Abstract
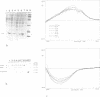
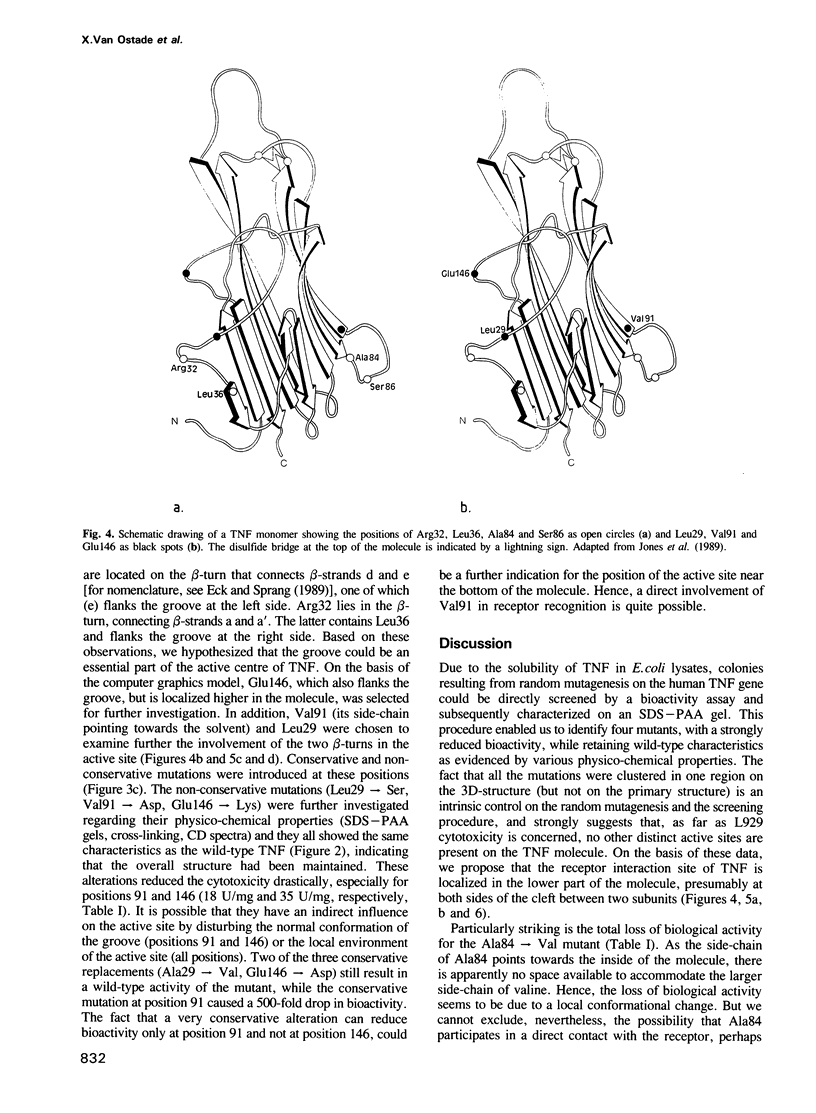
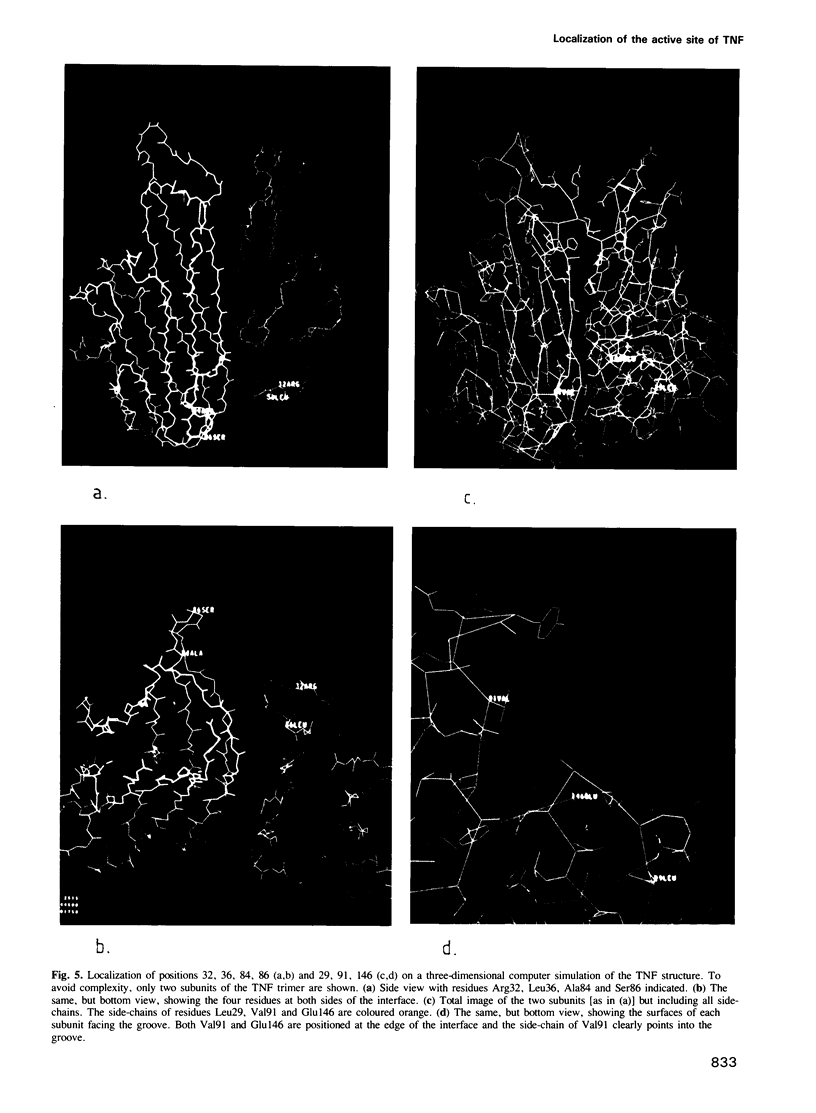
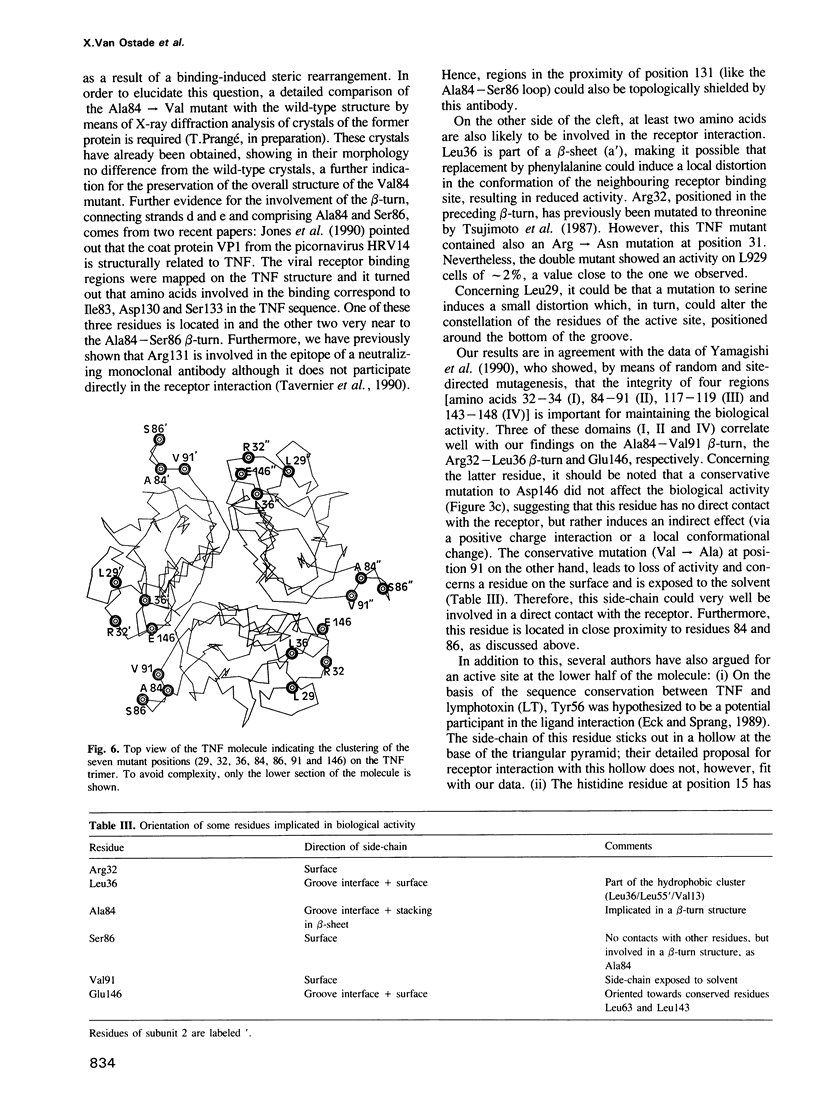
In order to define the active site(s) of human tumour necrosis factor (hTNF), we mutagenized its gene at random and directly screened the resulting population for loss of cytotoxic activity on L929 cells. Four biologically inactive mutant proteins (Arg32----Trp, Leu36----Phe, Ser86----Phe and Ala84----Val) behaved similar to the wild-type in various physico-chemical assays. The residues were positioned on a 3D structural model and were found to cluster together at the base of the molecule at each side of the groove that separates two monomers in the trimeric structure. A very conservative mutation at one of these sites (Ala84----Val) almost completely abolished cytotoxic activity. Amino acid alterations in three other residues in close proximity to this receptor binding site were introduced: replacements at positions 29 and 146 clearly reduced cytotoxicity only when non-conservative alterations were introduced (Leu29----Ser and Glu146----Lys), suggesting an indirect influence on the active site. However, a conservative mutation at position 91 (Val----Ala) caused a significant drop (500-fold) in bioactivity which suggests that Val91 may also play a direct role in receptor recognition. Our results favor a model in which each TNF molecule has three receptor-interaction sites (between the three subunits), thus allowing signal transmission by receptor clustering.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arakawa T., Yphantis D. A. Molecular weight of recombinant human tumor necrosis factor-alpha. J Biol Chem. 1987 Jun 5;262(16):7484–7485. [PubMed] [Google Scholar]
- Beutler B., Cerami A. The history, properties, and biological effects of cachectin. Biochemistry. 1988 Oct 4;27(20):7575–7582. doi: 10.1021/bi00420a001. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Schoenfeld H. J., Schlaeger E. J., Hunziker W., Lesslauer W., Loetscher H. Identification of two types of tumor necrosis factor receptors on human cell lines by monoclonal antibodies. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3127–3131. doi: 10.1073/pnas.87.8.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlino J. A., Lin L. S., Creasey A. A. Use of a sensitive receptor binding assay to discriminate between full-length and truncated human recombinant tumor necrosis factor proteins. J Biol Chem. 1987 Jan 25;262(3):958–961. [PubMed] [Google Scholar]
- Creasey A. A., Yamamoto R., Vitt C. R. A high molecular weight component of the human tumor necrosis factor receptor is associated with cytotoxicity. Proc Natl Acad Sci U S A. 1987 May;84(10):3293–3297. doi: 10.1073/pnas.84.10.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck M. J., Beutler B., Kuo G., Merryweather J. P., Sprang S. R. Crystallization of trimeric recombinant human tumor necrosis factor (cachectin). J Biol Chem. 1988 Sep 15;263(26):12816–12819. [PubMed] [Google Scholar]
- Eck M. J., Sprang S. R. The structure of tumor necrosis factor-alpha at 2.6 A resolution. Implications for receptor binding. J Biol Chem. 1989 Oct 15;264(29):17595–17605. doi: 10.2210/pdb1tnf/pdb. [DOI] [PubMed] [Google Scholar]
- Engelmann H., Novick D., Wallach D. Two tumor necrosis factor-binding proteins purified from human urine. Evidence for immunological cross-reactivity with cell surface tumor necrosis factor receptors. J Biol Chem. 1990 Jan 25;265(3):1531–1536. [PubMed] [Google Scholar]
- Espevik T., Brockhaus M., Loetscher H., Nonstad U., Shalaby R. Characterization of binding and biological effects of monoclonal antibodies against a human tumor necrosis factor receptor. J Exp Med. 1990 Feb 1;171(2):415–426. doi: 10.1084/jem.171.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers W., Brouckaert P., Devos R., Fransen L., Leroux-Roels G., Remaut E., Suffys P., Tavernier J., Van der Heyden J., Van Roy F. Lymphokines and monokines in anti-cancer therapy. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 1):587–595. doi: 10.1101/sqb.1986.051.01.071. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Aggarwal B. B., Benton C. V., Bringman T. S., Henzel W. J., Jarrett J. A., Leung D. W., Moffat B., Ng P., Svedersky L. P. Cloning and expression of cDNA for human lymphotoxin, a lymphokine with tumour necrosis activity. Nature. 1984 Dec 20;312(5996):721–724. doi: 10.1038/312721a0. [DOI] [PubMed] [Google Scholar]
- Hakoshima T., Tomita K. Crystallization and preliminary X-ray investigation reveals that tumor necrosis factor is a compact trimer furnished with 3-fold symmetry. J Mol Biol. 1988 May 20;201(2):455–457. doi: 10.1016/0022-2836(88)90153-2. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Remy R., Brockhaus M., van Loon A. P. Two different cell types have different major receptors for human tumor necrosis factor (TNF alpha). J Biol Chem. 1989 Sep 5;264(25):14927–14934. [PubMed] [Google Scholar]
- Jones E. Y., Stuart D. I., Walker N. P. Structure of tumour necrosis factor. Nature. 1989 Mar 16;338(6212):225–228. doi: 10.1038/338225a0. [DOI] [PubMed] [Google Scholar]
- Kramer W., Drutsa V., Jansen H. W., Kramer B., Pflugfelder M., Fritz H. J. The gapped duplex DNA approach to oligonucleotide-directed mutation construction. Nucleic Acids Res. 1984 Dec 21;12(24):9441–9456. doi: 10.1093/nar/12.24.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lewit-Bentley A., Fourme R., Kahn R., Prangé T., Vachette P., Tavernier J., Hauquier G., Niers W. Structure of tumour necrosis factor by X-ray solution scattering and preliminary studies by single crystal X-ray diffraction. J Mol Biol. 1988 Jan 20;199(2):389–392. doi: 10.1016/0022-2836(88)90323-3. [DOI] [PubMed] [Google Scholar]
- Loetscher H., Pan Y. C., Lahm H. W., Gentz R., Brockhaus M., Tabuchi H., Lesslauer W. Molecular cloning and expression of the human 55 kd tumor necrosis factor receptor. Cell. 1990 Apr 20;61(2):351–359. doi: 10.1016/0092-8674(90)90815-v. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Wang A., Levenson C. Site-specific mutagenesis to modify the human tumor necrosis factor gene. Methods Enzymol. 1987;154:403–414. doi: 10.1016/0076-6879(87)54087-3. [DOI] [PubMed] [Google Scholar]
- Narachi M. A., Davis J. M., Hsu Y. R., Arakawa T. Role of single disulfide in recombinant human tumor necrosis factor-alpha. J Biol Chem. 1987 Sep 25;262(27):13107–13110. [PubMed] [Google Scholar]
- Pine R., Huang P. C. An improved method to obtain a large number of mutants in a defined region of DNA. Methods Enzymol. 1987;154:415–430. doi: 10.1016/0076-6879(87)54088-5. [DOI] [PubMed] [Google Scholar]
- Schall T. J., Lewis M., Koller K. J., Lee A., Rice G. C., Wong G. H., Gatanaga T., Granger G. A., Lentz R., Raab H. Molecular cloning and expression of a receptor for human tumor necrosis factor. Cell. 1990 Apr 20;61(2):361–370. doi: 10.1016/0092-8674(90)90816-w. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C. A., Davis T., Anderson D., Solam L., Beckmann M. P., Jerzy R., Dower S. K., Cosman D., Goodwin R. G. A receptor for tumor necrosis factor defines an unusual family of cellular and viral proteins. Science. 1990 May 25;248(4958):1019–1023. doi: 10.1126/science.2160731. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Baglioni C. The active form of tumor necrosis factor is a trimer. J Biol Chem. 1987 May 25;262(15):6951–6954. [PubMed] [Google Scholar]
- Socher S. H., Riemen M. W., Martinez D., Friedman A., Tai J., Quintero J. C., Garsky V., Oliff A. Antibodies against amino acids 1-15 of tumor necrosis factor block its binding to cell-surface receptor. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8829–8833. doi: 10.1073/pnas.84.24.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soma G., Kitahara N., Tsuji Y., Kato M., Oshima H., Gatanaga T., Inagawa H., Noguchi K., Tanabe Y., Mizuno D. Improvement of cytotoxicity of tumor necrosis factor (TNF) by increase in basicity of its N-terminal region. Biochem Biophys Res Commun. 1987 Oct 29;148(2):629–635. doi: 10.1016/0006-291x(87)90923-5. [DOI] [PubMed] [Google Scholar]
- Stanssens P., Opsomer C., McKeown Y. M., Kramer W., Zabeau M., Fritz H. J. Efficient oligonucleotide-directed construction of mutations in expression vectors by the gapped duplex DNA method using alternating selectable markers. Nucleic Acids Res. 1989 Jun 26;17(12):4441–4454. doi: 10.1093/nar/17.12.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier J., Marmenout A., Bauden R., Hauquier G., Van Ostade X., Fiers W. Analysis of the structure-function relationship of tumour necrosis factor. Human/mouse chimeric TNF proteins: general properties and epitope analysis. J Mol Biol. 1990 Jan 20;211(2):493–501. doi: 10.1016/0022-2836(90)90367-U. [DOI] [PubMed] [Google Scholar]
- Tavernier J., van Ostade X., Hauquier G., Prange T., Lasters I., de Maeyer M., Lewit-Bentley A., Fourme R. Conserved residues of tumour necrosis factor and lymphotoxin constitute the framework of the trimeric structure. FEBS Lett. 1989 Nov 6;257(2):315–318. doi: 10.1016/0014-5793(89)81560-1. [DOI] [PubMed] [Google Scholar]
- Tsujimoto M., Tanaka S., Sakuragawa Y., Tsuruoka N., Funakoshi K., Butsugan T., Nakazato H., Nishihara T., Noguchi T., Vilcek J. Comparative studies of the biological activities of human tumor necrosis factor and its derivatives. J Biochem. 1987 Apr;101(4):919–925. doi: 10.1093/oxfordjournals.jbchem.a121960. [DOI] [PubMed] [Google Scholar]
- Van Ostade X., Tavernier J., Fiers W. Two conserved tryptophan residues of tumor necrosis factor and lymphotoxin are not involved in the biological activity. FEBS Lett. 1988 Oct 10;238(2):347–352. doi: 10.1016/0014-5793(88)80510-6. [DOI] [PubMed] [Google Scholar]
- Wingfield P., Pain R. H., Craig S. Tumour necrosis factor is a compact trimer. FEBS Lett. 1987 Jan 26;211(2):179–184. doi: 10.1016/0014-5793(87)81432-1. [DOI] [PubMed] [Google Scholar]
- Yamagishi J., Kawashima H., Matsuo N., Ohue M., Yamayoshi M., Fukui T., Kotani H., Furuta R., Nakano K., Yamada M. Mutational analysis of structure--activity relationships in human tumor necrosis factor-alpha. Protein Eng. 1990 Aug;3(8):713–719. doi: 10.1093/protein/3.8.713. [DOI] [PubMed] [Google Scholar]
- Yamamoto R., Wang A., Vitt C. R., Lin L. S. Histidine-15: an important role in the cytotoxic activity of human tumor necrosis factor. Protein Eng. 1989 May;2(7):553–558. doi: 10.1093/protein/2.7.553. [DOI] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]