Abstract
Background and Purpose
Chronic lateral hip and thigh pain is regularly treated by the physical therapist. Many issues can cause pain in this region, and trigger points may contribute to pain. Dry Needling (DN) is an intervention used by physical therapists where a monofilament needle is inserted into soft tissue to reduce pain thereby facilitating return to prior level of function. The purpose of this case series is to report the outcomes of DN and conventional physical therapy as a treatment intervention for subjects with chronic lateral hip and thigh pain.
Case Descriptions
Four subjects with chronic lateral hip and thigh pain attended between four and eight sixty‐minute sessions of dry needling and stretching/ strengthening activities over a four to eight week intervention course. Outcomes were tested at baseline and upon completion of therapy. A long‐term follow up averaging 12.25 months (range 3 to 20 months) was also performed. The outcome measures included the Visual Analog Scale (VAS) and the Lower Extremity Functional Scale (LEFS).
Outcomes
The LEFS and VAS indicated clinically meaningful improvements in disability and pain in the short term and upon long term follow up for each subject. The LEFSmean for the four subjects improved from 50.75 at baseline to 66.75 at the completion of treatment. At long‐term follow‐up, the LEFSmean was 65.50. Each subject met the minimal clinically important difference (MCID) and minimal detectable change (MDC) for the LEFS and the VAS. The VAS was broken down into best (VASB), current (VASC), and worst (VASW) rated pain levels and averaged between the four subjects. The VASB improved from 20 mm at the initial assessment to 0 mm upon completion of the intervention duration. The VASC improved from 25.75 mm to 11.75 mm, and the VASW improved from 85 mm to 32.5 mm. At the long‐term follow up (average 12.25 months), the VASB, VASC, and VASW scores were 0 mm, 14.58 mm, and 43.75 mm respectively.
Discussion
Clinically meaningful improvements in pain and disability were noted. Subjects reported improved sleep and functional mobility, which were commensurate with their different age ranges and initial reported limitations in mobility. The results of this case series show promising outcomes for the use of dry needling in the treatment of chronic lateral hip and thigh pain. Further controlled clinical trials are recommended to determine the effectiveness of adding dry needling as compared to other interventions for chronic lateral hip and thigh pain.
Level of Evidence
Level 4.
Keywords: Dry Needling, hip pain, iliotibial band, trochanteric bursitis
BACKGROUND AND PURPOSE
Dry needling (DN) effectiveness research continues to be important in the therapy community regarding the use of DN as a treatment strategy for various conditions. Currently, no randomized control trial (RCT) studies exist investigating the effectiveness of DN treatment of pain of the lateral hip and thigh. Recent systematic reviews regarding the effectiveness of dry needling for TrPs and myofascial pain syndromes have been conducted and demonstrate some positive clinical responses to DN interventions. Cagnie et al recommend the use of DN for neck pain with moderate evidence, though there is weak evidence for improved function and quality of life.1 Kietrys et al recommend DN versus sham or placebo needling for decreasing pain immediately and at a four‐week follow‐up for individuals with myofascial pain syndrome.2 Tough et al on the other hand, did not find statistically significant evidence that DN was superior to sham or placebo interventions based on their meta‐analysis, but noted limitations to the review included small sample sizes and poor methodological qualities of the reviewed studies.3 Tough et al did note that the use of DN “could” be a positive direction for the treatment of myofascial pain syndrome, but larger, higher quality studies were needed.
TrPs have been studied as a source of pain, and the most accurate way to identify a likely TrP consists of palpation of a tender nodule in a taut band of muscle with subject pain recognition of tender spot palpation.4 The ability of a clinician to accurately diagnose a TrP location using palpation lacks clinical reproducibility, which is due to the inability to palpate the specific location of a TrP.4‐7 Sciotti et al and Myburgh et al have shown positive inter‐rater reliability for TrP identification in the upper trapezius muscle if the examiners were experienced.8,9 The problem with these studies is that small sample sizes were utilized and Myburgh et al showed that pairing experienced and inexperienced examiners caused a reduction in identification of TrPs.9 Hong et al and additional authors continue to promote the local twitch response (LTR) as being necessary for maximum effectiveness of trigger point dry needling (TrP‐DN), yet there is much debate regarding the necessity of the LTR.10 Tough et al suggest that of the original four criteria most commonly used to diagnose TrPs (LTR, predicted pain referral pattern, palpable tender nodule in a taught band of tissue, and reproduction of pain symptoms), LTR and predicted pain referral pattern are no longer essential for diagnosis.4 It should be noted that commonly, both myofascial DN and TrP‐DN terminology is being used to denote types of DN intervention, yet DN is not just limited to myofascial pain or TrP intervention. DN may be used to treat peri‐neural conditions, myofascial TrPs, intramuscular conditions, symptomatic scar tissue and other various conditions that might benefit from the use of DN. The current terms used, including “myofascial” DN or “TrP” DN, may be too restrictive for the multitude of conditions and tissues that can be treated with DN. Regardless of these issues, interventions described in this case series were focused on treating myofascial TrPs in the painful regions reported by the subjects in the case series.
Lateral hip and thigh pain may be the result of various etiologies including, but not limited to: osteoarthritis of the hip joint, greater trochanteric bursitis (GTB), iliotibial band syndrome (ITBS)/ snapping hip syndrome, muscle weakness/ strength imbalances, flexibility deficits, friction issues, spinal pathology, and leg length discrepancies.11‐20 GTB is often commonly used as a label by medical providers to identify lateral hip pain. GTB is most likely to occur between the fourth and sixth decades of life, though cases in all age groups have been reported in the literature.21 Trochanteric pain syndrome was originally thought to be caused by inflammation of the trochanteric and/ or sub‐gluteus maximus bursa (i.e. bursitis), but authors of recent MRI and ultrasound studies question the idea that bursitis is the etiology for all trochanteric regional pain.18 A more general term, greater trochanteric pain syndrome (GTPS) includes a number of disorders of the lateral, peri‐trochanteric region of the hip, including trochanteric bursitis, gluteus medius and minimus tears, and external coxa saltans (snapping hip).19 The incidence of GTPS is reported to be approximately 1.8 subjects per 1000 per year, with the prevalence being higher in women, and in subjects with coexisting low back pain, osteoarthritis, ITB tenderness, and obesity.20 Symptoms include persistent pain in the lateral hip radiating down the lateral thigh to the knee, and occasionally into the buttock region. Physical examination typically indicates point tenderness in the posterolateral area of the greater trochanter.20
Iliotibial band (ITB) involvement, typically associated with lateral knee pain, commonly presents concurrently with GTPS in the author’s clinical experience. According to the literature, the lateral knee region is the most extensively researched and identified area of ITB pain pathology, but clinically it is common to have palpable tenderness along the entire length of the ITB. There is minimal published evidence that describes effective treatment strategies for ITBS. Treatments vary greatly and commonly include non‐steroidal anti‐inflammatory drug (NSAID) administration, phonophoresis, corticosteroid injections, deep friction massage, and correction of hip strength abnormalities.13,14 Given the inconsistency with accurate diagnosis of the etiology of chronic lateral hip and thigh pain, the possibility of TrP formation in the affected hip and thigh musculature is plausible. Clinical presentation of palpable tender nodules in taught bands of tissue and subject recognition of pain pattern supports this as a possible etiology. Though the ITB and GT region are not considered to be muscular in nature, many surrounding muscles are closely linked to the ITB and GTB regions, which could be sources of pain contribution.
Given the lack of research supporting diagnostic criterion and treatment strategies for lateral hip and thigh pain, there is a need for the documentation and presentation of clinically effective interventions that can improve pain, thereby improving general function present due to chronic pain. The purpose of this case series was to investigate DN coupled with conventional physical therapy (CPT) as a treatment strategy for subjects with chronic lateral hip and thigh pain.
CASE DESCRIPTIONS
This case series included four subjects with chronic lateral hip and thigh pain of duration > 90 days. Specific questions were asked to each subject, and included thorough questioning about sleep deficit due to pain when lying on the affected hip, mobility limitations associated with pain, and any limping mechanics during gait that may not have been observed upon presentation to the clinic. A review of subject histories found common functional deficits including difficulty sleeping due to pain caused by rolling onto the affected side, and limited functional mobility due to pain affecting walking tolerance > 5 to 10 minutes in duration. The subjects were all in good relative health without serious underlying pathology. They could each ambulate independently without an assistive device, though three out of four reported intermittent limping due to pain. All four subjects had been previously treated by physicians and physical therapists for interventions including, but not limited to: corticosteroid injections and/ or “traditional” physical therapy interventions including stretching and exercise activities, light and deep friction tissue mobilization (such as foam rolling and massage/ myofascial release techniques), and therapeutic ultrasound. Subjects had not been treated for at least one year prior to the intervention for this case series. Temporary relief was reported with the previous treatment strategies, but pain was not eliminated and there was no long‐term improvement per subjective reporting by each of the subjects.
CLINICAL IMPRESSION 1
Given the fact all four subjects had previous treatment consisting of CPT, and ongoing lateral hip and thigh pain since that time, the subjects were considered appropriate for inclusion in the case series to examine the effectiveness of adding DN to a CPT program. An examination of each subject was performed in order to assess common functional limitations, strength deficits, gait impairments, and to rule out serious neurovascular pathology that might require referral to another medical specialist based upon findings.
EXAMINATION
Examination took place at baseline, and upon completion of the therapy intervention period. The number of treatment sessions and duration of treatment depended on each subject’s response to the intervention. The number of treatment sessions ranged from four to eight. Treatment was not rendered > 8 weeks due to maximal measureable improvement being attained by each subject during that time frame.
Posture and gait mechanics were assessed. Posture assessment included observation of lumbar, innominate, and global spinal positioning, and observation of gait mechanics. Physical examination of each of the subjects revealed no to mild loss of lumbar lordosis, but given the layers of tissue covering the lumbar region, accurate palpation and observation of lumbar spinal curvature was difficult and found to be unreliable in each of the subjects. The ability to accurately assess pelvic symmetry with static or movement‐based positioning testing is generally believed to lack validity or reliability, therefore palpation assessment for positional faults of the SIJ were not performed.22‐24 No other postural abnormalities were noted.
Bilateral lower extremity (BLE) strength was assessed via manual muscle testing (MMT) in a short sitting position with the hips and knees flexed and the legs hanging off the table. Strength was not being measured as an assessed outcome for change in this case series, thus the manual muscle testing scores are not noted within. It was noted that all four subjects had mild bilateral hip abduction, extension, and hip flexion strength deficit, which was scored between 4 to 4+ out of a possible 5, with hip abductors and extensors being the weaker of the hip muscle groups consistently demonstrated between the four subjects.
A lower quarter neurological examination was performed to screen each subject for spinal origin symptoms. This included dermatomal, myotomal, and deep tendon reflexes (DTRs). Dermatomal testing assessed light touch sensory palpation to the T10‐S2 dermatomal regions of the trunk and lower extremities. Myotomal testing was assessed via MMT of the same nerve root representations just mentioned. DTRs were assessed via testing of the L4 and S1 nerve roots in short sitting at the patellar tendon and Achilles’ tendon bilaterally. Bilateral knee and ankle DTR’s were normal in all subjects. Seated slump testing (sensitivity= 0.84; specificity= 0.83)25 was performed to assess for neural tension/ lumbar disc involvement, and was negative in all subjects. There were no neurovascular abnormalities noted. It is noted that one subject did have a pacemaker, and therefore did not receive electrical stimulation applied to the needles as part of the intervention. This will be discussed later in the case series discussion section.
Symptom centralization testing for pathology of discogenic origin has been found to be valid and reliable.26 Subjects were tested via repeated flexion and extension movements in standing for perihperalization/ centralization phenomenon, which was negative for discogenic pain in all subjects. Sacroiliac joint (SIJ) involvement was ruled out using a test cluster as suggested by Van der Wurff et al27 as well as the active straight leg raise (ASLR) as described by Mens et al28 Van der Wurff identified the following cluster of five pain provocation tests: ASIS distraction and compression, Gaenslen, thigh thrust, and Patrick tests. A cutoff of three or more positive tests has the highest positive likelihood ratio of 4.02 (sensitivity of 85%, specificity of 79%, positive predictive value of 77%, and negative predictive value of 87%).27 All tests of the SIJ were negative for all subjects.
Palpation assessment revealed tender nodules in taut tissue bands and subject pain recognition in the vastus lateralis muscles and greater trochanteric bursa areas in each subject, suggesting possible TrPs in the affected musculature. The locations and number of tender nodules varied and were located throughout the affected regions in each subject in no particular pattern. There were no autonomic responses noted (e.g. temperature change, diaphoresis, etc.) and no sensory issues were identified. Trophic changes of the skin were also absent in all subjects.
Range of motion (ROM) was not assessed, as this case series was focusing on pain, changes in patient self‐report of functional mobility, and subjective reports of dysfunction such as sleep deficit.
CLINICAL IMPRESSION 2
Based upon examination findings, all four subjects were deemed appropriate to receive the intervention described in the “Intervention” section of the case series report. There were no contraindications that would preclude any of the four subjects from the application of DN and CPT. All subjects reported no previous limitations in sleep, mobility, or general function prior to the onset of their hip and thigh pain conditions. All four subjects had ongoing lateral hip and thigh pain affecting their daily activity tolerance and sought long‐term pain relief, which they had not received with prior treatment. Hip and thigh pain coupled with negative contraindications for DN intervention made the subjects appropriate for DN to be performed.
OUTCOME MEASURES
The outcome measures used in this case series were the Lower Extremity Functional Scale (LEFS) and the Visual Analog Scale (VAS) and are reported in Table 1. The VAS is a 100 mm scale where the subject marked a line at the area most closely associated with their respective pain levels. At baseline, the average VAS for “best, current, and worst” level scores was 20, 25.75, and 85 (out of 100) respectively. The VAS has moderate to good reliability (correlation coefficient 0.60‐0.77)29 to detect disability and high reliability for pain (correlation coefficient 0.76‐0.84).30
Table 1.
Outcome Measure Scores at Baseline and Upon Completion of Treatment
| Outcome Measure | Subject 1 | Subject 2 | Subject 3 | Subject 4 |
|---|---|---|---|---|
| LEFSa Baseline | 70/ 80 | 51/ 80 | 46/ 80 | 36/ 80 |
| LEFS Final | 80/ 80 | 60/ 80 | 67/ 80 | 60/ 80 |
| LEFS Follow‐Up | 80/ 80 | 58/ 80 | 58/ 80 | 66/ 80 |
| VASb (mm.) Baseline: | ||||
| Best | 0 | 30 | 20 | 30 |
| Current | 0 | 12 | 61 | 30 |
| Worst | 60 | 90 | 90 | 100 |
| VAS (mm.) Final: | ||||
| Best | 0 | 0 | 0 | 0 |
| Current | 0 | 7 | 3 | 1 |
| Worst | 0 | 40 | 4 | 5 |
| VAS (mm) Follow‐Up: | ||||
| Best | 0 | 0 | 0 | 0 |
| Current | 0 | 1 | 0 | 0 |
| Worst | 0 | 4 | 4 | 35 |
| LEFS Baseline mean: 50.75/ 80 | VAS Baseline mean(Best): 20 | VAS Final mean(Best): 0 | VAS Follow Up mean (Best): 0 | |
| LEFS Final mean: 66.75/ 80 | VAS Baseline mean (Current): 25.75 | VAS Final mean (Current): 11.75 | VAS Follow Up mean (Current): 14.58 | |
| LEFS Follow Up mean: 65.50/ 80 | VAS Baseline mean (Worst): 85 | VAS Final mean (Worst): 12.75 | VAS Follow Up mean (Worst): 43.75 |
LEFS: Lower Extremity Functional Scale
VAS: Visual Analog Scale
The LEFS was used to assess functional disability. The lower the score the greater the disability. The LEFS is a quick and reliable patient self‐report functional outcome tool that can be easily completed and has been found to be a reliable and sensitive to change when compared to the SF‐36, with a minimal detectible change of 9 points and the minimal clinically important difference of 9 points.31 Test‐ retest reliability per Watson et al32 was found to be high (for subjects with anterior knee pain), and Yeung et al33 reported a large responsiveness to change along with being reliable and valid to assess subject change.
INTERVENTION
Risks and potential complications were advised and written consent was obtained outlining common and serious adverse events associated with DN interventions. Common complications include muscle soreness, bruising, and vasovagal reaction. More serious (but rare) complications include infection, broken needle, and pneumothorax.34 Contraindications include, but are not limited to: local infection, recent cancer/ history of immune suppression, bleeding disorders, current/ chronic use of anti‐coagulant medications, pregnancy, compromised sterility of equipment, and lack of practitioner practical knowledge.34 There we no reported contraindications by the subjects that would prevent the use of DN.
Subjects were treated with CPT interventions including traditional stretching and strengthening exercise activities and a specific DN protocol focusing on the painful region of the hip and thigh. Informed consent to participate in the series was retrospectively obtained from the subjects. Human subjects research review was not required for this case series. Subjects were advised that all HIPPA protected health information standards would be upheld and none of their identifying information would be released per the policies and procedures of the clinic where the treatment was performed.
The subjects were treated for four to eight sessions, one to two times per week for up to eight weeks. Subjects were positioned in side lying with a pillow between their knees on a hi‐low table for subject and therapist comfort. The following soft tissues were treated: approximate mid center of the greater trochanter, and five along the lateral thigh (in either the ITB or the vastus lateralis), with each needle spaced four fingerbreadths distal to the previously inserted needle for a total of six needles. The location of the needles were determined based on the author’s clinical experience of performing DN to lateral hip and thigh pain, and this has become a semi‐standardized approach to the application of DN for this condition in the author’s private practice. Each subject performed the CPT exercise program exactly as listed in Table 2 prior to DN, without variation from one subject to the next.
Table 2.
Examples of Conventional Physical Therapy Activities
| Variable | Intervention | Duration | Illustration(s) |
|---|---|---|---|
| Therapeutic stretching and exercise |
|
|
See appendix A for images of stretches and exercises utilized in the case series. |
The needles used in this case series were solid monofilament Seirin J‐type sterile needles (Seirin Corp., 1007‐1 Sodeshi‐Cho, Shimizu‐ku, Shizuoka‐shi, Shizuoka 424‐0036 Japan), No. 8 (0.30 diameter) x 50 mm. Needles were held in the therapist’s dominant hand for application and manipulation of the needle within the tissue. Before needle insertion, an application of 70% isopropyl alcohol was performed to the areas and allowed to dry for a least ten‐seconds, which reduces the resident micro‐flora of the skin by 80‐91%.34 All DN interventions were performed according to the Dry Needling Institute (DNI) of the American Academy of Manipulative Therapy (AAMT) Fellowship training program.34 The DNI program does not focus specifically on TrP‐DN, as some other DN training programs do, however, the DNI does include TrP‐DN as one of several treatment strategies for DN, hence this is the basis for this specific treatment strategy for this case series. The electrical stimulation unit used to apply current to the needles was an AWQ‐104L digital electro‐acupunctoscope, four‐channel, eight‐lead device (Lahasa OMS, 230 Libbey Parkway, Weymouth, MA 02189).
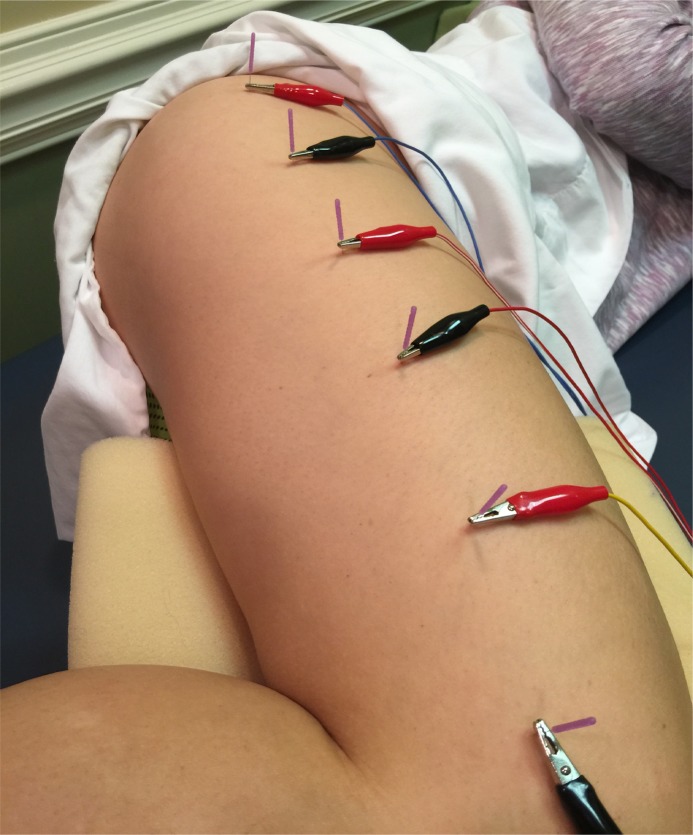
DN of the greater trochanteric bursa regions was performed as shown in Figure 1. Needles were inserted lateral to medial in the center of the greater trochanteric region to an approximate depth of 40 to 45 mm. Needles were wound clockwise to attain needle grasp of the between the needle and soft tissue, and left in‐situ for 15 minutes. DN techniques, including needling winding, may have a local and/ or remote therapeutic effect based on mechanical coupling of connective tissue and the needle thereby causing a “downstream” effect on the generation of a mechanical signal caused by needle grasp pulling. These downstream effects may include cell secretion, modification of extracellular matrix, enlargement and propagation of the pain signal along connective tissue planes, and afferent input modulation by changes in the connective milieu.35‐38
Figure 1.
Example of DN placement for treatment. Most proximal needle is placed over the greater trochanter, while each subsequent needle is placed approximately 4 fingerbreadths distal to the previous needle, in the middle of the ITB.
DN of the vastus lateralis/ ITB region was performed with four 30‐mm. needles as demonstrated in Figure 1. Flat palpation was used to first identify multiple tender points throughout the midline of the lateral thigh. Once the initial needle was inserted into the greater trochanteric region, five subsequent needles were inserted four fingerbreadths distal to each prior needle insertion location. The most distal needle was inserted to a depth of approximately 10‐15 mm, depending on the amount of tissue per subject, in order to avoid joint insertion. The needles were rotated clockwise to attain needle grasp and left in‐situ for 15 minutes.
The use of electrical stimulation applied to the needles was performed according to the following parameters outlined by the DNI:34 2 Hz, 250 microseconds, continuous asymmetric biphasic square wave with negative spike at an intensity described by the subjects as “mild to moderate”. As a side note, one subject was not treated with electrical‐stimulation of the tissues via the needles due to having a pacemaker, which may have contraindicated this portion of the intervention. Call bells were left with each subject receiving DN.
OUTCOMES
The demographic characteristics of the subjects are outlined in Table 3. The efficacy of DN was measured by pain reduction, reduction in disability level, and through subjective reports of improvement in the subject’s general daily activity tolerance. At baseline and upon completion of the intervention, pain and disability were assessed via the VAS and LEFS outcome measures. As noted earlier, objective measures such as hip strength, though mildly deficient, were not assessed as an outcome being measured indicating improvement. The reason for this was that strength deficit was not a reported deficit noted by the subjects; hence the outcomes were not looking to improve strength specifically, rather pain primarily. The results of these outcome measures are shown in Table 1. An average of the outcome measure scores were used to measure the overall improvement in pain and disability levels, as this gives a general representation of improvement between the four subjects. Each subject met the MCID and MDC for the LEFS as shown in Table 1. The final LEFS scores upon completion of the intervention ranged from 60 to 80 points versus the initial range of 36 to 70 points. The mean improvement between the four subjects demonstrated a mean improvement from 50.75 at baseline to 66.75 at completion of treatment, which at sixteen points, is well above the MDC/ MDIC indicating clinically meaningful improvement. At long term follow up (average of 12.25 months after completion of the treatment sessions); the LEFS average score was 65.50.
The VAS scores were broken down into reported best (VASB), current (VASC), and worst (VASW) levels. Individual VAS ranges were as follows: VASB at baseline, scores ranged from 0 mm to 30 mm and improved to 0 mm for all four subjects at completion of treatment. The VASC ranged from 0 mm to 61 mm and improved to 0 mm to 7 mm upon completion. The VASW scores at baseline ranged from 60 mm to 100 mm and improved to 0 mm to 40 mm upon completion. A mean was then calculated to average the four subject’s scores for ease of interpretation. The mean VASB score improved from 20 mm to 0 mm (at completion of treatment). The mean VASC improved from 25.75 mm to 11.75 mm. The mean VASW improved from 85 mm to 32.5 mm. At follow up, the mean VASB was 0 mm, the mean VASC was 14.58 mm, and the mean VASW was 43.75 mm. All four subjects verbally reported subjective reports of improved sleep, walking tolerance, and general improved tolerance to daily activities upon completion of treatment, and at the follow‐up. Sleep, walking tolerance, and general function was noted as limited prior to initiation of treatment. At the long‐term follow up, there were no significant reports of functional limitations reported by any of the four subjects.
DISCUSSION
Clinical results were positive, indicating improvements in pain and disability per the outcome measures used in this case series. Subjective reports of improved sleep, walking tolerance, and daily activity tolerance were also reported at follow up. All subjects reported specific improvements such as walking tolerance with no intermittent limping, which was a common presentation prior to intervention. There was one subject who received DN without the use of electrical stimulation due to having a pacemaker, and this subject also demonstrated remarkable improvement in reported pain and disability per the outcome measures. It should be noted that studies comparing the use of DN with and without electrical stimulation should be performed in the future, as there are no current studies examining DN alone versus DN with electrical stimulation in the treatment of lateral hip and thigh pain.
There are limitations to case series research. The small sample size (n=4) is an inherent limitation to a case series, though results are more clinically meaningful than a single case report. Given the lack of randomization and no specific inclusion or exclusion criterion, only descriptive outcomes can be reported, and statistical analysis cannot be inferred or provided. The small sample size also makes generalization of the intervention difficult, though the four subjects in the series were typical subjects seen in everyday practice and not statistically broken down to fit a certain criterion‐based inclusion. Also, all four subjects performed CPT exercises that may have contributed to the overall positive outcomes, which makes it difficult to determine how much of the improvements were specifically attributable to DN and how much were attributable to the exercise program prescribed. Larger randomized control studies looking at DN interventions need to be performed in order to fully assess the effectiveness of DN as an intervention strategy for GTPS and/ or ITB etiologies. Further research is recommended in order to determine if DN is clinically beneficial independent of, or coupled with other therapeutic interventions, such as other “manual” therapy techniques including “myofascial release” and massage or non‐thrust mobilization. Another area of further research should also compare the use of DN with electrical stimulation versus DN alone.
CONCLUSIONS
CPT and DN were tolerated well by the subjects, demonstrating improvements in pain and function, without significant adverse effects. Given the clinically meaningful reduction in pain and improvements in reported function, the addition of DN to CPT for chronic lateral hip and thigh pain etiologies shows promise. Future higher level research is needed to fully explore the effectiveness of DN for lateral hip and thigh pain.
APPENDIX A
Images of Common Physical Therapy (CPT) Activities
REFERENCES
- 1.Cagnie B Castelein B Pollie F Steelant L Verhoeyen H Cools A. Evidence for the Use of Ischemic Compression and Dry Needling in the Management of Trigger Points of the Upper Trapezius in Patients with Neck Pain: A Systematic Review. Am J of Phys Med & Rehab. 2015;94(7):573‐583. [DOI] [PubMed] [Google Scholar]
- 2.Kietrys DM Palombaro KM Azzaretto E, et al. Effectiveness of dry needling for upper‐quarter myofascial pain: a systematic review and meta‐analysis. J of Orthop Sports Phys Ther. 2013;43(9):620‐634. [DOI] [PubMed] [Google Scholar]
- 3.Tough EA White AR Cummings TM Richards SH Campbell JL. Acupuncture and dry needling in the management of myofascial trigger point pain: a systematic review and meta‐analysis of randomised controlled trials. Eur J Pain. 2009;13(1):3‐10. [DOI] [PubMed] [Google Scholar]
- 4.Tough EA White AR Richards S Campbell J. Variability of Criteria Used to Diagnose Myofascial Trigger Point Pain Syndrome—Evidence From a Review of the Literature. Clin J of Pain. 2007;23(3):278‐286. [DOI] [PubMed] [Google Scholar]
- 5.Gerwin RD Shannon S Hong C‐Z Hubbard D Gevirtz R. Interrater reliability in myofascial trigger point examination. Pain. 1997;69(1–2):65‐73. [DOI] [PubMed] [Google Scholar]
- 6.Myburgh C Larsen AH Hartvigsen J. A Systematic, Critical Review of Manual Palpation for Identifying Myofascial Trigger Points: Evidence and Clinical Significance. Arch Phys Med & Rehab. 2008;89(6):1169‐1176. [DOI] [PubMed] [Google Scholar]
- 7.Lucas N Macaskill P Irwig L Moran R Bogduk N. Reliability of Physical Examination for Diagnosis of Myofascial Trigger Points: A Systematic Review of the Literature. Clin J of Pain. 2009;25(1):80‐89 10.1097. [DOI] [PubMed] [Google Scholar]
- 8.Sciotti VM Mittak VL DiMarco L, et al. Clinical precision of myofascial trigger point location in the trapezius muscle. Pain. 2001;93(3):259‐266. [DOI] [PubMed] [Google Scholar]
- 9.Myburgh C Lauridsen HH Larsen AH Hartvigsen J. Standardized manual palpation of myofascial trigger points in relation to neck/shoulder pain; the influence of clinical experience on inter‐examiner reproducibility. Man Ther. 2011;16(2):136‐140. [DOI] [PubMed] [Google Scholar]
- 10.Hong C‐Z. Lidocaine Injection vs Dry Needling To Myofascial Trigger Point: The Importance of the Local Twitch Response. Am J of Phys Med & Rehab. 1994;73(4):256‐263. [DOI] [PubMed] [Google Scholar]
- 11.Pfirrmann CWA Chung CB Theumann NH Trudell DJ Resnick D. Greater Trochanter of the Hip: Attachment of the Abductor Mechanism and a Complex of Three Bursae—MR Imaging and MR Bursography in Cadavers and MR Imaging in Asymptomatic Volunteers. Rad. 2001;221(2):469‐477. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez‐Nemegyei J Canoso JJ. Evidence‐Based Soft Tissue Rheumatology: III: Trochanteric Bursitis. JCR: J of Clin Rheum. 2004;10(3):123‐124. [DOI] [PubMed] [Google Scholar]
- 13.Ellis R Hing W Reid D. Iliotibial band friction syndrome—A systematic review. Man Ther. 2007;12(3):200‐208. [DOI] [PubMed] [Google Scholar]
- 14.Fairclough J Hayashi K Toumi H, et al. Is iliotibial band syndrome really a friction syndrome? J Sci Med Sport. 2007;10(2):74‐76. [DOI] [PubMed] [Google Scholar]
- 15.Noehren B Davis I Hamill J. ASB Clinical Biomechanics Award Winner 2006: Prospective study of the biomechanical factors associated with iliotibial band syndrome. Clin Biomech. 2007;22(9):951‐956. [DOI] [PubMed] [Google Scholar]
- 16.Segal NA Felson DT Torner JC, et al. Greater Trochanteric Pain Syndrome: Epidemiology and Associated Factors. Arch Phys Med & Rehab. 2007;88(8):988‐992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sher I Umans H Downie S Tobin K Arora R Olson T. Proximal iliotibial band syndrome: what is it and where is it? Skel Radiol. 2011;40(12):1553‐1556. [DOI] [PubMed] [Google Scholar]
- 18.Silva F Adams T Feinstein J Arroyo RA. Trochanteric Bursitis: Refuting the Myth of Inflammation. JCR: J of Clin Rheum. 2008;14(2):82‐86. [DOI] [PubMed] [Google Scholar]
- 19.Strauss EJ Nho SJ Kelly BT. Greater Trochanteric Pain Syndrome. Sports Med & Arthrosc Rev. 2010;18(2):113‐119. [DOI] [PubMed] [Google Scholar]
- 20.Williams BS Cohen SP. Greater Trochanteric Pain Syndrome: A Review of Anatomy, Diagnosis and Treatment. Anesth & Analges. 2009;108(5):1662‐1670. [DOI] [PubMed] [Google Scholar]
- 21.Shbeeb MI Matteson EL. Trochanteric Bursitis (Greater Trochanter Pain Syndrome). Mayo Clinic Proceedings. 1996;71(6):565‐569. [DOI] [PubMed] [Google Scholar]
- 22.Holmgren U Waling K. Inter‐examiner reliability of four static palpation tests used for assessing pelvic dysfunction. Man Ther. 13(1):50‐56. [DOI] [PubMed] [Google Scholar]
- 23.Riddle DL Freburger JK Network NAORR. Evaluation of the Presence of Sacroiliac Joint Region Dysfunction Using a Combination of Tests: A Multicenter Intertester Reliability Study. Phys Ther. 2002;82(8):772‐781. [PubMed] [Google Scholar]
- 24.Robinson HS Brox JI Robinson R Bjelland E Solem S Telje T. The reliability of selected motion‐ and pain provocation tests for the sacroiliac joint. Man Ther. 12(1):72‐79. [DOI] [PubMed] [Google Scholar]
- 25.Majlesi J Togay H Ünalan H Toprak S. The Sensitivity and Specificity of the Slump and the Straight Leg Raising Tests in Patients With Lumbar Disc Herniation. JCR: J Clin Rheum. 2008;14(2):87‐91. [DOI] [PubMed] [Google Scholar]
- 26.Laslett M. Evidence‐Based Diagnosis and Treatment of the Painful Sacroiliac Joint. J Man & Manip Ther. 2008;16(3):142‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van der Wurff P Buijs EJ Groen GJ. A Multitest Regimen of Pain Provocation Tests as an Aid to Reduce Unnecessary Minimally Invasive Sacroiliac Joint Procedures. Arch of Phys Med & Rehab. 87(1):10‐14. [DOI] [PubMed] [Google Scholar]
- 28.Mens JMA Vleeming A Snijders CJ Stam HJ Ginai AZ. The active straight leg raising test and mobility of the pelvic joints. Eur Spine J. 1999;8(6):468‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boonstra AM Schiphorst Preuper HR Reneman MF Posthumus JB Stewart RE. Reliability and validity of the visual analogue scale for disability in patients with chronic musculoskeletal pain. Int’l J of Rehab Research. Internationale Zeitschrift fur Rehabilitationsforschung. Revue internationale de recherches de readaptation. 2008;31(2):165‐169. [DOI] [PubMed] [Google Scholar]
- 30.Bijur PE Silver W Gallagher EJ. Reliability of the visual analog scale for measurement of acute pain. Acad Emerg Med : Official journal of the Society for Academic Emergency Medicine. 2001;8(12):1153‐1157. [DOI] [PubMed] [Google Scholar]
- 31.Binkley JM Stratford PW Lott SA Riddle DL Network TNAORR. The Lower Extremity Functional Scale (LEFS): Scale Development, Measurement Properties, and Clinical Application. Phys Ther. 1999;79(4):371‐383. [PubMed] [Google Scholar]
- 32.Cynthia J. Watson MP Jennifer Ratner David L. Zeigler Patricia Horton Susan S. Smith. Reliability and Responsiveness of the Lower Extremity Functional Scale and the Anterior Knee Pain Scale in Patients With Anterior Knee Pain. J Orthop Sports Phys Ther. 2005;35(3):136‐146. [DOI] [PubMed] [Google Scholar]
- 33.Teresa S.M. Yeung JW Paul Stratford Joy MacDermid. Reliability, Validity, and Responsiveness of the Lower Extremity Functional Scale for Inpatients of an Orthopaedic Rehabilitation Ward. J Orthop Sports Phys Ther. 2009;39(6):468‐477. [DOI] [PubMed] [Google Scholar]
- 34.James Dunning D, MSc Manip. Ther., OCS, MSCP, MAACP (UK), FAAOMPT, MMACP (UK) DN‐1: Dry Needling for Craniofacial, Cervicothoracic & Upper Extremity COnditions: an Evidence‐Based Approach. Montgomery, AL: Dry Needling Institute of the American Academy of Manipulative Therapy; 2012:256.
- 35.Langevin HM Churchill DL Cipolla MJ. Mechanical signaling through connective tissue: a mechanism for the therapeutic effect of acupuncture. The FASEB J. 2001;15(12):2275‐2282. [DOI] [PubMed] [Google Scholar]
- 36.Langevin HM Churchill DL Fox JR Badger GJ Garra BS Krag MH. Biomechanical response to acupuncture needling in humans. J Applied Phys. 1985; 91(6): 2471‐2478. [DOI] [PubMed] [Google Scholar]
- 37.Langevin HM Bouffard NA Badger GJ Iatridis JC Howe AK. Dynamic fibroblast cytoskeletal response to subcutaneous tissue stretch ex vivo and in vivo. Am J Physio Cell Physiol. 2005;288(3): C747‐756. [DOI] [PubMed] [Google Scholar]
- 38.Langevin HM Bouffard NA Badger GJ Churchill DL Howe AK. Subcutaneous tissue fibroblast cytoskeletal remodeling induced by acupuncture: Evidence for a mechanotransduction‐based mechanism. J of Cell Phys. 2006;207(3):767‐774. [DOI] [PubMed] [Google Scholar]