Abstract
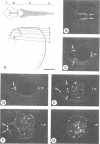
The Wnt-1 (int-1) gene was originally identified as an oncogene, but its normal function is in embryogenesis. The gene is the vertebrate homologue of the Drosophila segment polarity gene wingless, and encodes a secretory protein. In mouse embryos, Wnt-1 expression is necessary for proper development of the midbrain and anterior hindbrain. Here we describe the molecular cloning and primary structure of the zebrafish Wnt-1 gene (denoted wnt-1). Comparison with its mouse homologue reveals that both the genomic organization of wnt-1 and the amino acid sequence of the corresponding gene product have been extensively conserved during vertebrate evolution. Moreover, there is probably at least one Wnt-1-related sequence in the zebrafish genome. In zebrafish embryos, wnt-1 is expressed during differentiation of the neural tube. In situ hybridization analysis reveals that the transcripts are confined to the dorsal surfaces of the midbrain, hindbrain and spinal cord, and to lateral cells at the midbrain-hindbrain junction. Thus, the pattern of wnt-1 expression in the developing central nervous system of zebrafish is virtually identical to that seen in mouse embryos. Unexpectedly, despite the striking similarities of Wnt-1 structure and expression in fish and higher vertebrates, we could not identify sequences of obvious homology outside the coding regions, neither in the promoter nor in the introns.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker N. E. Embryonic and imaginal requirements for wingless, a segment-polarity gene in Drosophila. Dev Biol. 1988 Jan;125(1):96–108. doi: 10.1016/0012-1606(88)90062-0. [DOI] [PubMed] [Google Scholar]
- Baker N. E. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J. 1987 Jun;6(6):1765–1773. doi: 10.1002/j.1460-2075.1987.tb02429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley R. S., Brown A. M. The proto-oncogene int-1 encodes a secreted protein associated with the extracellular matrix. EMBO J. 1990 May;9(5):1569–1575. doi: 10.1002/j.1460-2075.1990.tb08276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. A., Joyner A. L. Expression patterns of the homeo box-containing genes En-1 and En-2 and the proto-oncogene int-1 diverge during mouse development. Genes Dev. 1988 Dec;2(12B):1736–1744. doi: 10.1101/gad.2.12b.1736. [DOI] [PubMed] [Google Scholar]
- Desplan C., Theis J., O'Farrell P. H. The sequence specificity of homeodomain-DNA interaction. Cell. 1988 Sep 23;54(7):1081–1090. doi: 10.1016/0092-8674(88)90123-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Sher E., Heemskerk-Jongens J., Kassis J. A., O'Farrell P. H. Two-tiered regulation of spatially patterned engrailed gene expression during Drosophila embryogenesis. Nature. 1988 Apr 14;332(6165):604–609. doi: 10.1038/332604a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiken H. G., Njølstad P. R., Molven A., Fjose A. A zebrafish homeobox-containing gene with embryonic transcription. Biochem Biophys Res Commun. 1987 Dec 31;149(3):1165–1171. doi: 10.1016/0006-291x(87)90530-4. [DOI] [PubMed] [Google Scholar]
- Gavin B. J., McMahon J. A., McMahon A. P. Expression of multiple novel Wnt-1/int-1-related genes during fetal and adult mouse development. Genes Dev. 1990 Dec;4(12B):2319–2332. doi: 10.1101/gad.4.12b.2319. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Kimmel C. B., Westerfield M., Walker C., Streisinger G. A neural degeneration mutation that spares primary neurons in the zebrafish. Dev Biol. 1988 Mar;126(1):115–128. doi: 10.1016/0012-1606(88)90245-x. [DOI] [PubMed] [Google Scholar]
- Immerglück K., Lawrence P. A., Bienz M. Induction across germ layers in Drosophila mediated by a genetic cascade. Cell. 1990 Jul 27;62(2):261–268. doi: 10.1016/0092-8674(90)90364-k. [DOI] [PubMed] [Google Scholar]
- Ingham P. W., Baker N. E., Martinez-Arias A. Regulation of segment polarity genes in the Drosophila blastoderm by fushi tarazu and even skipped. Nature. 1988 Jan 7;331(6151):73–75. doi: 10.1038/331073a0. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen B., Johnsen O. C., Valla S. The complete nucleotide sequence of the growth-hormone gene from Atlantic salmon (Salmo salar). Gene. 1989 Apr 30;77(2):317–324. doi: 10.1016/0378-1119(89)90079-6. [DOI] [PubMed] [Google Scholar]
- Kimmel C. B. Genetics and early development of zebrafish. Trends Genet. 1989 Aug;5(8):283–288. doi: 10.1016/0168-9525(89)90103-0. [DOI] [PubMed] [Google Scholar]
- Kokubu F., Hinds K., Litman R., Shamblott M. J., Litman G. W. Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. EMBO J. 1988 Jul;7(7):1979–1988. doi: 10.1002/j.1460-2075.1988.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- McMahon J. A., McMahon A. P. Nucleotide sequence, chromosomal localization and developmental expression of the mouse int-1-related gene. Development. 1989 Nov;107(3):643–650. doi: 10.1242/dev.107.3.643. [DOI] [PubMed] [Google Scholar]
- Molven A., Wright C. V., Bremiller R., De Robertis E. M., Kimmel C. B. Expression of a homeobox gene product in normal and mutant zebrafish embryos: evolution of the tetrapod body plan. Development. 1990 Jun;109(2):279–288. doi: 10.1242/dev.109.2.279. [DOI] [PubMed] [Google Scholar]
- Morata G., Lawrence P. A. The development of wingless, a homeotic mutation of Drosophila. Dev Biol. 1977 Apr;56(2):227–240. doi: 10.1016/0012-1606(77)90266-4. [DOI] [PubMed] [Google Scholar]
- Njølstad P. R., Molven A., Apold J., Fjose A. The zebrafish homeobox gene hox-2.2: transcription unit, potential regulatory regions and in situ localization of transcripts. EMBO J. 1990 Feb;9(2):515–524. doi: 10.1002/j.1460-2075.1990.tb08138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njølstad P. R., Molven A., Hordvik I., Apold J., Fjose A. Primary structure, developmentally regulated expression and potential duplication of the zebrafish homeobox gene ZF-21. Nucleic Acids Res. 1988 Oct 11;16(19):9097–9111. doi: 10.1093/nar/16.19.9097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordermeer J., Meijlink F., Verrijzer P., Rijsewijk F., Destrée O. Isolation of the Xenopus homolog of int-1/wingless and expression during neurula stages of early development. Nucleic Acids Res. 1989 Jan 11;17(1):11–18. doi: 10.1093/nar/17.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. The int genes in mammary tumorigenesis and in normal development. Trends Genet. 1988 Oct;4(10):291–295. doi: 10.1016/0168-9525(88)90172-2. [DOI] [PubMed] [Google Scholar]
- Nusse R., Theunissen H., Wagenaar E., Rijsewijk F., Gennissen A., Otte A., Schuuring E., van Ooyen A. The Wnt-1 (int-1) oncogene promoter and its mechanism of activation by insertion of proviral DNA of the mouse mammary tumor virus. Mol Cell Biol. 1990 Aug;10(8):4170–4179. doi: 10.1128/mcb.10.8.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R., Varmus H. E. Many tumors induced by the mouse mammary tumor virus contain a provirus integrated in the same region of the host genome. Cell. 1982 Nov;31(1):99–109. doi: 10.1016/0092-8674(82)90409-3. [DOI] [PubMed] [Google Scholar]
- Odenwald W. F., Garbern J., Arnheiter H., Tournier-Lasserve E., Lazzarini R. A. The Hox-1.3 homeo box protein is a sequence-specific DNA-binding phosphoprotein. Genes Dev. 1989 Feb;3(2):158–172. doi: 10.1101/gad.3.2.158. [DOI] [PubMed] [Google Scholar]
- Papkoff J., Brown A. M., Varmus H. E. The int-1 proto-oncogene products are glycoproteins that appear to enter the secretory pathway. Mol Cell Biol. 1987 Nov;7(11):3978–3984. doi: 10.1128/mcb.7.11.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Schryver B. Secreted int-1 protein is associated with the cell surface. Mol Cell Biol. 1990 Jun;10(6):2723–2730. doi: 10.1128/mcb.10.6.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N. H., Martin-Blanco E., Coleman K. G., Poole S. J., Ellis M. C., Kornberg T. B., Goodman C. S. Expression of engrailed proteins in arthropods, annelids, and chordates. Cell. 1989 Sep 8;58(5):955–968. doi: 10.1016/0092-8674(89)90947-1. [DOI] [PubMed] [Google Scholar]
- Rijsewijk F., Schuermann M., Wagenaar E., Parren P., Weigel D., Nusse R. The Drosophila homolog of the mouse mammary oncogene int-1 is identical to the segment polarity gene wingless. Cell. 1987 Aug 14;50(4):649–657. doi: 10.1016/0092-8674(87)90038-9. [DOI] [PubMed] [Google Scholar]
- Roelink H., Wagenaar E., Lopes da Silva S., Nusse R. Wnt-3, a gene activated by proviral insertion in mouse mammary tumors, is homologous to int-1/Wnt-1 and is normally expressed in mouse embryos and adult brain. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4519–4523. doi: 10.1073/pnas.87.12.4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackleford G. M., Varmus H. E. Expression of the proto-oncogene int-1 is restricted to postmeiotic male germ cells and the neural tube of mid-gestational embryos. Cell. 1987 Jul 3;50(1):89–95. doi: 10.1016/0092-8674(87)90665-9. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990 Aug 30;346(6287):847–850. doi: 10.1038/346847a0. [DOI] [PubMed] [Google Scholar]
- Wainwright B. J., Scambler P. J., Stanier P., Watson E. K., Bell G., Wicking C., Estivill X., Courtney M., Boue A., Pedersen P. S. Isolation of a human gene with protein sequence similarity to human and murine int-1 and the Drosophila segment polarity mutant wingless. EMBO J. 1988 Jun;7(6):1743–1748. doi: 10.1002/j.1460-2075.1988.tb03003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M., Eisen J. S. Neuromuscular specificity: pathfinding by identified motor growth cones in a vertebrate embryo. Trends Neurosci. 1988 Jan;11(1):18–22. doi: 10.1016/0166-2236(88)90044-6. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bailes J. A., McMahon A. P. Expression of the proto-oncogene int-1 is restricted to specific neural cells in the developing mouse embryo. Cell. 1987 Jul 3;50(1):79–88. doi: 10.1016/0092-8674(87)90664-7. [DOI] [PubMed] [Google Scholar]
- van Ooyen A., Kwee V., Nusse R. The nucleotide sequence of the human int-1 mammary oncogene; evolutionary conservation of coding and non-coding sequences. EMBO J. 1985 Nov;4(11):2905–2909. doi: 10.1002/j.1460-2075.1985.tb04021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., Nusse R. Structure and nucleotide sequence of the putative mammary oncogene int-1; proviral insertions leave the protein-encoding domain intact. Cell. 1984 Nov;39(1):233–240. doi: 10.1016/0092-8674(84)90209-5. [DOI] [PubMed] [Google Scholar]
- van den Heuvel M., Nusse R., Johnston P., Lawrence P. A. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell. 1989 Nov 17;59(4):739–749. doi: 10.1016/0092-8674(89)90020-2. [DOI] [PubMed] [Google Scholar]