ABSTRACT
Migration and organization of the nucleus are essential for the proliferation and differentiation of cells, including neurons. However, the relationship between the positioning of the nucleus and cellular morphogenesis remains poorly understood. Inherited recessive cerebellar ataxia has been attributed to mutations in SYNE1, a component of the linker of nucleoskeleton and cytoskeleton (LINC) complex. Regardless, Syne1-mutant mice present with normal cerebellar development. The Sad1-Unc-84 homology (SUN)-domain proteins are located at the inner nuclear membrane and recruit Syne proteins through the KASH domain to the outer nuclear membrane. Here, we report an unrecognized contribution of Sun1 and Sun2 to the postnatal development of murine cerebellum. Mice depleted of Sun1 showed a marked reduction in the cerebellar volume, and this phenotype is exacerbated with additional loss of a Sun2 allele. Consistent with these histological changes, Sun1−/− and Sun1−/−Sun2+/− mice exhibited defective motor coordination. Results of immunohistochemical analyses suggested that Sun1 is highly expressed in Purkinje cells and recruits Syne2 to the periphery of the nucleus. Approximately 33% of Purkinje cells in Sun1−/− mice and 66% of Purkinje cells in Sun1−/−Sun2+/− mice were absent from the surface of the internal granule layer (IGL), whereas the proliferation and migration of granule neurons were unaffected. Furthermore, the Sun1−/−Sun2+/− Purkinje cells exhibited retarded primary dendrite specification, reduced dendritic complexity and aberrant patterning of synapses. Our findings reveal a cell-type-specific role for Sun1 and Sun2 in nucleokinesis during cerebellar development, and we propose the use of Sun-deficient mice as a model for studying cerebellar ataxia that is associated with mutation of human SYNE genes or loss of Purkinje cells.
KEY WORDS: Sun1, LINC complex, Cerebellar ataxia
Summary: Mice lacking Sun proteins serve as a working model to study SYNE1-associated cerebellar ataxia; they will also be useful in identifying therapeutic targets for neurodegenerative diseases involving Purkinje cell loss.
INTRODUCTION
The positioning of the nucleus affects the proliferation, migration and differentiation of neurons (Higginbotham and Gleeson, 2007; Morris et al., 1998; Zhang et al., 2009). Most neurons arise in the germinal zone of the lateral ventricles and migrate toward the pial surface in an inside-out manner. Two processes that affect the movement of neurons are interkinetic nuclear migration and nucleokinesis (Burke and Roux, 2009). Interkinetic nuclear migration involves cell-cycle-dependent movement of the nucleus within the cell from the apical to basal regions (i.e. during G1-S phase), before returning to the apical region in preparation for mitosis (Kosodo et al., 2011). By contrast, nucleokinesis involves movement of the neuron in three steps: neurite extension, migration of the microtubule organization center (MTOC) and finally repositioning of the nucleus (Tsai and Gleeson, 2005). Both interkinetic nuclear migration and nucleokinesis require proper coordination and connection with the cytoskeletal structure and the nucleus for accurate nuclear positioning. Although the majority of neuronal migration in the mammalian brain occurs during the embryonic stage, substantial migration also occurs postnatally. Understanding the mechanisms that initiate, maintain and terminate neuronal migration is key to mapping brain circuitry, and to gaining insight into both normal and pathological neurodevelopment (Ghashghaei et al., 2007).
Mammalian linker of nucleoskeleton and cytoskeleton (LINC) complexes comprise Sad1-Unc-84 homology (SUN)-domain proteins and Klarsicht/ANC-1/Syne homology (KASH)-domain-containing Syne proteins, which mechanically couple the nucleus and cytoskeleton (Chi et al., 2007; Crisp et al., 2006; Haque et al., 2006; Ostlund et al., 2009; Padmakumar et al., 2005). C-termini of Sun proteins are embedded in the inner nuclear membrane (INM) with a trimeric coiled-coil that predisposes them to bind to three KASH peptides in the perinuclear space (Sosa et al., 2012). The N-termini of Sun proteins engage with nuclear lamins, which comprise the nucleoskeleton (Chen et al., 2012, 2014; Chi et al., 2007; Crisp et al., 2006; Haque et al., 2006; Padmakumar et al., 2005). Loss of function in this connection has been shown to affect mammalian development in a tissue-dependent manner (Lei et al., 2009; Yu et al., 2011; Zhang et al., 2010, 2007, 2009), and the nervous system seems to be particularly affected. For example, Lmnb1-deficient (Lmnb1Δ/Δ) and Lmnb2−/− mice show defective neocortical layering (Coffinier et al., 2011; Vergnes et al., 2004). The proteins Syne1 and Syne2 play crucial roles in anchoring synaptic and non-synaptic myonuclei, which are important for the proper innervation of motor neurons (Zhang et al., 2010, 2007). It appears that Sun1 and Sun2 form redundant complexes with Syne2 to regulate interkinetic and radial neuronal migration in the cerebral cortex (Zhang et al., 2009).
Knockout of the Sun1 gene in mice causes infertility and impairs telomere attachment to the nuclear envelope, resulting in persistent double-stranded DNA breaks, as well as inefficient homologous pairing and synapse formation in meiosis (Chi et al., 2009; Ding et al., 2007). Interestingly, unlike Sun1−/− animals, Sun2−/− mice have no grossly discernible abnormalities and are reproductively normal (Lei et al., 2009), suggesting that the two proteins do not simply serve redundant roles. However, mice that have a homozygous deficiency of both Sun1 and Sun2 do not survive birth and show skeletal muscles with destructive myonuclear positioning in the syncytial cells, as well as abnormal lamination in the cerebral cortex (Lei et al., 2009; Zhang et al., 2009).
TRANSLATIONAL IMPACT.
Clinical issue
Nucleokinesis (movement of the nucleus) is a key process that influences neuron migration during embryonic and postnatal development. It requires proper coordination of different proteins that are associated with the microtubule or actin component of the cytoskeleton. The mammalian linker of nucleoskeleton and cytoskeleton (LINC) complex has been shown to mechanically couple the nucleus and the cytoskeleton for migration of the nucleus. It comprises Klarsicht/ANC-1/Syne homology (KASH)-domain-containing Syne proteins (such as Syne1 and Syne2) and the SUN-domain proteins, including Sun1 and Sun2 – which form redundant complexes with Syne2 to regulate neuronal migration in the cerebral cortex. Mutations in SYNE1 have been associated with inherited autosomal recessive cerebellar ataxia type 1 (ARCA1) in humans. However, development of cerebellum is normal in Syne1−/− mice, which prevents the application of this mouse model to the study of this human disease.
Results
In this study, the authors generated genetically modified mice that lack Sun1 and/or Sun2 in different allelic combinations, and they report that Sun1 and Sun2 contribute to the development of murine cerebellum. They show that Sun1 is highly expressed in cerebellar Purkinje cells and recruits Syne2 to the nuclear periphery. Sun1 and Sun2 have a dosage-dependent but non-redundant effect on the migration and dendritic morphogenesis of Purkinje cells. Mice deficient for Sun1 show a marked reduction of the cerebellar volume, and this phenotype is exacerbated with additional loss of a single Sun2 allele. Consistent with the histological findings on cerebellar alterations, Sun-deficient mice exhibit defective motor coordination.
Implications and future directions
This study shows that Sun1 and Sun2 play an important role during cerebellar development, in particular in regulating nucleokinesis in Purkinje cells. In view of the functional linkage between Sun and Syne proteins, the Sun-deficient mice generated here represent a promising model to study human SYNE-associated cerebellar ataxia. In addition, they can be used to identify potential therapeutic targets for intervention in cerebellar ataxia, as well as in neurodegenerative diseases associated with Purkinje-cell loss.
Mutations in SYNE1 are associated with human autosomal recessive cerebellar ataxia [e.g. autosomal recessive cerebellar ataxia type 1 (ARCA1) or autosomal recessive spinocerebellar ataxia-8 (SCAR8)]. Intronic SYNE1 mutations in these individuals affect gene splicing, which can result in the premature termination of the proteins and a loss of the KASH domain. Curiously, Syne1 knockout mice do not appear to recapitulate the human ARCA1 pathological phenotypes, thereby precluding these mice as a useful animal model (Zhang et al., 2009). We have previously generated Sun1−/− mice. The Sun1 deficiency affects mammalian gametogenesis and hearing (Chi et al., 2009; Horn et al., 2013). The Sun1−/− mice in our previous study appeared normal at birth and showed no apparent pathologies, except for infertility (Chi et al., 2009). However, in long-term follow up, ataxic movements were observed in many of these Sun1−/− mice, which is suggestive of cerebellar dysfunction. Here, we report our findings on the selective defect in the nuclear positioning and primary dendrite specification of Sun-deficient Purkinje cells, which resulted in ataxia-producing cerebellar dysmorphogenesis. Thus, Sun-deficient mice provide a model for understanding the mechanistic basis of Purkinje-cell-loss-associated ataxias and cerebellar function.
RESULTS
Sun1-deficient mice demonstrate defective cerebellar development
Through systematically phenotyping organ systems in Sun1−/− mice, we discovered that the average weight of the brains of adult Sun1−/− (n=12, 402.0±7.2 mg) mice was significantly lower than wild-type (WT) counterparts (WT, n=10, 449.2±5.1 mg, P<0.001; supplementary material Fig. S1A,B). Sun1−/− compared with WT mice showed a ∼25% reduction in cerebellar volume (based on measurements of the width and height of the cerebellum; Fig. 1A,B). These findings are remarkable because the average body weights did not differ between Sun1−/− and WT animals (WT: 24.4±0.653 g, n=31 vs. Sun1−/−: 25.6±1.06 g, n=20, P=0.3378; supplementary material Fig. S1C).
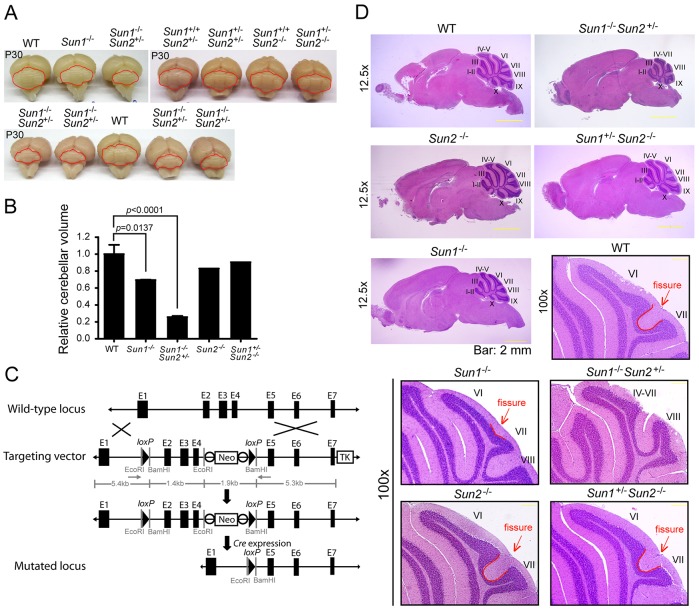
Fig. 1.
Sun1−/− mice show aberrant cerebellar development. (A) Pictures of mouse brains from 30-day-old mice with the indicated genotypes. The mouse cerebellums are outlined in red. (B) Statistics of relative cerebellum volume of the brains shown in A, calculated by using width×length2. The volume of cerebellums from Sun1−/− (P=0.0137) and Sun1−/−Sun2+/− (P<0.0001) mice is significantly reduced compared to that of WT. Student's t-test. (C) Generation of Sun2-knockout mice. The WT allele, the targeting vector and the mutated locus of Sun2 are presented in the scheme. The targeting vector contains the PGK-Neo gene (Neo) and the thymidine kinase gene (TK). The solid triangles represent loxP sites, and white circles are FRT sites. (D) H&E-stained sagittal sections of paraffin-embedded mouse brains from 30-day-old WT, Sun1−/−, Sun1−/−Sun2+/−, Sun2−/− and Sun1+/−Sun2−/− mice. Image magnifications (12.5× and 100×) are indicated. Numerals indicate the lobules, and the fissures between lobule VI and VII of cerebellums are outlined in red lines and indicated by red arrows. Scale bars: 2 mm (200 μm in magnification images).
Sun1−/−Sun2−/− double-null mice show profound growth retardation with severe multi-organ abnormalities and a failure to thrive at birth (Lei et al., 2009; Zhang et al., 2009). In Sun1−/−Sun2−/− mice, lamination of the cerebral neocortex is severely impaired owing to defective neuronal migration (Zhang et al., 2009). To study these observations in greater detail, we generated a new Sun2-conditional-knockout mouse (Fig. 1C; supplementary material Fig. S1C-E), in which exons 2 to 4 of Sun2 were deleted. Consistent with a previous report (Lei et al., 2009), whole-body depletion of Sun2 (i.e. Sun2−/−) did not lead to any distinguishing somatic organ abnormalities or changes in body weight, compared with WT mice (P=0.3185; supplementary material Fig. S1C). Our Sun1−/−Sun2−/− mice did not survive birth and therefore could not be studied for postnatal development. By contrast, when we bred our mice to create Sun1−/−Sun2+/− animals, this genotype did thrive postnatally, and the average body weight (21.59±0.866 g, n=21; supplementary material Fig. S1C) of these mice was only slightly less than that of WT controls (P=0.0108 comparing Sun1−/−Sun2+/− with WT). Sun1+/−Sun2−/− mice also appeared to be normal at birth, and the average body weight of these animals (24.25±0.726 g, n=16) was not statistically different (P=0.8797) from that of WT mice. In observations of gross anatomy, Sun1−/− and Sun1−/−Sun2+/−, but not Sun1+/−Sun2−/−, mice showed a marked decrease in cerebellar size compared with WT cohorts (Fig. 1A,B). Hematoxylin and eosin (H&E) staining revealed a difference in the foliation of cerebellum in neonatal Sun1−/− mice (supplementary material Fig. S1F). An absence of the intercrural fissure between lobules VI and VII (Fig. 1D, indicated by red arrows and red outlines) was significant in postnatal day (P)30 Sun1−/− mice. Moreover, foliation and fissuration of the cerebellar cortex was severely reduced in Sun1−/−Sun2+/− mice compared with WT animals (Fig. 1D). These results suggest discrete, non-redundant roles for Sun1 and Sun2 in cerebellar development.
Sun1−/− mice suffer from impaired motor coordination
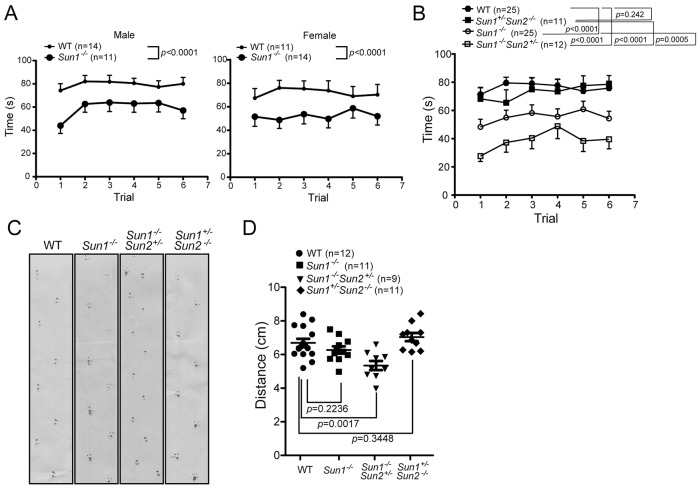
The histological differences described above and our observations of the behavior of Sun1−/−Sun2+/− mice (supplementary material Movies 1, 2) prompted us to investigate whether the Sun proteins are involved in cerebellar motor function. The rotarod test is an established method for evaluating motor coordination and balance in mice. Six rotarod trials were performed on age-matched (3-4 months old) WT, Sun1−/−, Sun1−/−Sun2+/− and Sun1+/−Sun2−/− mice, and the duration that the mice remained on the rod was recorded. No differences in gender were observed in the performance profiles (Fig. 2A); however, the motor coordination of Sun1−/− (P<0.0001) and Sun1−/−Sun2+/− (P<0.0001), but not Sun1+/−Sun2−/− (P=0.242), animals differed significantly from that of WT controls (Fig. 2B). A comparison of Sun1−/−Sun2+/− and Sun1−/− mice revealed that the former exhibited a worse motor coordination profile (P=0.0005) than the latter (Fig. 2B). The motor functions of our mice were further tested using footprint analysis. Sun1−/−Sun2+/− mice exhibited significant motor impairment, as demonstrated by a reduced length of the hindlimb stride compared with that of the WT cohort (P=0017; Fig. 2C). These results indicate that motor coordination is correlated with the extent of histological changes in the cerebellum of Sun1- or Sun2-deficient mice (Fig. 1).
Fig. 2.
The coordinated movement of mice deficient for Sun1 and/or Sun2. (A) Motor coordination of 3- to 4-month-old WT and Sun1−/− mice was tested using rotarod. The number of mice tested for each gender are indicated. The time to fall off the rotarod (latency) was recorded. (B) Similar to A, the time to fall off the rotarod from age-matched (3-4 months old) animals with genotypes WT, Sun1−/−, Sun1+/−Sun2−/−, and Sun1−/−Sun2+/−. Mean±s.e.m. for each genotype is presented. (C,D) Footprint analysis obtained from 5- to 6-month-old WT, Sun1−/−, Sun1+/−Sun2−/−, and Sun1−/−Sun2+/− animals. (D) Footprint analysis statistics and the mean±s.e.m. for each genotype is presented. Each data point represents the averaged stride length from 5 or 6 steps of each mouse. Student's t-test.
Differential contributions of Sun1 and Sun2 to the localization of Syne2 to the nuclear envelope in Purkinje cells
The cerebellar cortex of mature mice contains three well-defined cell layers surrounding the white matter and deep nuclei – the molecular layer, the Purkinje cell layer and the inner granule layer (IGL) (Sillitoe and Joyner, 2007). Immunofluorescent staining of WT cerebellum revealed that Sun1 was highly expressed in the nuclear envelope of Purkinje cells (marked by calbindin staining), compared with that in interneurons in the molecular layer or internal granular neurons in the IGL (Fig. 3A). By contrast, Sun2 was ubiquitously stained in all three layers of neurons in the cerebellar cortex (Fig. 3B).
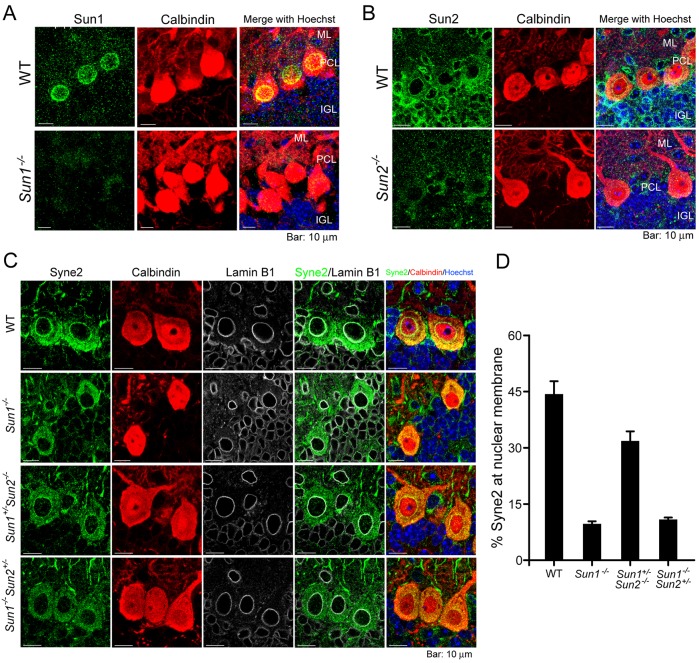
Fig. 3.
Sun1 recruits Syne2 to the nuclear membrane. (A) Expression and localization of Sun1 (green) in 14-day-old mouse cerebellum. The antibody against Sun1 specifically stained the nuclear rim of Purkinje cells. Parallel immunohistochemical staining of Sun1 in Sun1−/− cerebellum was used as a negative control. (B) Expression and localization of Sun2 (green) in 14-day-old mouse cerebellum by immunohistochemical staining. Parallel immunostaining of Sun2 in Sun2−/− cerebellum was used as a negative control. DNA was stained by Hoechst33342 (blue). ML, molecular layer; PCL, Purkinje cell layer; IGL, inner granule layer. (C) Expression and localization of Syne2 (green) in 14-day-old mouse cerebellum by immunofluorescent staining using paraffin-embedded tissue sections. Calbindin (red) and lamin B1 (gray) were co-immunostained to mark Purkinje cells and the nuclear membrane, respectively. (D) Percentage of Syne2 at the nuclear membrane. Intensities of fluorescence of Syne2 at the (nuclear membrane)/(total cells) were quantified by ImageJ. Eight to ten cells were quantified for each genotype. Mean±s.e.m. Scale bars: 10 μm.
Sun1 has been demonstrated to interact with the KASH domain of Syne proteins in the periplasmic space (Crisp et al., 2006; Ostlund et al., 2009; Sosa et al., 2012). The mammalian brain expresses several Syne isoforms (Zhang et al., 2010), including Syne1 and Syne2. To determine whether the Sun proteins serve to localize Syne isoforms in cerebellum, we examined Syne1 and Syne2 localization in cerebellar cells of Sun-deficient mice. Consistent with a previously published report (Gros-Louis et al., 2007), Syne1 immunoreactivity in WT Purkinje cells occurred primarily in the cytoplasm (supplementary material Fig. S2), but not in the nuclear membrane. By contrast, clear staining of Syne2 was observed at the nuclear membrane of WT Purkinje cells; however, this staining was significantly reduced when Sun1 was homozygously knocked out (i.e. in Sun1−/− and Sun1−/−Sun2+/− mice; Fig. 3C). Fig. 3D shows the relative quantities of Syne2 at the nuclear membrane in Purkinje cells from the indicated genotypes. Compared with WT cells, where 44% of Syne2 was located at the nuclear membrane, in Sun1-null cells (i.e. Sun1−/− and Sun1−/−Sun2+/−), approximately 14% of Syne2 was located at the nuclear membrane, whereas the heterozygous expression of a single Sun1 allele in a Sun2-null background (Sun1+/−Sun2−/−) increased the amount of Syne2 at the nuclear membrane to 32% (Fig. 3D). Thus, localization of Syne2 at the nuclear membrane is affected more by the presence of the Sun1-null genotype than by the Sun2-null genotype. The different physiological outcomes of cerebellar development in Sun1−/−Sun2+/− and Sun1+/−Sun2−/− mice could be attributed to the differential contributions of Sun1 and Sun2 to the recruitment of KASH-domain-containing Syne2 to the nuclear membrane of Purkinje cells.
Purkinje cells are positioned aberrantly in Sun1−/− mice
The mouse cerebrum reaches near maturity at birth; however, the cerebellum continues to grow postnatally (Goldowitz and Hamre, 1998; Millen and Gleeson, 2008). During cerebellar development, the granule cell precursors (GCPs) migrate to and proliferate at the surface of the cerebellum to form the external granule layer (EGL). In mice, the GCPs achieve peak proliferation at approximately P8 (Goldowitz and Hamre, 1998). GCPs subsequently exit the cell cycle, differentiate into mature granule cells and migrate radially, passing the developing Purkinje cells, to form the IGL. Therefore, we sought to determine whether the reduced size of Sun1−/− and Sun1−/−Sun2+/− cerebellums could be explained mechanistically through increased apoptosis or decreased cell proliferation. To investigate these possibilities, we performed staining of Ki67 (supplementary material Fig. S3A) and a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay (supplementary material Fig. S3B) on P7 littermates. No significant differences were observed between WT, Sun1−/− or Sun1−/−Sun2+/− cerebellums in the TUNEL or the Ki67 assays. Furthermore, the incorporation rate of BrdU at the EGL was not different between WT and Sun1−/− mice (supplementary material Fig. S3C,D). These results suggest that increased apoptosis or decreased cellular proliferation does not account for the reduction in cerebellar size in Sun1−/− or Sun1−/−Sun2+/− mice.
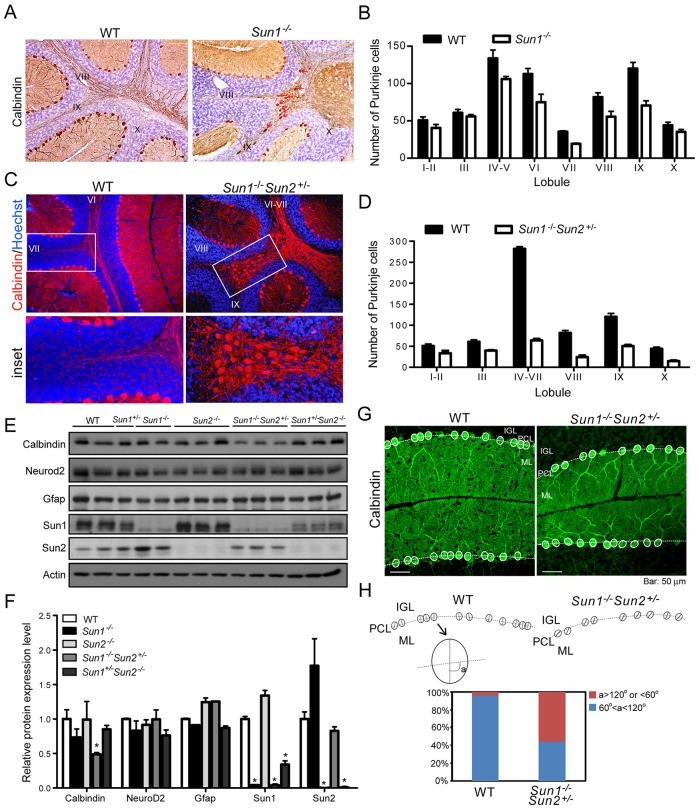
In the Sun1−/− and Sun1−/−Sun2+/− cerebellums, the newly generated granule cells in the EGL migrated completely in order to form the IGL, with no residual granule cells remaining at the pial surface (Fig. 1D). Thus, an EGL layer was not observed in the mature Sun1−/− or Sun1−/−Sun2+/− cerebellums (Fig. 1D). By contrast, although the newly generated Sun1−/− Purkinje cells were able to migrate radially from the ventricular zone to localize at the periphery of the cortex underneath the EGL, some of them failed to reach their final destination (supplementary material Fig. S1G). The positions of some Sun1−/− Purkinje cells were superimposed with the inner granule neurons in P0 cerebellum (supplementary material Fig. S1G, indicated by arrowheads). Furthermore, a large increase was observed in the number of Purkinje cells in the white matter of cerebellums in P30 Sun1−/− and Sun1−/−Sun2+/− mice (Fig. 4A-D). Detailed quantification revealed that, compared with WT cerebellums, the number of Purkinje cells at the surface of the IGL was reduced by 33% in Sun1−/− (Fig. 4B) and 66% in Sun1−/−Sun2+/− mice (Fig. 4D), with lobules IV to VII exhibiting the most significant reductions (Fig. 4B,D). Consistent with the histological findings, protein expression of calbindin, but not Neurod2 (expressed in mature neurons) or Gfap (expressed in radial glia), was significantly reduced in Sun1−/−Sun2+/− cerebellum compared with the WT (Fig. 4E,F). We noted that the expression level of Sun2 in Sun1−/− cerebellum increased approximately 70% compared with that of the WT (Chen et al., 2012), and increased approximately 90% compared with that of the Sun1−/−Sun2+/− cohorts. These results indicated that Sun2 might partially complement the function of Sun1.
Fig. 4.
Aberrant positioning of Purkinje cells in Sun1-null cerebellum. (A) Cerebellum from 30-day-old mouse stained for calbindin (brown) by immunohistochemistry. Some calbindin-positive cells were found in the white matter of Sun1−/− cerebellum (indicated by red arrows). Numerals indicate the lobules. (B) Quantification of Purkinje cells in each sagittal section at the surface of the IGL in WT and Sun1−/− cerebellum. Statistics (mean±s.e.m.) were obtained from independent measurements of four mice for each genotype. (C) Calbindin (red) in 30-day-old mouse cerebellum was stained using immunofluorescence. Many calbindin-positive cells were found in the white matter of Sun1−/−Sun2+/− cerebellum (boxed, inset). DNA was stained by Hoechst 33342 (blue). (D) Quantification of Purkinje cells in each sagittal section at the surface of the IGL in WT and Sun1−/−Sun2+/− cerebellum. Statistics (mean±s.e.m.) were obtained from independent measurements of four mice for each genotype. (E) Western blot analysis for protein expression of calbindin, Neurod2, Gfap, Sun1 and Sun2 in cerebellums of adult mice with the indicated genotypes. (F) Quantification of the relative protein expression levels shown in E. Statistics are mean±s.e.m. *P<0.05, Student's t-test. (G) Purkinje cells of 30-day-old WT and Sun1−/−Sun2+/− cerebellums were immunostained for calbindin (green). The Purkinje cell bodies are outlined with white ovals. The white dashed lines mark the border between the Purkinje cell layer (PCL) and the inner granule layer (IGL). ML, molecular layer. (H, upper scheme) Schematic presentation of the alignment of the Purkinje cell soma shown in G. The angle (indicated as ‘a’) of the long axis of the soma, and the border (dashed line) between the IGL and ML was measured for each Purkinje cell. (Lower graph) Quantification of ‘a’ in 50 Purkinje cells of each genotype. 96% of WT and 44% of Sun1−/−Sun2+/− Purkinje cells showed ‘a’ between 60° and 120°; and 4% of WT and 56% of Sun1−/−Sun2+/− Purkinje cells showed ‘a’ >120° or <60°. Scale bars: 50 μm.
Moreover, for the Sun1−/−Sun2+/− Purkinje cells that were correctly positioned at the surface of the IGL, more than 50% of the somas (Fig. 4G, outlined with the solid white line) appeared to have a distorted orientation with their cell soma oriented (measured as angle ‘a’) non-perpendicularly (defined as >120° or <60°) to the border (marked by white dashed lines) of the Purkinje cell layer and the IGL (Fig. 4H). These results suggest that the aberrant positioning and maturation of Purkinje cells, but not the proliferation or migration of granule cells, is the primary cause of the reduced cerebellar volume in Sun1-deficient mice.
Sun-deficient mice have defective dendritic arborization in Purkinje cells
Dendrite arborization specifies neuronal connectivity and integration. Sun proteins interact with Syne proteins to connect the cytoskeletal microtubules and actin, which are important for neuronal plasticity (Cingolani and Goda, 2008; Conde and Cáceres, 2009). Thus, we sought to determine whether, in addition to Purkinje cell loss, defective dendritic morphogenesis occurs in the Sun1−/−Sun2+/− cerebellum.
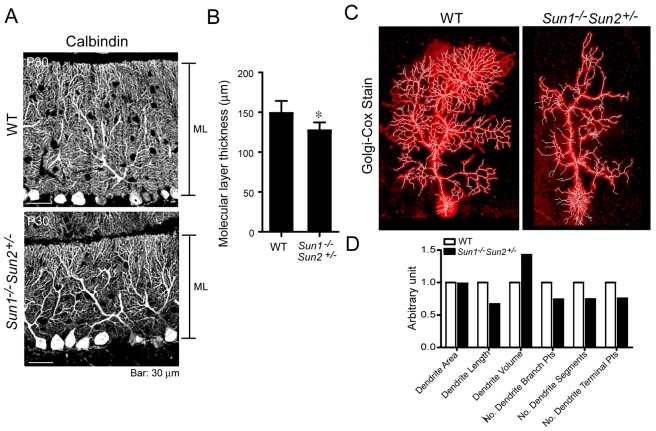
In P30 littermates, in which the cerebellum is mature, we observed a significant reduction in the thickness of the molecular layer in Sun1−/−Sun2+/− cerebellum, compared with that of WT (Fig. 5A,B). Dendritic arborization of a single Purkinje cell was studied using the Golgi–Cox silver-impregnation method (Fig. 5C; Ranjan and Mallick, 2010). Limited by the capricious nature of this technique (Pilati et al., 2008), we compared the dendrite pattern of a WT and a Sun1−/−Sun2+/− Purkinje cell of comparable dendritic area (Fig. 5C,D). The total dendrite length, segments and branching points (analyzed using Imaris 7.2 software) were reduced by approximately 20-30% in the Sun1−/−Sun2+/− Purkinje cell compared with those of the WT cell. These results suggest that Sun1 and Sun2 not only contribute to the positioning of Purkinje cells, but also to the branching of their dendrites.
Fig. 5.
Mice deficient for Sun genes show retarded dendritic arborization. (A) Immunolabeling of P30 WT and Sun1−/−Sun2+/− Purkinje cell dendrites using an antibody against calbindin (gray). ML, molecular layer. (B) Thickness of the ML in the cerebellum of P30 WT and Sun1−/−Sun2+/− mice shown in A. The mean±s.e.m. was obtained from four mice of each genotype. *P<0.05, Student's t-test. (C) Golgi-Cox silver stain (red) of a WT (left panel) and a Sun1−/−Sun2+/− (right panel) Purkinje cell. The total dendritic area, length, volume, segments, branching points, segments and terminal points was analyzed using the Imaris 7.2 Filament Tracer function (white lines). The quantification of the data and statistics are shown in D. Scale bars: 30 μm.
Specification of Purkinje primary dendrites and patterning of synapses are affected in Sun-deficient cerebellum
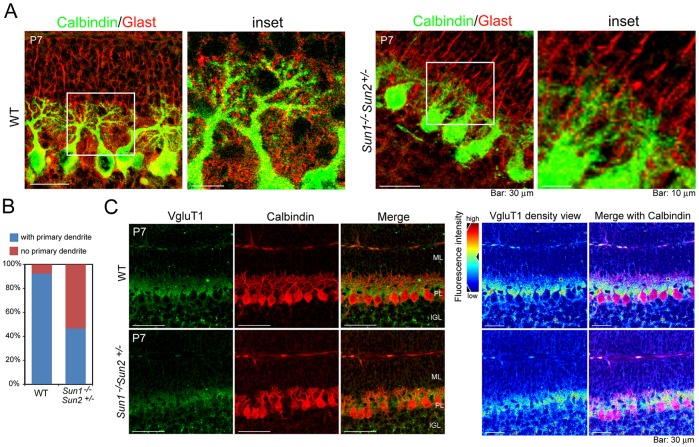
We next determined dendrite morphogenesis in developing cerebellum. In mice, Purkinje cell somas extend multiple dendrites in random orientations during the first postnatal week (supplementary material Fig. S4; Tanaka et al., 2006). A single or a few primary dendrites are determined during the second postnatal week, which extend and branch later, forming the most elaborate dendritic tree among the neurons in the central nervous system (McKay and Turner, 2005). Radial glia provide a structural basis for the directional growth of Purkinje cell dendrites (Lordkipanidze and Dunaevsky, 2005). In WT Purkinje cells, dendrites grew directionally in consonance with radial glia processes [visualized by staining for glutamate astrocyte-specific transporter (Glast), a marker of radial glia; Fig. 6A]. By contrast, the Purkinje cells in Sun1−/−Sun2+/− cerebellum that localized at the IGL surface showed distinctly retarded extension of primary dendrites and dendritic arbor, whereas the radial glia processes formed normally (Fig. 6A). Only 46.6% (27 out of 58) of P7 Purkinje cells in Sun1−/−Sun2+/− cerebellum showed significant extension of primary dendrites compared with 92.3% (48 out of 52) in WT cohorts (Fig. 6B).
Fig. 6.
Aberrant synaptic patterning in the developing cerebellum upon deficiency of Sun genes. (A) Cerebellum sagittal sections from 7-day-old WT and Sun1−/−Sun2+/− mice were immunostained for calbindin (green) to mark Purkinje cells and Glast (red) to label radial glia. (B) Quantification of primary dendrites of P7 WT and Sun1−/−Sun2+/− Purkinje cells, which show promising primary dendritic outgrowth. Approximately 50 Purkinje cells were quantified for each genotype. (C, left panel) Immunofluorescent staining of calbindin (red) and VgluT1 (green) in P7 WT and Sun1−/−Sun2+/− cerebellum. (Right panel) Density view of VgluT1. The color code of the expression levels is presented on the left. The ‘warm’ colors represent high expression levels, and the ‘cold’ colors represent low expression levels.
In developing murine cerebellum, vesicular glutamate transporter 1 (VgluT1 encoded by the Slc17a7 gene) that is localized to the excitatory axon terminals of parallel fibers arising from granule cells has been shown to mediate glutamate uptake into synaptic vesicles of Purkinje neurons (Hioki et al., 2003). Vesicular glutamate transporters are crucial for the balance between excitation and inhibition (Fremeau et al., 2004), and are involved in brain disorders, such as schizophrenia, Alzheimer's disease and Parkinson's disease (Harrison et al., 2003; Kashani et al., 2007; Kirvell et al., 2006). Using immunofluorescent staining, we found that VgluT1 was clustered at the interface of the molecular layer and the GCL in P7 WT cerebellum, forming goblet-like structures, surrounding the newly extended primary dendrites of Purkinje cells (Fig. 6C). By contrast, in Sun1−/−Sun2+/− cerebellum, the distribution of VgluT1 was relatively amorphous (Fig. 6C). The aberrant synaptic patterning of VgluT1 suggests that the efficiency of neurotransmission is affected by the retarded morphogenesis of the Purkinje dendritic tree upon deficiency of Sun1 or Sun2.
DISCUSSION
Early and adult neurogenesis in mammals requires the accurate translocation of neuronal precursors. Genes that are associated with the microtubules or actin cytoskeleton have been shown to non-redundantly influence mammalian brain size through effects on neurite formation, extension and branching, and synaptogenesis (Bond and Woods, 2006; Hoogenraad and Bradke, 2009; Hotulainen and Hoogenraad, 2010). The inner nuclear membrane proteins Sun1 and Sun2 bridge the nucleoskeleton and cytoskeleton, and have been reported to play a role in embryonic neurogenesis (Zhang et al., 2009). In this study, we further demonstrate that Sun1 and Sun2 have a dosage-dependent but nonreciprocal effect on the migration and dendritic morphogenesis of Purkinje cells during postnatal cerebellar development. Unlike Sun1−/−Sun2−/− or Syne1−/−Syne2−/− mice, which suffer from defective neocortical lamination and fail to thrive after birth (Zhang et al., 2009), the viable Sun1−/− and Sun−/−Sun2+/− animals, which present with cerebellar ataxia, might serve as a working model for the study of the molecular mechanisms underlying SYNE1-associated ARCA1 (Dupré et al., 2007; Gros-Louis et al., 2007), as well as for the identification of therapeutic targets in neurodegenerative diseases involving Purkinje cell loss (Millen and Gleeson, 2008). In addition, the Sun2-conditional-knockout mouse developed in this study could be further applied in investigations of LINC-associated human diseases and tissue patterning during development.
The nucleus is the most prominent organelle involved in repositioning the soma, also known as nucleokinesis (Tsai and Gleeson, 2005). Neuronal migration is generally initiated by chemotaxis, which triggers a process of elongation and the translocation of the nucleus (Marín et al., 2006). In this study, we showed that Sun1 is selectively expressed in Purkinje cells and mediates the positioning of the nucleus and cellular morphogenesis (Figs 3-5), whereas migration of granule neurons is not affected by the depletion of Sun1 or Sun2 (Fig. 1D). During development, Purkinje cells and granule neurons are generated from two anatomically distinct progenitor zones – the ventricular zone and the more dorsally located rhombic lip, respectively (Millen and Gleeson, 2008; Sillitoe and Joyner, 2007). Upon differentiation, the postmitotic Purkinje cells leave the ventricular zone and migrate radially (inside-out) within the developing anlage. Conversely, cells exiting the rhombic lip migrate over the anlage and proliferate to form the EGL, finally migrating radially through the Purkinje cell layer in an outside-in manner to form the IGL of the mature cerebellum (Millen and Gleeson, 2008; Sillitoe and Joyner, 2007). In Sun1−/−Sun2+/− cerebellum, approximately 66% of the Purkinje cells failed to present at the surface of the IGL, whereas the granule neurons migrated normally (Fig. 1D; Fig. 4C,D). One plausible explanation for the selective interference of Sun1 depletion in the migration of Purkinje cells but not granule neurons is that Sun1 is only required for the inside-out and not outside-in radial migration of neurons. Another possible explanation is that Purkinje cells require stronger pulling forces for the nucleus owing to the large size of their nuclei compared with those of other neurons. Nevertheless, these results indicate that the radial migration of Purkinje cells but not of granule neurons during cerebellar development is dependent on the expression of Sun1.
Isoforms of Syne proteins have been reported to have tissue-specific expression (Rajgor et al., 2012; Randles et al., 2010; Yu et al., 2011; Zhang et al., 2007, 2009). Thus, Sun proteins are responsible for recruiting KASH-domain-containing Syne isoforms but the roles they play might be dependent on the tissue. The failure to detect localization of Syne1 at the nuclear membrane (supplementary material Fig. S2) might be due to variations in the expression of the Syne1 isoforms (Rajgor et al., 2012; Randles et al., 2010). It has previously been reported that singular depletion of either Sun1 or Sun2 does not affect the nuclear membrane localization of Syne1 or Syne2 in the skeletal muscle or retinal cells (Lei et al., 2009; Yu et al., 2011). However, a separate study has demonstrated that Sun1 is required for the recruitment of Nesprin-4 to the outer nuclear membrane in order to maintain the basal localization of the nuclei in outer hair cells (Horn et al., 2013). In this study, we found that Sun1 is selectively expressed in Purkinje cells (Fig. 3A) and is more important than Sun2 in recruiting Syne2 to the nuclear membrane (Fig. 3C,D). The cerebellar phenotypes in Sun1−/−Sun2+/− and Sun1+/−Sun2−/− mice suggest that Sun1 and Sun2 contribute differently to the robustness of the LINC complex in a dosage- and cell-type-dependent manner (Horn et al., 2013; Lei et al., 2009; Yu et al., 2011; Zhang et al., 2009).
In addition to Sun1, Sun2, Syne1 and Syne2, proteins that interact with the LINC complex – such as the nuclear lamins B1 and B2 (encoded by Lmnb1 and Lmnb2, respectively) – are also associated with neuronal migration and brain development. Lmnb1-deficient mice (Lmnb1Δ/Δ) die shortly after birth with immature lungs, abnormalities in bones and layering of neurons in the brain (Coffinier et al., 2011; Vergnes et al., 2004). Lmnb2−/− embryos develop normally with respect to size, except for the brain (Coffinier et al., 2010). The Lmnb2−/− cerebral cortex and cerebellum are small, with abnormal layering of neurons. The common features of defective neuronal migration in Sun1 and Sun2 double knockout, Syne1 and Syne2 double knockout, Lmnb1Δ/Δ, and Lmnb2−/− mice indicate that the connection between the nuclear lamina and the cytoskeleton plays a unique role in neurogenesis (Young et al., 2012; Zhang et al., 2009).
Most eukaryotic cells are spatially asymmetric or polarized. Cell polarity is determined by the geometry of the nucleus, the MTOC, intracellular organelles and the cytoskeleton. Cytoskeletal microtubules and actin filaments are active players during initial neuronal polarization (Bryant and Mostov, 2008; Hoogenraad and Bradke, 2009). Specification of primary dendrites of cerebellar Purkinje cells has been shown to be regulated by cell polarity (Tanabe et al., 2010). Therefore, it would be not surprising if compromising the link between the nucleoskeleton and cytoskeleton through the LINC complex results in retarded primary dendrite specification (Fig. 6A,B). The phenotype in Sun1−/− and Sun1−/−Sun2+/− cerebellum is similar to that of reeler mutants, the neurons of which do not recognize their proper localization and orientation upon completing the migration pathway (Falconer, 1951; Niu et al., 2008; Tissir and Goffinet, 2003). In the reeler brain, normal neuronal classes are formed, connections between axons and dendritic trees present no serious defects, and gliogenesis and myelination are not directly altered. However, signal transduction that is mediated by cell-cell interaction is disturbed, as well as the expression of cell-cell interaction molecules (Tissir and Goffinet, 2003). The reduced expression of reelin is associated with schizophrenia and bipolar disorder (Impagnatiello et al., 1998). Intriguingly, a large-scale genome-wide association analysis of bipolar disorder identified SYNE1 as one of the susceptible loci (Psychiatric GWAS Consortium Bipolar Disorder Working Group, 2011). Therefore, future research addressing whether mice that are deficient in Sun1 and/or Sun2, or Syne1 and/or Syne2 show cognition phenotypes such as schizophrenia or bipolar disorder could be beneficial in the field of psychiatric medicine.
MATERIALS AND METHODS
Generation of Sun1- and Sun2-knockout mice
The Sun1−/− animals were created as described previously (Chi et al., 2009). To generate the Sun2-knockout mouse, the conditional knockout vector that targets exons 2-4 of mouse Sun2 was constructed (Fig. 1C) by cloning a 5.4-kb fragment containing exon 1 of the mouse Sun2 gene into vector pL253 using the recombineering method (Liu et al., 2003). A loxP locus was inserted at the 3′-end of exon 1, followed by a 1.4-kb sequence of the Sun2 gene containing exons 2-4. A FRT locus, Neo gene, and a second FRT and loxP loci were cloned at the 3′ end of exon 4, followed by a 5.3-kb sequence of the Sun2 gene containing exons 5-7. The HSV-TK gene was placed at the 3′-end for negative selection. The constructed Sun2 target vector was introduced into 129/Sv embryonic stem (ES) cells by using electroporation and were then double-selected using G418 and ganciclovir. Expression of the construct in surviving clones was confirmed by Southern blotting using probes amplified from genomic DNA with primers (Probe 5′-1F: 5ʹ-CAGCACCCTCACATGAGTTGGGT-3ʹ; Probe5′-1R: 5ʹ-TGTACAGGACTGTACTTCCGGCCA-3ʹ). Genomic DNAs extracted from ES cells were digested with BamHI. The WT clones showed a band at 10,800 bp, whereas the successfully integrated clones gave a signal at 8500 bp (supplementary material Fig. S1D). The heterozygous ES cells were electroporated with a vector that expressed Cre recombinase. ES cells that lost exons 2-4 of Sun2 were confirmed by PCR analyses (supplementary material Fig. S1E) using primers [WT forward (CU): 5ʹ-ATAGTCGACGCATGCTATATGGAGCTGTA-3ʹ; knockout forward (GU): 5ʹ-ATAGTCGACTGGGAACTTCCCATCTCCTC-3ʹ; common reverse (JD): 5ʹ-ATAGCGGCCGCTCCCACTCCATGGTGACCTC-3ʹ]. The PCR product of the WT clones is 489 bp and that of the knockout clones is 795 bp, and their sequences were verified by sequencing. The Sun2+/− ES cells were injected into C57BL/6 blastocysts. Founder animals that were >90% mosaic were crossbred with C57BL/6 mice, and F1 mice were screened for germline transmission by using PCR. Two successful Sun2+/− germline-transmitted litters were obtained and were then crossed with WT C57BL/6Nar1 mice to reduce the genetic background of 129/Sv. These Sun2-knockout mice (deleted for exons 2-4) differed from other Sun2-knockout mice (deleted for exons 11-16 of Sun2) that were previously generated by Lei et al. (2009). Sun2+/− animals were crossed with Sun1+/− animals to generate Sun1+/−Sun2+/− double haplo-deficient mice, and then Sun1+/−Sun2+/− mice were crossed with Sun1+/−Sun2+/− mice to generate double-knockout animals.
Immunohistochemistry
Mice were anesthetized and perfused with PBS followed by 4% paraformaldehyde in PBS. Mouse brains were dehydrolyzed and embedded in paraffin. For immunohistochemistry, tissue paraffin sections were deparaffinized using xylene and ethanol. After rehydration, antigen retrieval was achieved by placing the slides in 100°C citrate buffer, pH 6.4 for 50 min. Slides were then rinsed with ddH2O and PBS successively. Endogenous peroxides were quenched by 0.5% H2O2 for 10 min. To prevent non-specific binding, slides were blocked with 1% BSA for 30 min and then incubated with primary antibodies diluted in PBS at room temperature for 1.5 h. After three washes with PBS, a biotin-free MM HRP-polymer (Biocare Medical) was added, followed by development of the color using 3,3′-diaminobenzidine (DAB) substrate-chromogen (Biocare Medical). The nucleus was stained with hematoxylin.
Immunofluorescent staining and confocal microscopy
Cryopreserved or deparaffinized tissue sections were post-fixed in 4% paraformaldehyde for 30 min at room temperature and permeabilized with 0.5% Triton X-100 in PBS for 30 min at room temperature. To block nonspecific binding, tissues were incubated with 1% BSA or 2% goat serum in PBS for 30 min. Primary antibodies were then applied and incubated for 1.5 h at room temperature. Fluorescence-conjugated (Alexa-488, Alexa-568 or Alexa-633) secondary antibodies were used for detection. Cell nuclei were counterstained with Hoechst 33342 (Invitrogen), and the slides were mounted with Prolong Gold antifade reagents (Invitrogen).
Antibodies
The mouse anti-Sun1 antibody was raised in rabbit and purified with protein-A agarose as described previously (Chi et al., 2009). The rat anti-BrdU and rabbit anti-Syne1, rabbit anti-Sun2, rabbit anti-VgluT1, rabbit anti-Neurod2 and rabbit anti-Gfap antibodies were obtained from Abcam; the mouse anti-calbindin and mouse anti-β-Actin antibodies were from Sigma-Aldrich; the rabbit anti-Ki67 antibody was from Thermo; the guinea pig anti-Glast antibody was from Millipore; the rabbit anti-Syne2 antibody was from Genetex.
TUNEL assay
DNA strand breaks were detected by using the TUNEL assay, as described by the manufacturer (Chemicon). Terminal deoxynucleotidyl transferase (TdT) catalyzes a template-independent addition of nucleotide triphosphates to the 3′-OH ends of double-stranded or single-stranded DNA. In brief, perfused brain cryo tissue sections were fixed in 1% paraformaldehyde in PBS at room temperature for 10 min. After two washes with PBS, tissues were post-fixed in precooled ethanol:acetic acid 2:1 for 5 min at −20°C. After another two washes of PBS for 5 min, the tissue specimens were incubated with equilibrium buffer at room temperature for 10 min, followed by application of the working strength TdT enzyme and FITC-conjugated nucleotides in a humidified chamber at 37°C for 1.5 h. The reaction was stopped by using working strength stop/wash buffer, and the nuclei were counterstained with Hoechst 33342.
Rotarod behavior test
Age-matched animals (3-4 months old) were placed on the rod with a constant rotating speed of 20 rpm in order to get familiarized with the rotarod apparatus (Rotamex-5, Columbus Instruments) 1 day before the testing. On the trial day, animals were tested by increasing the rod speed from 0 to 30 rpm in 30 s, the test then continued at 30 rpm for an additional 60 s. The time to fall off the rotarod (latency) was recorded; animals were then returned to the home cage. Six trials were recorded within 1 day, with an interval of 30 min between each trial. The procedure and use of the animals were approved (protocol number: NHRI-IACUC-098086-A and NHRI-IACUC-098043-A) by the Institutional Animal Care and Use Committee (IACUC) of National Health Research Institutes (NHRI, Zhunan, Miaoli County, Taiwan).
Hindpaw footprint test
The hind paws of mice at 5-6 months old were dipped into black non-toxic paint. Mice were allowed to walk through a plastic tunnel (9×9×40 cm), the floor of which was covered with a sheet of white paper (9×40 cm). Step length for each mouse was analyzed. The step length is defined as the average distance of 5-6 steps. The procedure was approved by IACUC of NHRI (protocol number: NHRI-IACUC-098043-A).
Golgi-Cox stain
The Golgi-Cox method for staining neurons in the brain was performed according to the protocol described by Ranjan and Mallick (2010). In brief, brains from adult mice (3-4 months old) were removed and washed with distilled water followed by freshly prepared Golgi-Cox solution (5% sodium dichromate, 5% mercuric chloride, 5% potassium dichromate). Cerebellums were sliced sagittally (approximately 5-mm thick) and then covered with Golgi-Cox solution and incubated at 37°C in the dark for 7 days. At the end of incubation, 200-μm thick sections were prepared from each block using a vibratome (VT1200, Leica). Slices were rinsed twice (5 min each) in distilled water to remove traces of impregnating solution and then processed with the following procedures: dehydrated in 50% alcohol for 5 min, soaked in ammonia solution (3:1, ammonia:distilled water) for 5 min, rinsed twice (5 min each) in distilled water, soaked in 5% sodium thiosulfate for 10 min in dark, rinsed twice for 2 min each in distilled water, and dehydrated successively twice (10 min each) in 70%, 80%, 95%, and 99.5% ethanol, cleared in xylene, and mounted in Permount mounting medium (Surgipath) on glass slides. Images were taken on a Nikon Ti-E microscope, and the images were processed using NIS element or Helicon Focus (Heliconsoft) software.
Western blotting
Total cell lysates from vermis of mouse cerebellums were extracted using RIPA buffer [50 mM HEPES, pH 7.3, 150 mM NaCl, 2 mM EDTA, 20 mM β-gylcerophosphate, 0.1 mM Na3VO4, 1 mM NaF, 0.5 mM DTT and protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN)] containing 0.5% NP-40 plus rigorous sonication. The lysates were analyzed by using SDS-PAGE, followed by transfer to polyvinylidene fluoride (PVDF, Millipore) membranes and blotting with primary antibodies. Corresponding alkaline-phosphatase-conjugated secondary antibodies (Sigma-Aldrich) were added, and the blots were developed by chemiluminescence following the manufacturer's protocol (PerkinElmer).
Supplementary Material
Acknowledgements
We thank staff at the NHRI Animal Center and NHRI Optic Core Facility for technical support. We thank the technical services provided by the ‘Transgenic Mouse Model Core Facility of the National Core Facility Program for Biotechnology, Ministry of Science and Technology, Taiwan’ and the ‘Gene Knockout Mouse Core Laboratory of National Taiwan University Center of Genomic Medicine’. We also thank Lily I. Chen, Elizabeth Williams and Jerrold M. Ward for support with pathology analyses of the animals. This paper is dedicated to the memory of Dr Kuan-Teh Jeang.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Y.-H.C. and K.-T.J. designed the study. Y.-H.C., J.-Y.W., I.-S.Y., S.-W.L., C.-Y.C., C.-C.H. and W.-P.W. generated Sun2-knockout mice and performed the experiments. Y.-H.C. analyzed the data. Y.-H.C. and K.-T.J. wrote the manuscript.
Funding
This work was supported by intramural grants to Y.-H.C. from the National Health Research Institutes, Taiwan [NHRI 01A1-CSPP13-014]; and Ministry of Science and Technology, Taiwan [NSC101-2311-B-400-004-MY3]; and by intramural funds of National Institute of Allergy and Infectious (NIAID), National Institutes of Health; and an NIAID contract to SoBran, Inc.
Supplementary material
Supplementary material available online at http://dmm.biologists.org/lookup/suppl/doi:10.1242/dmm.019240/-/DC1
References
- Bond J. and Woods C. G. (2006). Cytoskeletal genes regulating brain size. Curr. Opin. Cell Biol. 18, 95-101. 10.1016/j.ceb.2005.11.004 [DOI] [PubMed] [Google Scholar]
- Bryant D. M. and Mostov K. E. (2008). From cells to organs: building polarized tissue. Nat. Rev. Mol. Cell Biol. 9, 887-901. 10.1038/nrm2523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B. and Roux K. J. (2009). Nuclei take a position: managing nuclear location. Dev. Cell 17, 587-597. 10.1016/j.devcel.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Chen C.-Y., Chi Y.-H., Mutalif R. A., Starost M. F., Myers T. G., Anderson S. A., Stewart C. L. and Jeang K.-T. (2012). Accumulation of the inner nuclear envelope protein sun1 is pathogenic in progeric and dystrophic laminopathies. Cell 149, 565-577. 10.1016/j.cell.2012.01.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z.-J., Wang W.-P., Chen Y.-C., Wang J.-Y., Lin W.-H., Tai L.-A., Liou G.-G., Yang C.-S. and Chi Y.-H. (2014). Dysregulated interactions between lamin A and SUN1 induce abnormalities in the nuclear envelope and endoplasmic reticulum in progeric laminopathies. J. Cell Sci. 127, 1792-1804. 10.1242/jcs.139683 [DOI] [PubMed] [Google Scholar]
- Chi Y.-H., Haller K., Peloponese J.-M. Jr. and Jeang K.-T. (2007). Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J. Biol. Chem. 282, 27447-27458. 10.1074/jbc.M703098200 [DOI] [PubMed] [Google Scholar]
- Chi Y.-H., Cheng L. I., Myers T., Ward J. M., Williams E., Su Q., Faucette L., Wang J.-Y. and Jeang K.-T. (2009). Requirement for Sun1 in the expression of meiotic reproductive genes and piRNA. Development 136, 965-973. 10.1242/dev.029868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani L. A. and Goda Y. (2008). Actin in action: the interplay between the actin cytoskeleton and synaptic efficacy. Nat. Rev. Neurosci. 9, 344-356. 10.1038/nrn2373 [DOI] [PubMed] [Google Scholar]
- Coffinier C., Chang S. Y., Nobumori C., Tu Y., Farber E. A., Toth J. I., Fong L. G. and Young S. G. (2010). Abnormal development of the cerebral cortex and cerebellum in the setting of lamin B2 deficiency. Proc. Natl. Acad. Sci. USA 107, 5076-5081. 10.1073/pnas.0908790107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffinier C., Jung H.-J., Nobumori C., Chang S., Tu Y., Barnes R. H., Yoshinaga Y., de Jong P. J., Vergnes L., Reue K. et al. (2011). Deficiencies in lamin B1 and lamin B2 cause neurodevelopmental defects and distinct nuclear shape abnormalities in neurons. Mol. Biol. Cell 22, 4683-4693. 10.1091/mbc.E11-06-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde C. and Cáceres A. (2009). Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 10, 319-332. 10.1038/nrn2631 [DOI] [PubMed] [Google Scholar]
- Crisp M., Liu Q., Roux K., Rattner J. B., Shanahan C., Burke B., Stahl P. D. and Hodzic D. (2006). Coupling of the nucleus and cytoplasm: role of the LINC complex. J. Cell Biol. 172, 41-53. 10.1083/jcb.200509124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Xu R., Yu J., Xu T., Zhuang Y. and Han M. (2007). SUN1 is required for telomere attachment to nuclear envelope and gametogenesis in mice. Dev. Cell 12, 863-872. 10.1016/j.devcel.2007.03.018 [DOI] [PubMed] [Google Scholar]
- Dupré N., Gros-Louis F., Chrestian N., Verreault S., Brunet D., de Verteuil D., Brais B., Bouchard J.-P. and Rouleau G. A. (2007). Clinical and genetic study of autosomal recessive cerebellar ataxia type 1. Ann. Neurol. 62, 93-98. 10.1002/ana.21143 [DOI] [PubMed] [Google Scholar]
- Falconer D. S. (1951). Two new mutants, ‘Trembler’ and ‘Reeler’, with neurological actions in the house mouse (Mus musculus L.). J. Genet. 50, 192-205. 10.1007/BF02996215 [DOI] [PubMed] [Google Scholar]
- Fremeau R. T. Jr, Voglmaier S., Seal R. P. and Edwards R. H. (2004). VGLUTs define subsets of excitatory neurons and suggest novel roles for glutamate. Trends Neurosci. 27, 98-103. 10.1016/j.tins.2003.11.005 [DOI] [PubMed] [Google Scholar]
- Ghashghaei H. T., Lai C. and Anton E. S. (2007). Neuronal migration in the adult brain: are we there yet? Nat. Rev. Neurosci. 8, 141-151. 10.1038/nrn2074 [DOI] [PubMed] [Google Scholar]
- Goldowitz D. and Hamre K. (1998). The cells and molecules that make a cerebellum. Trends Neurosci. 21, 375-382. 10.1016/S0166-2236(98)01313-7 [DOI] [PubMed] [Google Scholar]
- Gros-Louis F., Dupré N., Dion P., Fox M. A., Laurent S., Verreault S., Sanes J. R., Bouchard J.-P. and Rouleau G. A. (2007). Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat. Genet. 39, 80-85. 10.1038/ng1927 [DOI] [PubMed] [Google Scholar]
- Haque F., Lloyd D. J., Smallwood D. T., Dent C. L., Shanahan C. M., Fry A. M., Trembath R. C. and Shackleton S. (2006). SUN1 interacts with nuclear lamin A and cytoplasmic nesprins to provide a physical connection between the nuclear lamina and the cytoskeleton. Mol. Cell. Biol. 26, 3738-3751. 10.1128/MCB.26.10.3738-3751.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. J., Law A. J. and Eastwood S. L. (2003). Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann. N. Y. Acad. Sci. 1003, 94-101. 10.1196/annals.1300.006 [DOI] [PubMed] [Google Scholar]
- Higginbotham H. R. and Gleeson J. G. (2007). The centrosome in neuronal development. Trends Neurosci. 30, 276-283. 10.1016/j.tins.2007.04.001 [DOI] [PubMed] [Google Scholar]
- Hioki H., Fujiyama F., Taki K., Tomioka R., Furuta T., Tamamaki N. and Kaneko T. (2003). Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neuroscience 117, 1-6. 10.1016/S0306-4522(02)00943-0 [DOI] [PubMed] [Google Scholar]
- Hoogenraad C. C. and Bradke F. (2009). Control of neuronal polarity and plasticity--a renaissance for microtubules? Trends Cell Biol. 19, 669-676. 10.1016/j.tcb.2009.08.006 [DOI] [PubMed] [Google Scholar]
- Horn H. F., Brownstein Z., Lenz D. R., Shivatzki S., Dror A. A., Dagan-Rosenfeld O., Friedman L. M., Roux K. J., Kozlov S., Jeang K.-T. et al. (2013). The LINC complex is essential for hearing. J. Clin. Invest 123, 740-750. 10.1172/JCI66911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P. and Hoogenraad C. C. (2010). Actin in dendritic spines: connecting dynamics to function. J. Cell Biol. 189, 619-629. 10.1083/jcb.201003008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Impagnatiello F., Guidotti A. R., Pesold C., Dwivedi Y., Caruncho H., Pisu M. G., Uzunov D. P., Smalheiser N. R., Davis J. M., Pandey G. N. et al. (1998). A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. USA 95, 15718-15723. 10.1073/pnas.95.26.15718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashani A., Betancur C., Giros B., Hirsch E. and El Mestikawy S. (2007). Altered expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in Parkinson disease. Neurobiol. Aging 28, 568-578. 10.1016/j.neurobiolaging.2006.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirvell S. L., Esiri M. and Francis P. T. (2006). Down-regulation of vesicular glutamate transporters precedes cell loss and pathology in Alzheimer's disease. J. Neurochem. 98, 939-950. 10.1111/j.1471-4159.2006.03935.x [DOI] [PubMed] [Google Scholar]
- Kosodo Y., Suetsugu T., Suda M., Mimori-Kiyosue Y., Toida K., Baba S. A., Kimura A. and Matsuzaki F. (2011). Regulation of interkinetic nuclear migration by cell cycle-coupled active and passive mechanisms in the developing brain. EMBO J. 30, 1690-1704. 10.1038/emboj.2011.81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei K., Zhang X., Ding X., Guo X., Chen M., Zhu B., Xu T., Zhuang Y., Xu R. and Han M. (2009). SUN1 and SUN2 play critical but partially redundant roles in anchoring nuclei in skeletal muscle cells in mice. Proc. Natl. Acad. Sci. USA 106, 10207-10212. 10.1073/pnas.0812037106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P., Jenkins N. A. and Copeland N. G. (2003). A highly efficient recombineering-based method for generating conditional knockout mutations. Genome Res. 13, 476-484. 10.1101/gr.749203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lordkipanidze T. and Dunaevsky A. (2005). Purkinje cell dendrites grow in alignment with Bergmann glia. Glia 51, 229-234. 10.1002/glia.20200 [DOI] [PubMed] [Google Scholar]
- Marín O., Valdeolmillos M. and Moya F. (2006). Neurons in motion: same principles for different shapes? Trends Neurosci. 29, 655-661. 10.1016/j.tins.2006.10.001 [DOI] [PubMed] [Google Scholar]
- McKay B. E. and Turner R. W. (2005). Physiological and morphological development of the rat cerebellar Purkinje cell. J. Physiol 567, 829-850. 10.1113/jphysiol.2005.089383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millen K. J. and Gleeson J. G. (2008). Cerebellar development and disease. Curr. Opin. Neurobiol. 18, 12-19. 10.1016/j.conb.2008.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. R., Efimov V. P. and Xiang X. (1998). Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 8, 467-470. 10.1016/S0962-8924(98)01389-0 [DOI] [PubMed] [Google Scholar]
- Niu S., Yabut O. and D'Arcangelo G. (2008). The Reelin signaling pathway promotes dendritic spine development in hippocampal neurons. J. Neurosci. 28, 10339-10348. 10.1523/JNEUROSCI.1917-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostlund C., Folker E. S., Choi J. C., Gomes E. R., Gundersen G. G. and Worman H. J. (2009). Dynamics and molecular interactions of linker of nucleoskeleton and cytoskeleton (LINC) complex proteins. J. Cell Sci. 122, 4099-4108. 10.1242/jcs.057075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmakumar V. C., Libotte T., Lu W., Zaim H., Abraham S., Noegel A. A., Gotzmann J., Foisner R. and Karakesisoglou I. (2005). The inner nuclear membrane protein Sun1 mediates the anchorage of Nesprin-2 to the nuclear envelope. J. Cell Sci. 118, 3419-3430. 10.1242/jcs.02471 [DOI] [PubMed] [Google Scholar]
- Pilati N., Barker M., Panteleimonitis S., Donga R. and Hamann M. (2008). A rapid method combining Golgi and Nissl staining to study neuronal morphology and cytoarchitecture. J. Histochem. Cytochem. 56, 539-550. 10.1369/jhc.2008.950246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychiatric GWAS Consortium Bipolar Disorder Working Group (2011). Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. Nat. Genet. 43, 977-983. 10.1038/ng.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajgor D., Mellad J. A., Autore F., Zhang Q. and Shanahan C. M. (2012). Multiple novel nesprin-1 and nesprin-2 variants act as versatile tissue-specific intracellular scaffolds. PLoS. ONE 7, e40098 10.1371/journal.pone.0040098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randles K. N., Lam L. T., Sewry C. A., Puckelwartz M., Furling D., Wehnert M., McNally E. M. and Morris G. E. (2010). Nesprins, but not sun proteins, switch isoforms at the nuclear envelope during muscle development. Dev. Dyn. 239, 998-1009. 10.1002/dvdy.22229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A. and Mallick B. N. (2010). A modified method for consistent and reliable Golgi-cox staining in significantly reduced time. Front. Neurol. 1, 157 10.3389/fneur.2010.00157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillitoe R. V. and Joyner A. L. (2007). Morphology, molecular codes, and circuitry produce the three-dimensional complexity of the cerebellum. Annu. Rev. Cell Dev. Biol. 23, 549-577. 10.1146/annurev.cellbio.23.090506.123237 [DOI] [PubMed] [Google Scholar]
- Sosa B. A., Rothballer A., Kutay U. and Schwartz T. U. (2012). LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell 149, 1035-1047. 10.1016/j.cell.2012.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe K., Kani S., Shimizu T., Bae Y.-K., Abe T. and Hibi M. (2010). Atypical protein kinase C regulates primary dendrite specification of cerebellar Purkinje cells by localizing Golgi apparatus. J. Neurosci. 30, 16983-16992. 10.1523/JNEUROSCI.3352-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M., Yanagawa Y., Obata K. and Marunouchi T. (2006). Dendritic morphogenesis of cerebellar Purkinje cells through extension and retraction revealed by long-term tracking of living cells in vitro. Neuroscience 141, 663-674. 10.1016/j.neuroscience.2006.04.044 [DOI] [PubMed] [Google Scholar]
- Tissir F. and Goffinet A. M. (2003). Reelin and brain development. Nat. Rev. Neurosci. 4, 496-505. 10.1038/nrn1113 [DOI] [PubMed] [Google Scholar]
- Tsai L.-H. and Gleeson J. G. (2005). Nucleokinesis in neuronal migration. Neuron 46, 383-388. 10.1016/j.neuron.2005.04.013 [DOI] [PubMed] [Google Scholar]
- Vergnes L., Peterfy M., Bergo M. O., Young S. G. and Reue K. (2004). Lamin B1 is required for mouse development and nuclear integrity. Proc. Natl. Acad. Sci. USA 101, 10428-10433. 10.1073/pnas.0401424101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Jung H.-J., Coffinier C. and Fong L. G. (2012). Understanding the roles of nuclear A- and B-type lamins in brain development. J. Biol. Chem. 287, 16103-16110. 10.1074/jbc.R112.354407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Lei K., Zhou M., Craft C. M., Xu G., Xu T., Zhuang Y., Xu R. and Han M. (2011). KASH protein Syne-2/Nesprin-2 and SUN proteins SUN1/2 mediate nuclear migration during mammalian retinal development. Hum. Mol. Genet. 20, 1061-1073. 10.1093/hmg/ddq549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xu R., Zhu B., Yang X., Ding X., Duan S., Xu T., Zhuang Y. and Han M. (2007). Syne-1 and Syne-2 play crucial roles in myonuclear anchorage and motor neuron innervation. Development 134, 901-908. 10.1242/dev.02783 [DOI] [PubMed] [Google Scholar]
- Zhang X., Lei K., Yuan X., Wu X., Zhuang Y., Xu T., Xu R. and Han M. (2009). SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64, 173-187. 10.1016/j.neuron.2009.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Felder A., Liu Y., Guo L. T., Lange S., Dalton N. D., Gu Y., Peterson K. L., Mizisin A. P., Shelton G. D. et al. (2010). Nesprin 1 is critical for nuclear positioning and anchorage. Hum. Mol. Genet. 19, 329-341. 10.1093/hmg/ddp499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.