Abstract
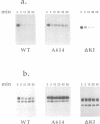
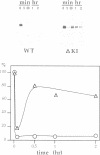
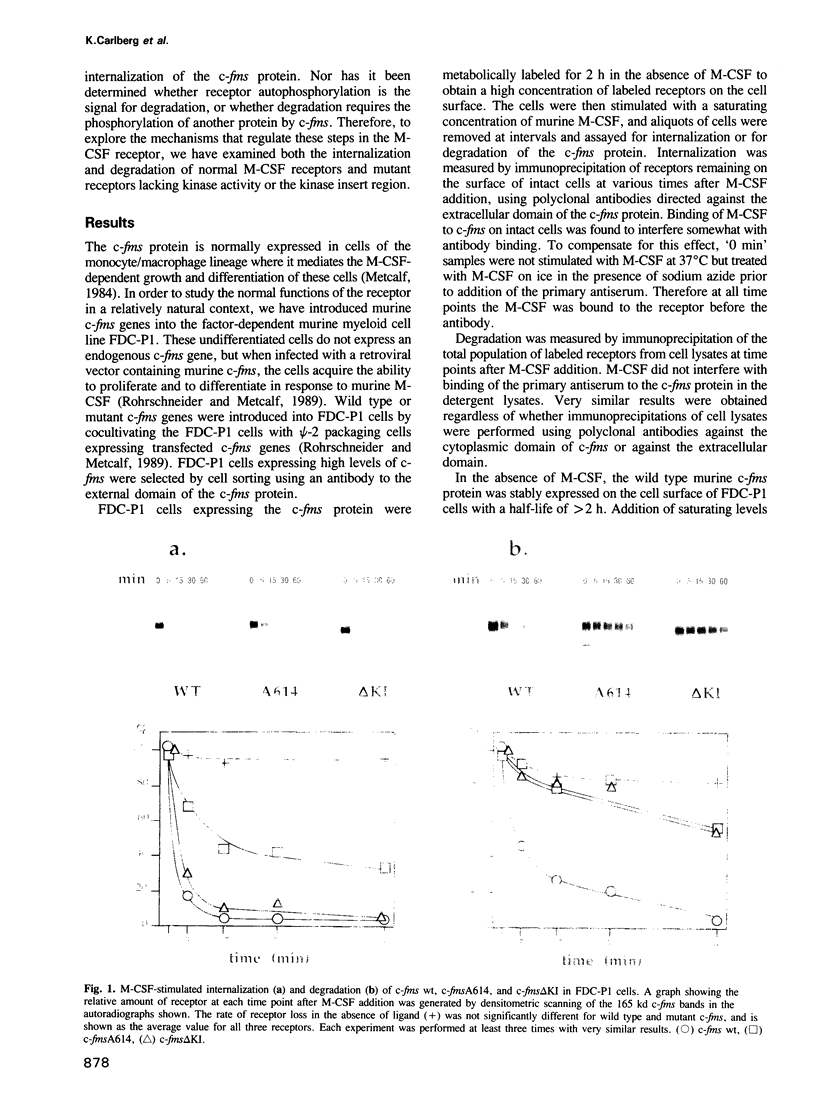
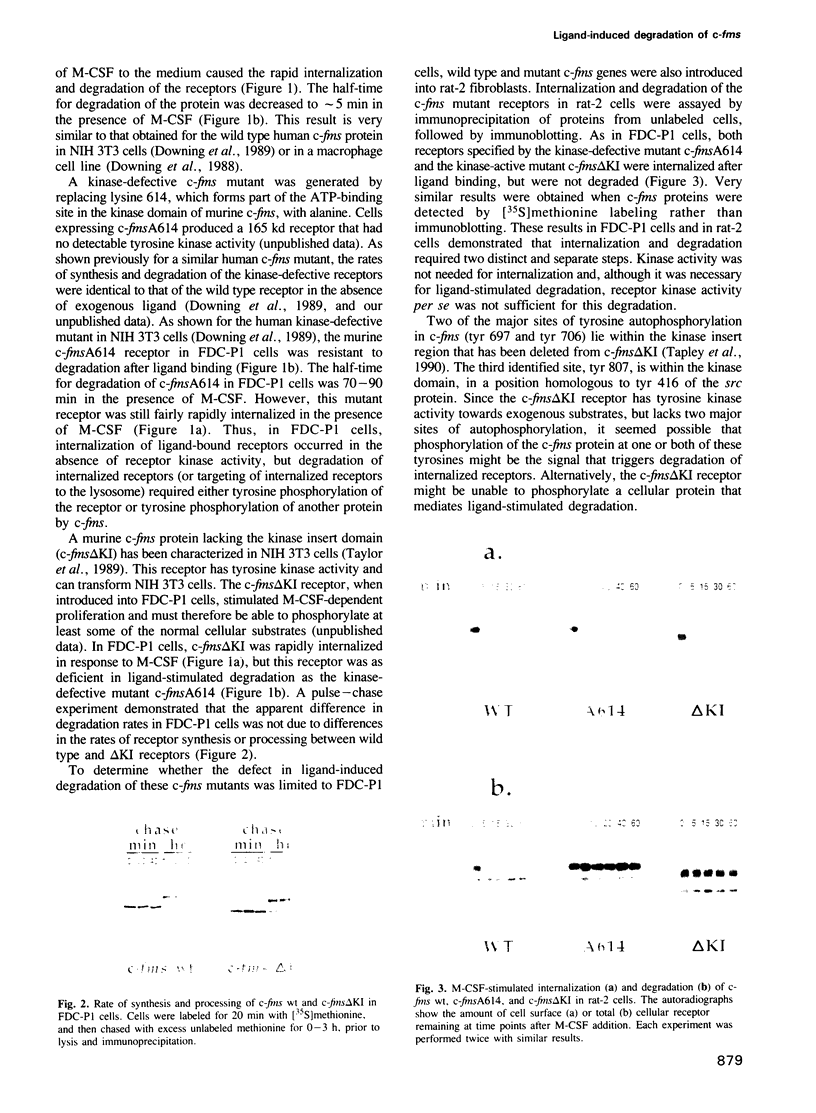
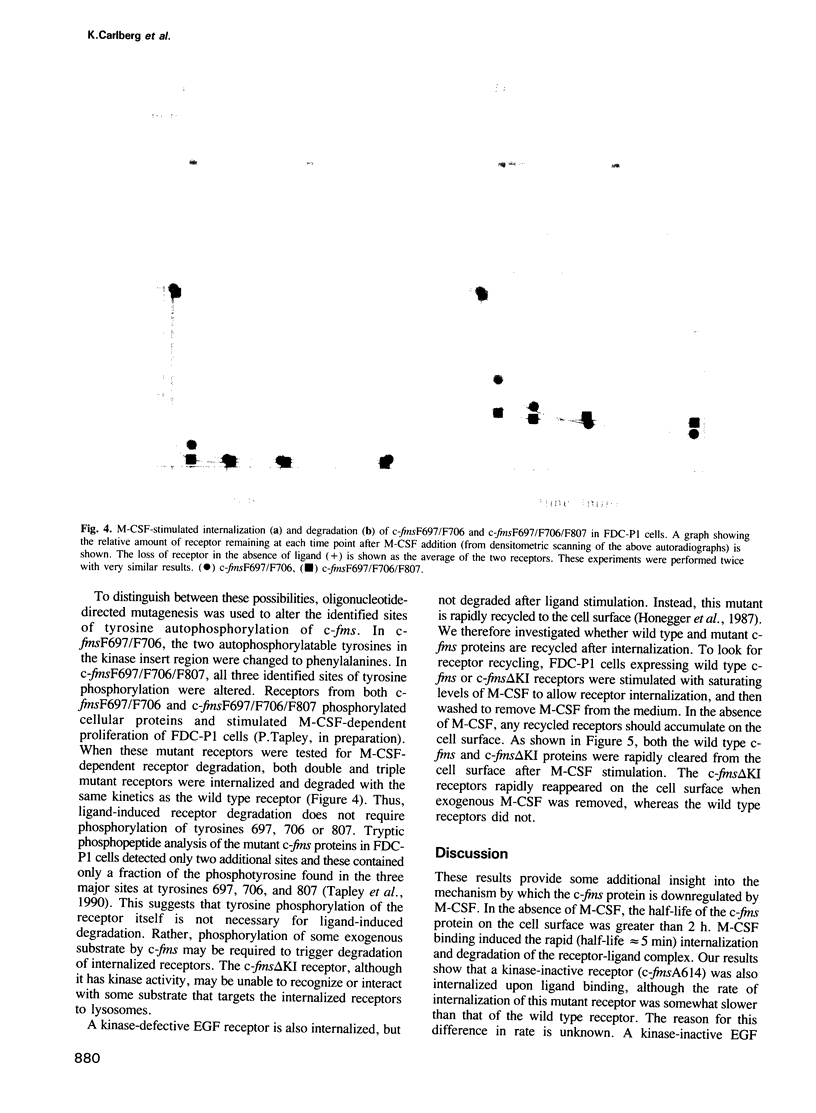
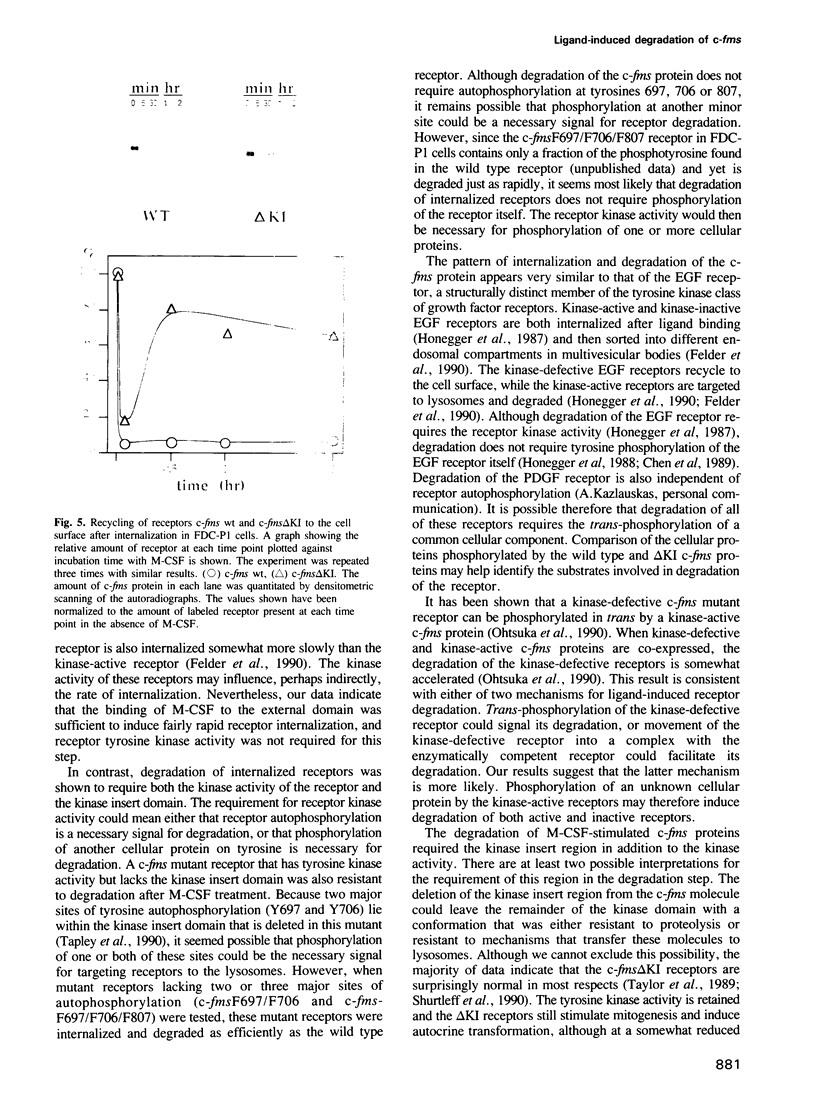
Molecular steps in endocytosis and degradation of the c-fms protein were analyzed by following the fate of mutated c-fms molecules after M-CSF binding. A mutant c-fms protein lacking tyrosine kinase activity was rapidly internalized after M-CSF binding but not degraded. Another mutant c-fms molecule that lacked most of the kinase insert region was similarly internalized after M-CSF binding and also not degraded. This indicates that the signal for internalization is separate from that directing degradation of the receptor. It has been shown previously that a c-fms mutant in which the kinase insert domain is deleted retains tyrosine kinase activity but lacks two major sites of autophosphorylation. The degradation step therefore requires both kinase activity and the kinase insert region whereas the internalization step is independent of these factors. The major sites of tyrosine autophosphorylation within the kinase insert region were next mutated to determine whether autophosphorylation in the kinase insert region of c-fms might be the signal that triggers degradation of internalized receptors. These mutant receptors were still rapidly degraded in response to M-CSF. Therefore, ligand-induced degradation of c-fms may require tyrosine phosphorylation of a protein other than the c-fms receptor itself and the kinase insert region may be necessary for recognition of this substrate.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backer J. M., Kahn C. R., White M. F. Tyrosine phosphorylation of the insulin receptor is not required for receptor internalization: studies in 2,4-dinitrophenol-treated cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3209–3213. doi: 10.1073/pnas.86.9.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne P. V., Guilbert L. J., Stanley E. R. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981 Dec;91(3 Pt 1):848–853. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Chen W. S., Lazar C. S., Lund K. A., Welsh J. B., Chang C. P., Walton G. M., Der C. J., Wiley H. S., Gill G. N., Rosenfeld M. G. Functional independence of the epidermal growth factor receptor from a domain required for ligand-induced internalization and calcium regulation. Cell. 1989 Oct 6;59(1):33–43. doi: 10.1016/0092-8674(89)90867-2. [DOI] [PubMed] [Google Scholar]
- Coussens L., Van Beveren C., Smith D., Chen E., Mitchell R. L., Isacke C. M., Verma I. M., Ullrich A. Structural alteration of viral homologue of receptor proto-oncogene fms at carboxyl terminus. Nature. 1986 Mar 20;320(6059):277–280. doi: 10.1038/320277a0. [DOI] [PubMed] [Google Scholar]
- Downing J. R., Rettenmier C. W., Sherr C. J. Ligand-induced tyrosine kinase activity of the colony-stimulating factor 1 receptor in a murine macrophage cell line. Mol Cell Biol. 1988 Apr;8(4):1795–1799. doi: 10.1128/mcb.8.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing J. R., Roussel M. F., Sherr C. J. Ligand and protein kinase C downmodulate the colony-stimulating factor 1 receptor by independent mechanisms. Mol Cell Biol. 1989 Jul;9(7):2890–2896. doi: 10.1128/mcb.9.7.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Felder S., Miller K., Moehren G., Ullrich A., Schlessinger J., Hopkins C. R. Kinase activity controls the sorting of the epidermal growth factor receptor within the multivesicular body. Cell. 1990 May 18;61(4):623–634. doi: 10.1016/0092-8674(90)90474-s. [DOI] [PubMed] [Google Scholar]
- Gentry L. E., Rohrschneider L. R., Casnellie J. E., Krebs E. G. Antibodies to a defined region of pp60src neutralize the tyrosine-specific kinase activity. J Biol Chem. 1983 Sep 25;258(18):11219–11228. [PubMed] [Google Scholar]
- Gliniak B. C., Rohrschneider L. R. Expression of the M-CSF receptor is controlled posttranscriptionally by the dominant actions of GM-CSF or multi-CSF. Cell. 1990 Nov 30;63(5):1073–1083. doi: 10.1016/0092-8674(90)90510-l. [DOI] [PubMed] [Google Scholar]
- Guilbert L. J., Stanley E. R. The interaction of 125I-colony-stimulating factor-1 with bone marrow-derived macrophages. J Biol Chem. 1986 Mar 25;261(9):4024–4032. [PubMed] [Google Scholar]
- Honegger A. M., Dull T. J., Felder S., Van Obberghen E., Bellot F., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Point mutation at the ATP binding site of EGF receptor abolishes protein-tyrosine kinase activity and alters cellular routing. Cell. 1987 Oct 23;51(2):199–209. doi: 10.1016/0092-8674(87)90147-4. [DOI] [PubMed] [Google Scholar]
- Honegger A. M., Schmidt A., Ullrich A., Schlessinger J. Separate endocytic pathways of kinase-defective and -active EGF receptor mutants expressed in same cells. J Cell Biol. 1990 May;110(5):1541–1548. doi: 10.1083/jcb.110.5.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger A., Dull T. J., Bellot F., Van Obberghen E., Szapary D., Schmidt A., Ullrich A., Schlessinger J. Biological activities of EGF-receptor mutants with individually altered autophosphorylation sites. EMBO J. 1988 Oct;7(10):3045–3052. doi: 10.1002/j.1460-2075.1988.tb03169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manger R., Najita L., Nichols E. J., Hakomori S., Rohrschneider L. Cell surface expression of the McDonough strain of feline sarcoma virus fms gene product (gp 140fms). Cell. 1984 Dec;39(2 Pt 1):327–337. doi: 10.1016/0092-8674(84)90011-4. [DOI] [PubMed] [Google Scholar]
- McClain D. A., Maegawa H., Lee J., Dull T. J., Ulrich A., Olefsky J. M. A mutant insulin receptor with defective tyrosine kinase displays no biologic activity and does not undergo endocytosis. J Biol Chem. 1987 Oct 25;262(30):14663–14671. [PubMed] [Google Scholar]
- Müller R., Slamon D. J., Adamson E. D., Tremblay J. M., Müller D., Cline M. J., Verma I. M. Transcription of c-onc genes c-rasKi and c-fms during mouse development. Mol Cell Biol. 1983 Jun;3(6):1062–1069. doi: 10.1128/mcb.3.6.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsuka M., Roussel M. F., Sherr C. J., Downing J. R. Ligand-induced phosphorylation of the colony-stimulating factor 1 receptor can occur through an intermolecular reaction that triggers receptor down modulation. Mol Cell Biol. 1990 Apr;10(4):1664–1671. doi: 10.1128/mcb.10.4.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reedijk M., Liu X. Q., Pawson T. Interactions of phosphatidylinositol kinase, GTPase-activating protein (GAP), and GAP-associated proteins with the colony-stimulating factor 1 receptor. Mol Cell Biol. 1990 Nov;10(11):5601–5608. doi: 10.1128/mcb.10.11.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regenstreif L. J., Rossant J. Expression of the c-fms proto-oncogene and of the cytokine, CSF-1, during mouse embryogenesis. Dev Biol. 1989 May;133(1):284–294. doi: 10.1016/0012-1606(89)90319-9. [DOI] [PubMed] [Google Scholar]
- Rettenmier C. W., Roussel M. F., Ashmun R. A., Ralph P., Price K., Sherr C. J. Synthesis of membrane-bound colony-stimulating factor 1 (CSF-1) and downmodulation of CSF-1 receptors in NIH 3T3 cells transformed by cotransfection of the human CSF-1 and c-fms (CSF-1 receptor) genes. Mol Cell Biol. 1987 Jul;7(7):2378–2387. doi: 10.1128/mcb.7.7.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Metcalf D. Induction of macrophage colony-stimulating factor-dependent growth and differentiation after introduction of the murine c-fms gene into FDC-P1 cells. Mol Cell Biol. 1989 Nov;9(11):5081–5092. doi: 10.1128/mcb.9.11.5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrschneider L. R., Rothwell V. M., Nicola N. A. Transformation of murine fibroblasts by a retrovirus encoding the murine c-fms proto-oncogene. Oncogene. 1989 Aug;4(8):1015–1022. [PubMed] [Google Scholar]
- Rothwell V. M., Rohrschneider L. R. Murine c-fms cDNA: cloning, sequence analysis and retroviral expression. Oncogene Res. 1987 Sep-Oct;1(4):311–324. [PubMed] [Google Scholar]
- Russell D. S., Gherzi R., Johnson E. L., Chou C. K., Rosen O. M. The protein-tyrosine kinase activity of the insulin receptor is necessary for insulin-mediated receptor down-regulation. J Biol Chem. 1987 Aug 25;262(24):11833–11840. [PubMed] [Google Scholar]
- Schlessinger J. Allosteric regulation of the epidermal growth factor receptor kinase. J Cell Biol. 1986 Dec;103(6 Pt 1):2067–2072. doi: 10.1083/jcb.103.6.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Shurtleff S. A., Downing J. R., Rock C. O., Hawkins S. A., Roussel M. F., Sherr C. J. Structural features of the colony-stimulating factor 1 receptor that affect its association with phosphatidylinositol 3-kinase. EMBO J. 1990 Aug;9(8):2415–2421. doi: 10.1002/j.1460-2075.1990.tb07417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M., Nüsslein-Volhard C. The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature. 1989 Apr 6;338(6215):478–483. doi: 10.1038/338478a0. [DOI] [PubMed] [Google Scholar]
- Stanley E. R., Guilbert L. J., Tushinski R. J., Bartelmez S. H. CSF-1--a mononuclear phagocyte lineage-specific hemopoietic growth factor. J Cell Biochem. 1983;21(2):151–159. doi: 10.1002/jcb.240210206. [DOI] [PubMed] [Google Scholar]
- Tapley P., Kazlauskas A., Cooper J. A., Rohrschneider L. R. Macrophage colony-stimulating factor-induced tyrosine phosphorylation of c-fms proteins expressed in FDC-P1 and BALB/c 3T3 cells. Mol Cell Biol. 1990 Jun;10(6):2528–2538. doi: 10.1128/mcb.10.6.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G. R., Reedijk M., Rothwell V., Rohrschneider L., Pawson T. The unique insert of cellular and viral fms protein tyrosine kinase domains is dispensable for enzymatic and transforming activities. EMBO J. 1989 Jul;8(7):2029–2037. doi: 10.1002/j.1460-2075.1989.tb03611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Wells A., Welsh J. B., Lazar C. S., Wiley H. S., Gill G. N., Rosenfeld M. G. Ligand-induced transformation by a noninternalizing epidermal growth factor receptor. Science. 1990 Feb 23;247(4945):962–964. doi: 10.1126/science.2305263. [DOI] [PubMed] [Google Scholar]
- Woolford J., McAuliffe A., Rohrschneider L. R. Activation of the feline c-fms proto-oncogene: multiple alterations are required to generate a fully transformed phenotype. Cell. 1988 Dec 23;55(6):965–977. doi: 10.1016/0092-8674(88)90242-5. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]