Abstract
To elucidate the function of lysine-rich histone, yeast cells, which are believed to lack this histone, were transformed with an expression vector carrying the sea urchin histone H1 gene under control of an inducible promoter. Expression of full-length protein was tested by immunoblotting and the intracellular distribution was monitored by immunoelectron microscopy. Even low amounts of exogenous H1 led to dramatic changes in intracellular morphology and cell death. The cells that survived had lost either the plasmid or the ability to express the exogenous protein. Thus, even low amounts of canonical histone H1 are lethal to yeast cells.
Full text
PDF

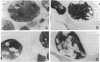
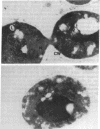
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bates D. L., Thomas J. O. Histones H1 and H5: one or two molecules per nucleosome? Nucleic Acids Res. 1981 Nov 25;9(22):5883–5894. doi: 10.1093/nar/9.22.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breeuwer M., Goldfarb D. S. Facilitated nuclear transport of histone H1 and other small nucleophilic proteins. Cell. 1990 Mar 23;60(6):999–1008. doi: 10.1016/0092-8674(90)90348-i. [DOI] [PubMed] [Google Scholar]
- Carter B. L. The yeast nucleus. Adv Microb Physiol. 1978;17:243–302. doi: 10.1016/s0065-2911(08)60059-4. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M. A model fungal gene regulatory mechanism: the GAL genes of Saccharomyces cerevisiae. Microbiol Rev. 1987 Dec;51(4):458–476. doi: 10.1128/mr.51.4.458-476.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Linder C., Thoma F. Histone H1 expressed in Saccharomyces cerevisiae binds to chromatin and affects survival, growth, transcription, and plasmid stability but does not change nucleosomal spacing. Mol Cell Biol. 1994 Apr;14(4):2822–2835. doi: 10.1128/mcb.14.4.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowary P. T., Widom J. Higher-order structure of Saccharomyces cerevisiae chromatin. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8266–8270. doi: 10.1073/pnas.86.21.8266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzolini L., Vaeck M., van Montagu M. Conserved epitopes on plant H1 histones recognized by monoclonal antibodies. Eur J Biochem. 1989 Jan 2;178(3):779–787. doi: 10.1111/j.1432-1033.1989.tb14509.x. [DOI] [PubMed] [Google Scholar]
- Moreland R. B., Langevin G. L., Singer R. H., Garcea R. L., Hereford L. M. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987 Nov;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B., Saunders C., Davie J. R., Hamkalo B. A. Ultrastructural organization of yeast chromatin. J Cell Biol. 1982 Apr;93(1):217–222. doi: 10.1083/jcb.93.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Kunz G., Daetwyler H., Telford J., Smith H. O., Birnstiel M. L. Genes and spacers of cloned sea urchin histone DNA analyzed by sequencing. Cell. 1978 Jul;14(3):655–671. doi: 10.1016/0092-8674(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Harris M. R., Sigournay C. M., Mayes E. L., Bustin M. A survey of H1o-and H5-like protein structure and distribution in higher and lower eukaryotes. Eur J Biochem. 1984 Jan 16;138(2):309–317. doi: 10.1111/j.1432-1033.1984.tb07916.x. [DOI] [PubMed] [Google Scholar]
- Srebreva L., Zlatanova J., Miloshev G., Tsanev R. Immunological evidence for the existence of H1-like histone in yeast. Eur J Biochem. 1987 Jun 1;165(2):449–454. doi: 10.1111/j.1432-1033.1987.tb11459.x. [DOI] [PubMed] [Google Scholar]
- Thoma F., Koller T., Klug A. Involvement of histone H1 in the organization of the nucleosome and of the salt-dependent superstructures of chromatin. J Cell Biol. 1979 Nov;83(2 Pt 1):403–427. doi: 10.1083/jcb.83.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino K. L., Crumrine D. A., Reichardt L. F. Lowicryl K4M embedding of brain tissue for immunogold electron microscopy. J Histochem Cytochem. 1985 Sep;33(9):969–973. doi: 10.1177/33.9.2991364. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Higher-order chromosome structure in yeast. J Cell Sci. 1990 May;96(Pt 1):1–3. doi: 10.1242/jcs.96.1.1. [DOI] [PubMed] [Google Scholar]
- Zlatanova J. S., Srebreva L. N., Banchev T. B., Tasheva B. T., Tsanev R. G. Cytoplasmic pool of histone H1 in mammalian cells. J Cell Sci. 1990 Jul;96(Pt 3):461–468. doi: 10.1242/jcs.96.3.461. [DOI] [PubMed] [Google Scholar]
- Zlatanova J. Histone H1 and the regulation of transcription of eukaryotic genes. Trends Biochem Sci. 1990 Jul;15(7):273–276. doi: 10.1016/0968-0004(90)90053-e. [DOI] [PubMed] [Google Scholar]
- Zlatanova J., Van Holde K. Histone H1 and transcription: still an enigma? J Cell Sci. 1992 Dec;103(Pt 4):889–895. doi: 10.1242/jcs.103.4.889. [DOI] [PubMed] [Google Scholar]