S.Z. analyzed the data. K.M.D. and G.W. wrote the manuscript. All authors reviewed and contributed to the final manuscript.
References
- 1.McDonough MA, Loenarz C, Chowdhury R, Clifton IJ, Schofield CJ. Structural studies on human 2OGdependent oxygenases. Curr Opin Struct Biol. 2010;20:659–672. doi: 10.1016/j.sbi.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Gorres KL, Raines RT. Prolyl 4-hydroxylase. Crit. Rev. Biochem. Mol. Biol. 2010;45:106–124. doi: 10.3109/10409231003627991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minor EM, Court BL, Young JL, Wang G. Ascorbate induces Ten-eleven translocation (Tet) methylcytosine dioxygenase-mediated generation of 5-hydroxymethylcytosine. J. Biol. Chem. 2013;288:13669–13674. doi: 10.1074/jbc.C113.464800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito S, D'Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He YF, Li BZ, Li Z, Liu P, Wang Y, Tang Q, Ding J, Jia Y, Chen Z, Li L, Sun Y, Li X, Dai Q, Song CX, Zhang K, He C, Xu GL. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science. 2011;333:1303–1307. doi: 10.1126/science.1210944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito S, Shen L, Dai Q, Wu SC, Collins LB, Swenberg JA, He C, Zhang Y. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maiti A, Drohat AC. Thymine DNA glycosylase can rapidly excise 5-formylcytosine and 5-carboxylcytosine: potential implications for active demethylation of CpG sites. J. Biol. Chem. 2011;286:35334–35338. doi: 10.1074/jbc.C111.284620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blaschke K, Ebata KT, Karimi MM, Zepeda-Martínez JA, Goyal P, Mahapatra S, Tam A, Laird DJ, Hirst M, Rao A, Lorincz MC, Ramalho-Santos M. Vitamin C induces Tet-dependent DNA demethylation and a blastocyst-like state in ES cells. Nature. 2013;500:222–226. doi: 10.1038/nature12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Szulwach KE, Li X, Li Y, Song CX, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, Vasanthakumar A, Godley LA, Chang Q, Cheng X, He C, Jin P. 5hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat. Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Zhang X, Clark E, Mulcahey M, Huang S, Shi YG. TET1 is a DNA-binding protein that modulates DNA methylation and gene transcription via hydroxylation of 5-methylcytosine. Cell Res. 2010;20:1390–1393. doi: 10.1038/cr.2010.156. [DOI] [PubMed] [Google Scholar]
- 13.Lane DJ, Lawen A. Non-transferrin iron reduction and uptake are regulated by transmembrane ascorbate cycling in K562 cells. J. Biol. Chem. 2008;283:12701–12708. doi: 10.1074/jbc.M800713200. [DOI] [PubMed] [Google Scholar]
- 14.Lane DJ, Robinson SR, Czerwinska H, Bishop GM, Lawen A. Two routes of iron accumulation in astrocytes: ascorbate-dependent ferrous ironuptake via the divalent metal transporter (DMT1) plus an independent route for ferric iron. Biochem. J. 2010;432:123–132. doi: 10.1042/BJ20101317. [DOI] [PubMed] [Google Scholar]
- 15.Lane DJ, Chikhani S, Richardson V, Richardson DR. Transferrin iron uptake is stimulated by ascorbate via an intracellular reductive mechanism. Biochim. Biophys. Acta. 2013;1833:1527–1541. doi: 10.1016/j.bbamcr.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 16.Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim SH, Ito S, Yang C, Wang P, Xiao MT, Liu LX, Jiang W, Liu J, Zhang JY, Wang B, Frye S, Zhang Y, Xu YH, Lei QY, Guan KL, Zhao SM, Xiong Y. Oncometabolite 2-hydroxyglutarate is a competitive inhibitor of α-ketoglutarate-dependent dioxygenases. Cancer Cell. 2011;19:17–30. doi: 10.1016/j.ccr.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lian CG, Xu Y, Ceol C, Wu F, Larson A, Dresser K, Xu W, Tan L, Hu Y, Zhan Q, Lee CW, Hu D, Lian BQ, Kleffel S, Yang Y, Neiswender J, Khorasani AJ, Fang R, Lezcano C, Duncan LM, Scolyer RA, Thompson JF, Kakavand H, Houvras Y, Zon LI, Mihm MC, Kaiser UB, Schatton T, Woda BA, Murphy GF, Shi YG. Loss of 5-hydroxymethylcytosine is an epigenetic hallmark of melanoma. Cell. 2012;150:1135–1146. doi: 10.1016/j.cell.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monfort A, Wutz A. Breathing-in epigenetic change with vitamin C. EMBO Rep. 2013;14:337–346. doi: 10.1038/embor.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilson JX. Regulation of vitamin C transport. Annu. Rev. Nutr. 2005;25:105–125. doi: 10.1146/annurev.nutr.25.050304.092647. [DOI] [PubMed] [Google Scholar]
- 20.Koshiishi I, Mamura Y, Liu J, Imanari T. Evaluation of an acidic deproteinization for the measurement of ascorbate and dehydroascorbate in plasma samples. Clinical Chemistry. 1998;44:863–868. [PubMed] [Google Scholar]
- 21.May JM, Qu ZC. Transport and intracellular accumulation of vitamin C in endothelial cells: relevance to collagen synthesis. Arch. Biochem. Biophys. 2005;434:178–186. doi: 10.1016/j.abb.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 22.Rumsey SC, Kwon O, Xu GW, Burant CF, Simpson I, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 23.May JM, Qu ZC, Qiao H. Transfer of ascorbic acid across the vascular endothelium: mechanism and self-regulation. Am J Physiol Cell Physiol. 2009;297:C169–C178. doi: 10.1152/ajpcell.00674.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Killilea DW, Wong SL, Cahaya HS, Atamna H, Ames BN. Iron accumulation during cellular senescence. Ann N Y Acad Sci. 2004;1019:365–367. doi: 10.1196/annals.1297.063. [DOI] [PubMed] [Google Scholar]
- 25.Dusek P, Schneider SA. Neurodegeneration with brain iron accumulation. Curr Opin Neurol. 2012;25:499–506. doi: 10.1097/WCO.0b013e3283550cac. [DOI] [PubMed] [Google Scholar]
- 26.Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, An J, Lamperti ED, Koh KP, Ganetzky R, Liu XS, Aravind L, Agarwal S, Maciejewski JP, Rao A. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature. 2010;468:839–843. doi: 10.1038/nature09586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsukaguchi H, Tokui T, Mackenzie B, Berger UV, Chen XZ, Wang Y, Brubaker RF, Hediger MA. A family of mammalian Na+-dependent L-ascorbic acid transporters. Nature. 1999;399:70–75. doi: 10.1038/19986. [DOI] [PubMed] [Google Scholar]
- 28.Corpe CP, Lee JH, Kwon O, Eck P, Narayanan J, Kirk KL, Levine M. 6-Bromo-6-deoxy-L-ascorbic acid: an ascorbate analog specific for Na+-dependent vitamin C transporter but not glucose transporter pathways. J. Biol. Chem. 2005;280:5211–5220. doi: 10.1074/jbc.M412925200. [DOI] [PubMed] [Google Scholar]